 Research Article
Research Article
Acetylcholine Transmission Related to Age Evaluated by the Quantitative Electroencephalographic Vigilance-Index
Rolf Ekedahl* and NeuFydi Stockholm
Department of Neurology, NeuFydi Stockholm, Sweden
Rolf Ekedahl, Department of Neurology, NeuFydi Stockholm, Sweden.
Received Date: August 27, 2019; Published Date: August 30, 2019
Abstract
Introduction: The objective for this study is to evaluate if there is an age-related decrease in the amount of available Acetylcholine for neurotransmission and to ensure that standard value for Vigilance-index is not age-dependent.
Methods:The average value for Vigilance-index, the ratio of Eyes open (E.O.) / Eyes closed (E.Cl.) average powers, based on four Electroencephalographic EEG epochs with eyes closed, and four epochs with eyes open calculated for a group of 30 healthy individuals aged between 17-67 years. The average Vigilance-index for this group compared with the average Vigilance-index for a baseline examination of 40 patients aged 66-93 years with memory complaints and examined for suspected dementia, with Vigilance-index below < 0.3, the normal value. Also, the correlation between age and Vigilance-index evaluated for the two groups and compared with the Pearson correlation coefficient and Spearman’s rank correlation coefficient.
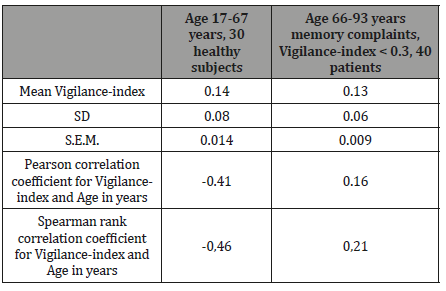
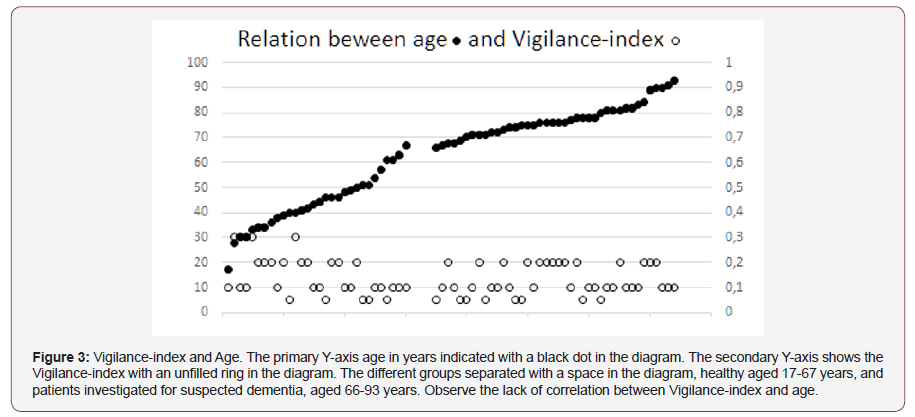
Results:There was no apparent correlation between age and Vigilance-index when the diagram for 17-93 years of age (Figure 3) inspected visually. The Vigilance-index average value was similar for the younger group (0.14) and the elderly group (0.13). The correlation coefficients were inverse for the younger group, Pearson`s, -0.41 and Spearman`s rank correlation coefficient, -0.46 showing a decreasing Vigilance-index to age. A weak positive correlation to age for the elderly group, Pearson`s 0.16, and Spearman`s rank correlation coefficient 0.21, though within normal values range for Vigilance-index.
ConclusionThere were no substantial relation to age for Vigilance-index and no decisive age-related decrease in cholinergic transmission. Vigilance-index can, therefore reliable be used to determine pathological cholinergic status and identify dementia diseases with a cholinergic deficit, such as Alzheimer`s and Lewy body dementia irrespective of age. Other causes of dementia and diseases or conditions with dementia symptoms without cholinergic deficit also be separated irrespective of age.
