 Case Report
Case Report
A Case of Branch Retinal Vein Occlusion with Macular Edema Presenting as Sub-Acute Vision Loss
Gomes RR1*, Basak DK2 and Rahman M3
1Associate Professor, Medicine, Ad-din Women’s Medical College and Hospital, Bangladesh
2Assiociate Consultant, Medicine, Sqaure Hospitals Limited, Bangladesh
3Emergency Medical Officer, Opthalmology, National Institute of Opthalmology, Bangladesh
Richmond Ronald Gomes, Associate Professor, Medicine, Ad-din Women’s Medical College and Hospital, Dhaka, Bangladesh.
Received Date: July 03, 2020; Published Date: July 21, 2020
Abstract
Branch retinal vein occlusion is the most common retinal vein occlusion. RVO is divided into central (CRVO), hemi (HRVO) and branch retinal vein (BRVO) occlusion. BRVO is venous occlusion at any branch of central retinal vein. Here our case illustrates present a 40 years old gentleman presented with sub-acute vision loss. He was found to have superior temporal branch retinal vein occlusion where hypertension, diabetes mellitus and dyslipidemia were thought to be the main risk factors. Close follow up, tight blood pressure are crucial to prevent the similar scenario in the fellow eye.
Keywords:Retinal vein; Sub-acute vision loss; Hypertension; Dyslipidemia; Diabetes mellitus
Background
Retinal vein occlusion (RVO) is the second most common retinal vascular disorder after diabetic retinopathy [1]. BRVO is classified according to the anatomical location as major or macular. Major BRVO refers to occlusion of a retinal vein that drains one of the quadrants. Macular BRVO refers to occlusion of a venule within the macula. The incidence of BRVO is most common in the supero-temporal quadrant (58.1-66%) [2,3]. BRVO is further classified into perfused (non-ischemic) or nonperfused (ischemic). BRVO is the most common RVO with an incidence of 0.44%-1.6% [3,4]. BRVO has many known ophthalmic and systemic risk factors including age, hypertension, glaucoma, hyperlipidemia, ocular hypertension [5,6]. There is a significant association of advancing age with BRVO, and the incidence of BRVO increases with age. However, there was no association of diabetes mellitus with BRVO (unlike the association found with CRVO in the same study) [5,7]. Other factors that are significantly associated with BRVO are glaucoma and body mass index (BMI) 5,8 Various meta-analysis studies have examined prevalence and association of vasculitis and thrombophilic risk factors and BRVO. The only significant associations are hyperhomocysteinemia and anti-cardiolipin antibodies with BRVO [8,9,10].
Case Report
A 40 years old nonsmoker, nonalcoholic gentleman known to have hypertension for 2 years (on irregular medication) presented at the outpatient department of Medicine, Ad-din Sakina Medical College, Jashore with sudden painless vision loss of his left eye for last 2 weeks. Since onset, he experienced progressive generalized blurring of the central vision. There was neither photopsia nor floaters. Systemic review was not significant. He had no symptoms and signs of systemic vasculitis such as rashes, joint pains or mucosal surface ulcers. Sexual history was not significant, and he has no history of substance abuse or smoking. There was no family history of vascular events as well. He has been taking atenolol on irregular basis. He was a medium built individual with a body mass index 25.14 kg/m2 (height 162 cm, body weight 66 kg). Blood pressure was 160/95 mm of Hg with a regular pulse rate of 82beats/minute. The visual acuity of his left eye was 6/24, with near visual acuity of N10 at 33 cm. The right eye had visual acuity of 6/6 and near vision of N5 at 33 cm. Confrontation test revealed no constriction of visual fields. Relative afferent papillary defect was absent. Slit lamp examination showed normal anterior segments with open angles bilaterally. The intraocular pressure was 18 mm Hg bilaterally.