Keywords: Electroencephalography; Desynchronization; Dementia; Acetylcholine; Cholinergic agents; Aged; Humans; Biomarkers; Reference Values; Synaptic Transmission
Introduction
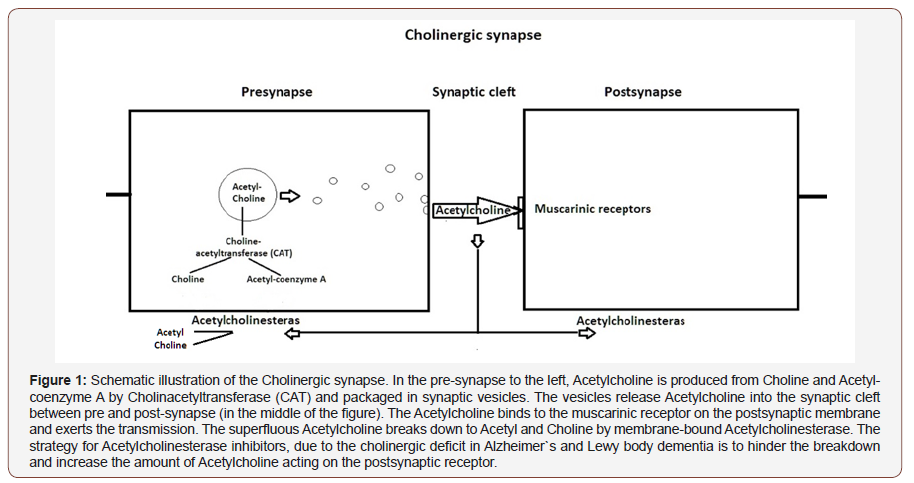
The recently presented method to measure cholinergic status via the Vigilance-index, a Quantitative Electroencephalographic (EEG) analysis, can identify early probable Alzheimer`s and Lewy body dementia even when cognitive testing is non-pathological with mini-mental examination scores (MMSE) [1-2]. The Vigilanceindex can evaluate the effect of Acetylcholinesterase (AchE) inhibitor treatment [2], due to the Vigilance-index reflects the postsynaptic impact of Acetylcholine, hindering the breakdown from Acetylcholinesterase in the synaptic cleft and improve the postsynaptic effect by increasing the amount of available Acetylcholine. The reliability to measure Acetylcholine effect and use it to identify Acetylcholine for pathological conditions with a cholinergic deficit as in Alzheimer’s (AD) and Lewy body dementia (LBD) makes it necessary to exclude age-related decrease in the Acetylcholine effect so a normal value can also be ensured for aged patients. Historically, different attempts to study neurons containing Acetylcholine and their relation to dementia disease, particularly for Alzheimer’s dementia, have been studied. From histologic and neurochemical necropsy studies of Alzheimer’s patient’s brains, a selective loss of central cholinergic neurons demonstrated which overlapped with the areas that had the highest density of neurofibrillary tangles and senile plaques [3-4].
There was also a relation between the reduction of Choline acetyltransferase (CAT), which synthesize Acetylcholine, and correlation to intellectual impairment measured with memory tests. The muscarinic cholinergic receptor binding activity was close to what has been observed in healthy brains [5]. These results suggest a close relation between Alzheimer’s dementia and the cholinergic system (Figure 1). In mentioned studies above the pathological reductions in the demented patients was compared with elderly non-demented patients used for a standard value. It has been speculated, if there is an age-related decline in Cholinergic function based on healthy young subjects that received Scopolamine, a central functioning anticholinergic drug, and aged individuals which demonstrated the same impact on memory functions profile compared to healthy young subjects without Scopolamine [6]. Necropsy findings indicated an increasing reduction of CAT in the Temporal lobe due to age in non-demented cases [7]. Cerebrospinal fluid levels of Acetylcholinesterase (AchE) increased after treatment with Acetylcholinesterase inhibitor treatment of AD patients [8], which might reflect increased levels of Acetylcholine in the synaptic cleft and enhancing the effect on the postsynaptic membrane and a compensatory upregulation of (AchE) to increase the breakdown of Acetylcholine. However, the Acetylcholine transmission and its relation to age, have not been directly studied except the observations in the studies of Vigilanceindex, there several of the aged patients had Vigilance-index values comparable with healthy young subjects and also had nonpathological MMSE [1-2] (Figure 1).
Materials and Methods
Two groups were analyzed, one group of 30 healthy persons, 17- 67 years of age and one group of 40 persons with normal Vigilanceindex (< 0.3), sent to a memory clinic due to suspected dementia. The group healthy subjects had no complaints of memory disturbance or any self-reported alcohol or drug abuse, nor any anticholinergic medication. The group with memory complaints were examined at memory clinic was selected due to Vigilance-index < 0.3, which represented normal cholinergic status [2]. EEG:s recorded at awake patients, sitting on regular chairs and instructed to open eyes for 30 seconds and close eyes for 90 seconds, four of these EEG epochs with eyes closed and with eyes open analyzed and an average value calculated for respective eyes opened and eyes closed epochs. This procedure ensured alertness and vigilance of the patients, which also monitored by the EEG assistant during the recording session. A few patients and parts of the EEG recordings that showed signs of drowsiness, and patients who were very tense or nervous with low EEG amplitudes during eye-closure comparable to magnitudes at eye-opening [8], were excluded from the analyses. Of the EEG epochs, approximately eighty seconds of eyes closed and twentyfive seconds of eyes open analyzed.
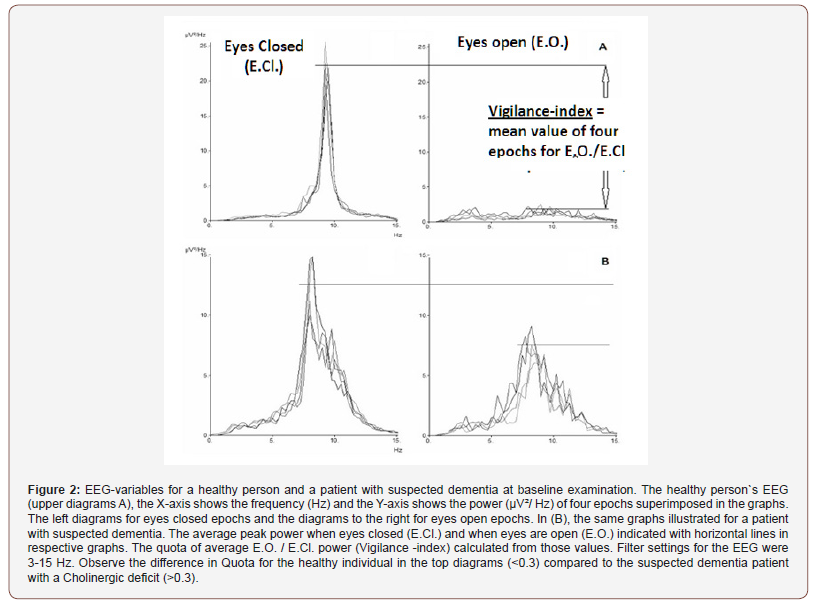
To avoid analyzes of arousal reaction and eye movement artifacts he first ten seconds excluded from the closed eyes epochs, of the similar reason the first three seconds omitted of the open eyes epochs. Rarely slightly shorter epochs analyzed, due to brief episodes of recording interference. Average peak-frequency and peak power for the four eyes closure epochs and the peak power for the four eyes open epochs calculated. The average peak power for eyes open epochs measured within ± 1 Hz deviation from peak-frequency for eyes closed epochs.The Vigilance-index was calculated from the ratio of the average peak power for eyes open divided with the average peak power for eyes closed (E.O. power / E.Cl. power) to quantify the relative desynchronization of the eyes open EEG epochs (Figure 2). The peak power was defined with an EEG filter setting of 3-15 Hz, covering the alpha and theta frequency (4-13 Hz) bands to avoid analysis of eye movement and muscle artifacts. EEG:s analyzed from the T6 or T5 area (10- 20 EEG system) which reflects the posterior part of the temporal lobe, known to be affected by Alzheimer’s and Lewy body dementia and also a part of the cortex where basic rhythm is prominent. Between the compared groups, the calculated data of average value for Vigilance-index, Standard deviation (S.D.), Standard error of the mean (S.E.M.). Pearson correlation coefficient and, Spearman rank correlation coefficient calculated and compared for age and Vigilance-index between the groups (Figure 2).