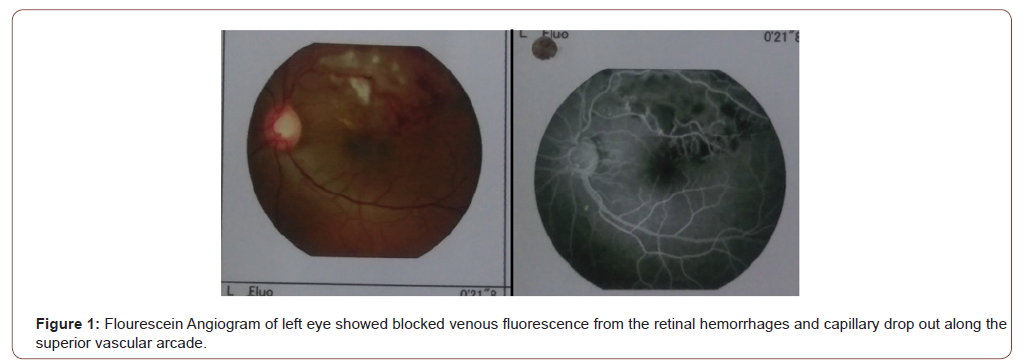
Posterior segment examination of left eye revealed blot retinal hemorrhages along an arcuate course, corresponding to supero- temporal retinal nerve fiber layer. Macular edema involved the fovea and was associated with hard exudates deposition. The patient has never had any laser treatment before. The supero-temporal retinal vein was dilated and tortuous along its entire course. The arterio-venous ratio was 2:3 infero-temporally but was 1:3 supero-temporally. The optic disc was pink with well defined margin and cup-disc ratio of 0.5. The infero-temporal retinal vein was neither dilated nor tortuous. There were no cotton wool spots, the vitreous was clear, and there was no evidence of retinal periarteritis or periphlebitis. Posterior segment findings of right eye were not significant (Figure 1).
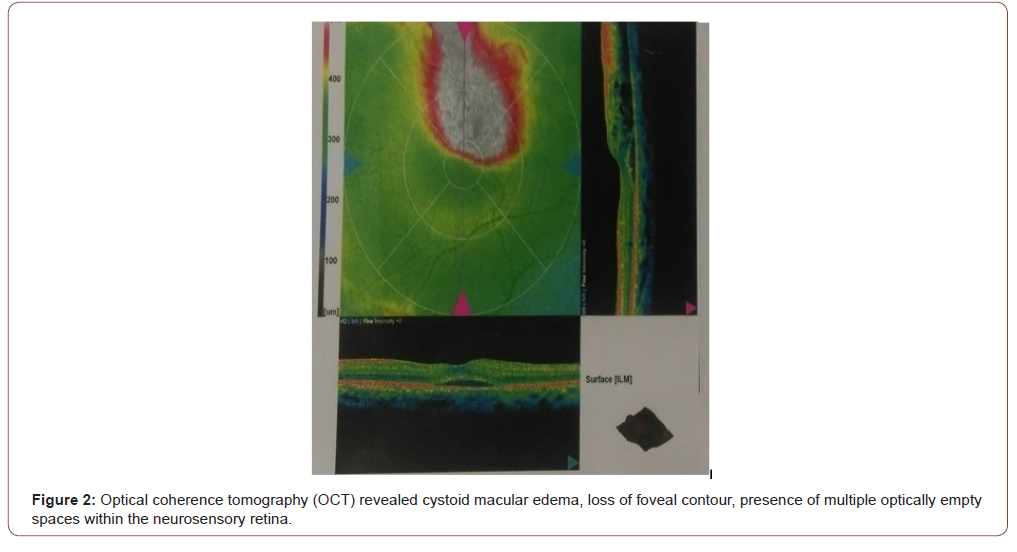
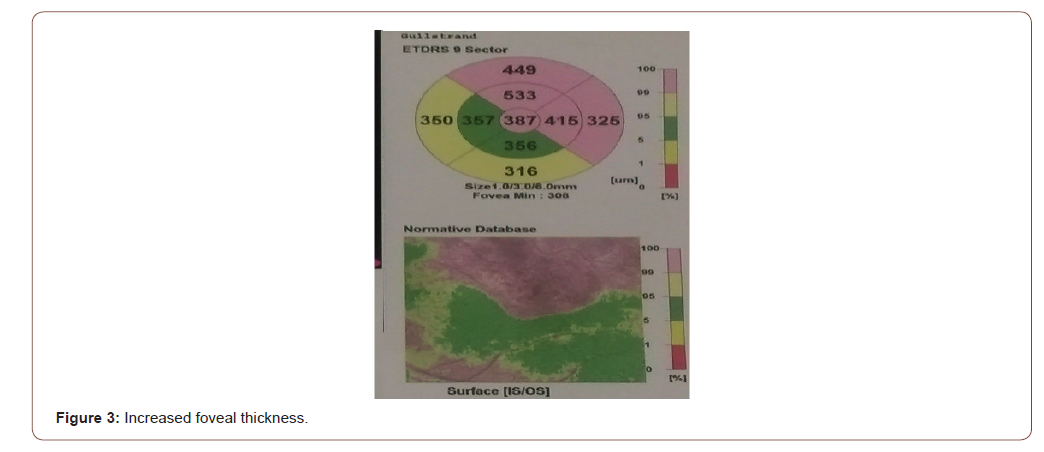
Flourescein Angiogram of left eye showed blocked venous fluorescence from the retinal hemorrhages and capillary drop out along the superior vascular arcade (Figure 1). Optical coherence tomography (OCT) revealed cystoid macular edema, loss of foveal contour, presence of multiple optically empty spaces within the neurosensory retina (Figure 2) and increased foveal thickness (Figure 3).
Full blood count, renal function tests, liver function tests and thyroid function tests were normal. ESR was 10 mm in 1st hour. Preliminary connective tissue screening (ANA, RA test) was negative. He was found to have newly detected diabetes mellitus with fasting blood sugar 9.7 mmol/L. Fasting lipid profile showed total cholesterol 263 mg/dl, LDL cholesterol 217 mg/dl Triglyceride 331 mg/dl.
He was given Inj. bevacizumab along with rosuvastatin 10 mg OD and combination of metformin and vildagliptin. Adequate dietary and lifestyle measures were instructed and ensured. Antihypertensive medications were rescheduled to ARB, telmisartan.
Discussion
Retinal vascular diseases are ocular manifestations of underlying systemic vascular disorders. The eyes are the only place in the body which allows direct visualization of the blood vessels. Therefore fundus examination offers a valuable opportunity for the early detection of occult systemic vascular disorders. Cugati et al in his analysis of 2 population-based cohorts (Beaver Dam Eye Study and Blue Mountains Eye Study) found out that participants aged less than 70 years old with retinal vein occlusion (RVO) at baseline were associated with higher cardiovascular mortality [11]. Being a part of the entity, Retinal vein occlusion (RVO) is the second most common retinal vascular disorder after diabetic retinopathy1. BRVO