Results
There was no apparent correlation to age for Vigilance-index (Figure 3). In the analysis, both groups average values, standard deviation (S.D.), standard error of the mean (S.E.M), did not reveal any significant differences for Vigilance-index (Table 1). The Pearson correlation coefficient between age and Vigilance-index showed, on the other hand, differences between the two groups. The group of healthy subjects ranging from 17-67 years of age showed an inverse correlation between age and Vigilance-index (-0.41), the elderly group of persons examined due to memory complaints, aged 66-93 years of age had a coefficient of 0.16 between age and Vigilance-index. (Table 1). The Spearman rank correlation coefficient for the healthy group was -0.46 and for the elderly group 0.21. For the healthy subjects, Vigilance-index did not increase with age rather a tendency to a decrease of Vigilance-index due to increasing age. For the elderly patients, a weak tendency to increasing Vigilance-index due to increasing age, but still within the standard value of <0.30. Conclusively, it seems that there is not any substantial age-related decrease of Vigilance-index reflecting a decrease of cholinergic transmission in healthy persons and elderly persons without a dementia disease due to the cholinergic deficit (Figure 3 & Table 1).
Table 1: Statistical comparison of the age groups examined. The different statistical measurements in the left column. In the heading, the compared groups. The statistical analysis shows no substantial evidence that Vigilance-index increases with age.
Discussion
What is measured with the Vigilance-index? The EEG variables which used to calculate Vigilance-index is based on pharmacological studies of the effects of cholinergic and anticholinergic substances [10-15,16] and AChE inhibitor medication [17-18,19-21]. A reduction of the desynchronization of EEG, or Berger reactivity when the eyes open, observed following the administration of anticholinergic drugs, such as Atropine and Scopolamine to healthy subjects [12,22]. Comparison between healthy subjects and patients with AD revealed significantly reduced desynchronization reaction at eye-opening in patients with AD [23]. The Vigilanceindex measure the desynchronization reaction but not actual amount of Acetylcholine instead the available Acetylcholine for the transmission to the post-synapse neuron and therefore indirect the amount of Acetylcholine which is recorded by the EEG response from eyes open reaction. Confirmed from a study of patients examined for suspected dementia at baseline and follow-up EEG examination after approximately one year and evaluated and compared by AchE inhibitor treatment or not during the observation time. The AchE inhibitor-treated had unchanged Vigilance-index at followup while the untreated patients had significantly increased index, which can be expected from a progressive decline of Acetylcholine transmission at dementia disease [2].
Another issue of importance for using the Vigilance-index to predict cholinergic deficit, is if there are an age-related decrease in Acetylcholine for non-demented elderly persons. Former necropsy studies have observed a selective loss of cholinergic neurons in Alzheimer`s disease [3-5,24] based on analysis of the enzymes Choline acetyltransferase (CAT) and Acetylcholinesterase (Figure 1), without evidence of loss of enzymes related to neurotransmitters GABA, Dopamine, Noradrenaline, and Serotonin [3]. From autopsy studies of the temporal lobe of non-demented subjects, a significant reduction in Choline acetyltransferase (CAT) seen with increasing age between 61 to 92 years [25], which might perhaps affect the amount of Acetylcholine and potentially reduce available Acetylcholine acting on the postsynaptic neuron. However, no direct evidence been observed for Acetylcholine transmission relation to age.
No differences in Vigilance-index average values between the younger healthy individuals and the elderly group and the correlation was instead inverse for the young group (17-67 years) in this study, which speaks powerfully against decreasing cholinergic transmission by Acetylcholine. For the elderly group, a very weak correlation to age and decreasing Acetylcholine transmission, and still a Vigilance-index within normal values for Vigilance-index (<0.3), been observed (Table 1). Therefore, it seems that even if the Acetylcholine synthesizing enzyme Choline acetyltransferase (CAT) decreases with age, it can still produce enough Acetylcholine to uphold the neurotransmission even at an advanced age (93 years). The elderly group used in the study for comparison with the younger group was not explicitly evaluated for cognition, but they came to a memory clinic due to memory complaints. From an earlier study, cognition with mini-mental score examination (MMSE) compared to Vigilance-index for a similar group of patients like the elderly group in this study, showed that the patients with lowest Vigilanceindex (< 0.3) values had the highest MMSE values > 25, both at baseline and follow-up examination. The non-pathological followup MMSE values, almost overlapped with the non-pathological baseline values for Vigilance-index after approximately two years of observation time [2].
Conclusion
The Vigilance-index can reliably be used to identify cholinergic transmission deficit irrespective of age and be used to identify pathological conditions with cholinergic deficit such as Alzheimer`s and Lewy body dementia and distinguish other causes of dementia without cholinergic deficits even for elderly persons, but also identify medications with unwanted secondary central cholinergic disturbances leading to dementia symptoms.
Acknowledgement
The author thanks Gunilla Brohme´ at Nacka hospital, Dr. Maria Norström and Dr. Mats Brådman at Bromma hospital for valuable help getting access to data.
Funding sources
This research not funded by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, therefore a non-conflict of interests exist.
References
- kedahl R (2019) Comparison of Mini Mental Score Examination, quantitative Electroencephalography and Cerebrospinal fluid biomarkers in clinical practice examining dementia. Int J Psychol Behav Anal 5(162): 1-5
- Ekedahl R (2019) Comparison of cholinergic status with quantitative EEG parameters in healthy subjects and patients suspected of dementia. Arch Biomed Eng & Biotechnol 1(3): 1-6.
- Davies P, Maloney A (1976) Selective loss of central Cholinergic neurons in Alzheimer`s disease. Lancet 308: 1403.
- rrelation of Cholinergic abnormalities with senile plaques and mental test scores in senile dementia. BMJ 2: 1457-1459.
- Perry E, Perry R, Blessed G, Tomlinson B (1977) Necropsy evidence of central Cholinergic deficits in senile dementia. Lancet 309: 189.
- Drachman D, Leavitt J (1974) Human memory and the Cholinergic system. Arch Neurol 30: 113-121.
- Perry E, Blessed G, Tomlinson B, Perry R, Crow T, et al. (1981) Neurochemical activities in human Temporal lobe related to aging and Alzheimer-type changes. Neurobiol aging 2: 252-256.
- Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, et.al. (2001) Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer`s disease. Neuroscience Letters. 300: 157-160.
- Niedermeyer E (2007) The normal EEG of the waking adult. In: Niedermeyer E, Silva FLd, editors. Electroencephalography. Fifth ed, Lippincott, Williams & Wilkins Philadelphia, USA, pp. 167-192.
- Agnoli A, Martucci N, Manna V, Conti L, Fioravanti M (1983) Effect of Cholinergic and anticholinergic drugs on short term memory in Alzheimer´s dementia: a computerized Electroencephalographic study. Clin Neuropharmacol 6: 311-323.