is classified according to the anatomical location as major or macular. The incidence of BRVO is most common in the supero-temporal quadrant (58.1-66%), followed by the infero-temporal quadrant (29%), and least common in the nasal quadrants (12.9%) [2,3]. The increased incidence in the supero-temporal quadrant is thought to be due to increased arteriovenous crossings in that quadrant. BRVO is further classified into perfused (non-ischemic) or nonperfused (ischemic). The true prevalence of retinal vein occlusive disorder is difficult to establish as many of them are asymptomatic and only diagnosed incidentally, unless it is complicated and visual disturbances manifest. Peripheral branch RVOs are asymptomatic. Symptomatic RVOs are due to macular involvement in which patients present loss of central vision. Macular edema is the major cause of central loss in RVOs [12]. BRVO is the most common RVO with an incidence of 0.44%-1.6% [3,4]. There was an association of higher prevalence with race, but not with gender. Overall the prevalence of any RVO and BRVO in increasing order by ethnicity was: whites, blacks, Asians, and then Hispanics. (The prevalence of BRVO was 0.282% in whites, 0.353% in blacks, 0.498% in Asians, and 0.598% in Hispanics.)4
BRVO has many known ophthalmic and systemic risk factors including age, hypertension, glaucoma, hyperlipidemia, ocular hypertension [5,6]. There is a significant association of advancing age with BRVO, and the incidence of BRVO increases with age. After adjusting for age The Beaver Dam Eye Study showed that BRVO was associated with hypertension, elevated systolic and diastolic pressure, pulse pressure, ocular perfusion, focal arteriolar narrowing, and arteriovenous nicking. A large meta-analysis by O’Mahoney et al showed a significant association of hypertension and hyperlipidemia with BRVO. However, there was no association of diabetes mellitus with BRVO (unlike the association found with CRVO in the same study) [5,7]. Other factors that are significantly associated with BRVO are glaucoma and body mass index (BMI) [5,8]. Various meta-analysis studies have examined prevalence and association of vasculitis and thrombophilic risk factors and BRVO. The only significant associations are hyperhomocysteinemia and anti-cardiolipin antibodies with BRVO [8-10].
The pathogenesis of BRVO is multifactorial in origin and not completely defined. Possible mechanisms include a combination of mechanical compression, degenerative changes in vessel walls, and/ or hypercoagulable factors. The arteriosclerotic changes (specifically arteriovenous crossing) are believed to result in venule occlusion through endothelial cell damage and thrombosis. Another hypothesis is that arteriosclerosis results in arteriolar insufficiency leading to BRVO. The association between BRVO and arteriovenous crossing has been established in multiple studies. In almost all cases of BRVO (97.6-100%) [13-15] the thick-walled artery is found anterior to the thin-walled vein. The artery and vein also share a common adventitial sheath at these crossings, which contributes to the predisposition of vein occlusion at these crossings. Arteriolar sclerosis increases the rigidity of the artery and further provides support for the mechanical basis of BRVO at arteriovenous crossings [13,16]. Mechanical obstruction of the vein by the rigid artery results in turbulent blood flow at arteriovenous crossings, resulting in venous intima media and endothelial damage which leads to vein occlusion [2,17].
In histological studies of BRVO the endothelium and intima media are found to be thickened and altered at the arteriovenous crossings, with no blood thrombus obliterating the venous lumen at the arteriovenous crossing, which suggests a compression as a major factor in the pathogenesis of BRVO. Post- mortem and post enucleation histological studies of chronic BRVO have shown the thickening of the common adventitial tissue of the artery and vein at the arteriovenous crossings [18]. Macular edema is the main cause of vision loss in BRVO. The pathogenesis of macular edema is believed to be a result of multiple inflammatory cascades. Analysis of vitreous samples from patients with BRVO has established the increased levels of VEGF, IL-6, IL-8, and monocyte chemoattractant protein-1 compared to control [19-21]. Excess VEGF is produced from retinal epithelial cells, endothelial cells and Muller cells in setting of BRVO, resulting in vascular permeability and contributing to macular edema [22]. The treatment for BRVO is aimed at treatment/ prevention of the complications that cause vision loss including macular edema, macular ischemia and neovascularization. The systemic risk factors should be optimized in consultation the primary care doctor. Prior to the advent of anti-vascular endothelial growth factor (VEGF) agents, laser photocoagulation was considered the gold standard for the treatment of BRVO as established by the BVOS Group [23,24]. Photocoagulation can be considered in patients with perfused macular edema with VA ≤ 20/40 without improvement in visual acuity for at least 3 months. Corticosteroids have been shown to be effective for treatment of macular edema in BRVO [25]. However; intraocular corticosteroids have significant side effects including progression of cataracts formation and elevation of intraocular pressure.