- Sannita W, Maggio L, Rosadino G (1987) Effects of Scopolamine (0.25- 0.75 mg i.m.) on the Quantitative EEG and the Neuropsychological status of healthy volunteers. Neuropsychobiology 17: 199-205.
- Sloan E, Fenton G, Standage K (1992) Anticholinergic drug effects on quantitative Electroencephalogram, visual evoked potentials and verbal memory. Biol Psychiatry 31: 600-606.
- Neufeld M, Rabey M, Parmet Y, Sifris P, Treves T, et al. (1994) Effects of single intravenous dose of scopolamine on the quantitative EEG in Alzheimer´s disease patients and age-matched controls. Electroencephalogr Clin Neurophysiol 91:407-412.
- White R, Rinaldi F, Himmich H (1956) Central and peripheral nervous effect of Atropine sulfate and Mepiperphenidol bromide (Darstine) on human subjects. J Appl Physiol 8: 635-642.
- Martin W, Eades C (1960) A Comparative Study of the Effect of Drugs on Activating and Vasomotor Responses Evoked by Mid Brain Stimulation: Atropine, Pentobarbital, Chlorpromazine and Chlorpromazine Sulfoxide. Psychopharmacology 1: 303-335.
- Longo W (1966) Behavorial and Electroencephalographic effects of Atropine and related compounds. Pharmacol Rev 18: 971-974.
- Alhainen K, Partanen J, Reiniainen K, Laulumaa V, Soininen H, et al. (1991) Discrimination of Tetrahydoaminoacridine responders by a single dose pharmaco-EEG in patients with Alzheimer´s disease. Neurosci Lett 127: 113-116.
- Reeves R, Struve F, Patrick G (2002) The Effects of Donepezil on Quantitative EEG in Patients with Alzheimer´s Disease. Clin Electroencephalogr 33: 93-96.
- Holl G, Straschill M, Thomsen T, Fischer J, Kewitz H (1992) Effect of the cholinesterase inhibiting substance Galanthamine on Human EEG and visual evoked potentials. Electroencephalogr Clin Neurophysiol 82: 445- 452.
- Babiloni C, Casseta F, Forno GD, Percio CD, Ferreri F, et al. (2006) Donepezil effects on sources of cortical rhythms in mild Alzheimer´s disease: Responders vs. Non-Responders. Neuroimage 31: 1650-1665.
- Babiloni C, Percio CD, Bordet R, Bourriez J-L, Bentivoglio M, et al. (2013) Effects of Acetylcholinesterase inhibitors and Memantine on restingstate electroencephalographic rhythms in Alzheimer´s disease patients. J Clin Neurophysiol 124: 837-850.
- White R, Rinaldi F, Himmich H (1956) Central and peripheral nervous effect of Atropine sulfate and Mepiperphenidol bromide (Darstine) on human subjects. J Appl Physiol 8: 635-642.
- Hiele Kvd, Bollen E, Vein A, Reijntjes R, Westendorp R, et al. (2008) EEG markers of future cognitive performance in the elderly. J Clin Neurophysiol 25: 83-89.
- Francis P, Palmer A, Sims N, Bowen D, Davison A, et al. (1985) Neurochemical studies of early onset Alzheimer`s disease. N Engl J Med 313:7-11.
- Perry E, Blessed G, Tomlinson B, Perry R, Crow T, et al. (1981) Neurochemical activities in human Temporal lobe related to aging and Alzheimer-type changes. Neurobiol aging 2: 251-256.
-
Rolf Ekedahl, NeuFydi Stockholm. Acetylcholine Transmission Related to Age Evaluated by the Quantitative Electroencephalographic Vigilance-Index. Arch Neurol & Neurosci. 4(5): 2019. ANN.MS.ID.000596.
-
Electroencephalography; Desynchronization; Dementia; Acetylcholine; Cholinergic agents; Aged; Humans; Biomarkers; Reference Values; Synaptic Transmission
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