In patients with BRVO, retinal ischemia results in elevated secretion of VEGF leading to increased vascular permeability and vasodilatation [20-22]. There are several anti-VEGF agents available to treat macular edema due to BRVO including ranibizumab (Lucentis), bevacizumab (Avastin), and aflibercept (Eylea). Regarding surgical care, Muqit et al published on long-term prospective visual outcomes of arteriovenous sheathotomy. In those cases where neovascular complications such as non-clearing vitreous hemorrhage, pars-plana vitrectomy may be considered usually in combination with intraoperative endolaser to the portion of the retina affected by the BRVO. Some retinal surgeons also consider pars-plana vitrectomy with internal limiting membrane peeling for treatment of recalcitrant RVO-associated macular edema, but this remains controversial. Chen et al published the first results of isovolemic hemodilution in patients with BRVO and decreased visual acuity with hematocrit ≥ 35%. Patients were randomized to treatment with volume replacement using hydroxyethylstarch compared to untreated patients. At one year follow up the final VA was 20/40 in the treated group compared with 20/80 in the untreated group [26]. Due to the systemic invasiveness of the treatment and the many systemic complications from isovolemic hemodilution it is not generally accepted to treat BRVO [16]. The major complications that result in vision loss in BRVO include macular edema, macular ischemia, and neovascularization. The typical follow up should be tailored on an individual basis to monitor for the development of these complications. After initial presentation, the typical follow up should be every month or two months to monitor the development of macular edema and/or neovascularization. If macular edema develops, treatment with anti-VEGF therapy with/without laser should be initiated and monitored for resolution. Once edema has resolved or stabilized, the follow up interval can be extended to three to six months or longer for stable chronic cases. Patients with nonperfused BRVO (> 5 disc diameters), that has not been treated with laser should be monitored every three months due to increased risk of neovascularization. The most common cause of decreased vision in BRVO is macular edema. Other complications include ischemic maculopathy, retinal neovascularization, macroaneurysmal formation, retinal telangiectasia, retinal detachment and vitreous hemorrhage.
BRVO has good visual prognosis with 50-60% of patients have a final visual acuity ≥ 20/40 even without treatment. The natural course of BRVO depends on the type of occlusion, degree of occlusion and development of collaterals. Poor prognostic indicators are chronic macular edema, macular BRVO, and NV resulting in VH [27-30]. Developing BRVO in one eye increases the risk of BRVO in the fellow to 7-10% [23,24,28,31,32]. The Beaver Dam Eye Study showed no association of increased all-cause mortality or ischemic heart disease death, after controlling for age, in subjects with BRVO.
Conclusion
BRVOs in adults require careful systemic evaluation for the presence of cardiovascular risk factors as well as to exclude hypercoagulabilities or collagen vascular diseases. Our case illustrates an interesting presentation of unilateral ischemic branch retinal vein occlusion, where hypertension and dyslipidemia were thought to be the main risk factors. While many interventions for fixed visual loss associated with BRVO have largely not proven to be of benefit, our management focused on controlling blood pressure and hyperlipidemia as preventive measures to protect the fellow eye. Meanwhile, close follow up was emphasized to investigate for signs of iris/angle neovascularization.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Jaulim A, Ahmed B, Khanam T, Chatziralli IP (2013) Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina 33(5): 901-991.
- Zhao J, Sastry SM, Sperduto RD, Chew EY, Remaley NA (1993) Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Ophthalmology 100(3): 423-428.
- Klein R, Klein BE, Moss SE, Meuer SM (2000) The epidemiology of retinal vein occlusion: The Beaver Dam Eye Study. Trans Am Ophthalmol Soc 98: 133-141.
- Rogers S, Rachel L. McIntosh, Ning Cheung, Lyndell Lim, et al. (2010) The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 117: 313-319.
- Anonymous (1993) Risk factors for branch retinal vein occlusion. The Eye Disease Case-control Study Group. Am J Ophthalmol 116(3): 286-296.
- Kolar P (2014) Risk Factors for Central and Branch Retinal Vein Occlusion: A Meta-Analysis of Published Clinical Data. J Ophthalmol :724780.
- O’Mahoney PR, Wong DT, Ray JG (2008) Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol 126: 692-629.
- Yau JW, Lee P, Wong TY, J Best, A Jenkins, et al. (2008) Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J 38: 904-910.
- Ajith TA, Ranimenon (2015) Homocysteine in ocular diseases. Clin Chim Acta 450: 316-321.
- Janssen MC, Martin den Heijer, Johannes R M Cruysberg, Hub Wollersheim, Sebastian J H Bredie (2005) Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost 93(6): 1021-1026.
- Cugati S, Wang JJ, Knutdson MD, et al. (2007) Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology 114: 520-524.
- Campochiaro PA (2010) Management of retinal vein occlusion. Adv StudOphthamol 7: 42-47.
- Duker JS, Brown GC (1989) Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol 107: 998-1000.
- Christoffersen NL, Larsen M (1999) Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology 106: 2054-2062.
- Weinberg D, Dodwell DG, Fern SA (1990) Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am J Ophthalmol 109: 298-302.
- Rehak J, Rehak M (2008) Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res 33: 111-131.
- Jefferies P, R Clemett, T Day (1993) An anatomical study of retinal arteriovenous crossings and their role in the pathogenesis of retinal branch vein occlusions. Aust N Z J Ophthalmol 21: 213-217.
- Frangieh GT, W R Green, E Barraquer-Somers, D Finkelstein (1982) Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol 100(7): 1132-1140.
- Fraenkl SA, Mozaffarieh M, Flammer J (2010) Retinal vein occlusions: the potential impact of a dysregulation of the retinal veins. EPMA J 1: 253-261.
- Yoshimura T, Koh-Hei Sonoda , Mika Sugahara, Yasutaka Mochizuki, Hiroshi Enaida, et al. (2009) Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One 4(12): e8158.
- Noma H, Hideharu Funatsu, Makiko Yamasaki, Hidetoshi Tsukamoto, Tatsuya Mimura, et al. (2005) Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 140(2): 256-261.
- Karia N (2010) Retinal vein occlusion: pathophysiology and treatment options. Clinical Ophthalmology 4: 809-816.
- Anonymous (1984) Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol 98(3): 271-282.
- Anonymous (1986) Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Group. Arch Ophthalmol 104(1): 34-41.
- McAllister IL, et al. (2009) Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol 147: 838-846.
- Chen HC, et al. (1998) Effect of isovolaemic haemodilution on visual outcome in branch retinal vein occlusion. Br J Ophthalmol 82(2): 162-167.
- Hayreh SS (2014) Ocular vascular occlusive disorders: Natural history of visual outcome. Prog Retina Eye Res 0: 1-25.
- Hayreh SS, Rojas P, Podhajsky P, et al. (1983) Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology 90: 488-506.
- Finkelstein D (1986) Argon laser photocoagulation Ophthalmology. For macular edema in branch vein occlusion. 93(7): 975-957.
- Hayreh SS, et al. (2014) Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol 132(1): 13-22.
- Rogers SL, et al. (2010) Natural History of Branch Retinal Vein Occlusion: An Evidence-Based Systematic Review. Ophthalmology 117(6): 1094-1101.
- Michels RG, et al. (1974) The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol 78(2): OP166-OP177.
-
Gomes RR, Basak DK, Rahman M. A Case of Branch Retinal Vein Occlusion with Macular Edema Presenting as Sub-Acute Vision Loss. 8(2): 2020. ANN.MS.ID.000685.
-
Acute meningoradiculoneuritis, Sjögren syndrome, Lymphocytic meningitis, Polyradiculopathy, Neuro-Sjögren
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






