 Research Article
Research Article
Continuous Spike and Waves During Slow Sleep in the Neurological Department of Fann National University Hospital of Dakar: Topographic Aspects and Age
SECK Lala Bouna1, SARR Mamadou Moustapha2, AKRIM Hanane3, KA Mamadou3, DIOP Alassane Mamadou3, DIOP-SENE Marème Soda4, GAYE Arame3, BASSE FAYE Anna Modji4, SOW Adjaratou Djeynabou4, NDIAYE Moustapha4 and DIOP Amadou Gallo4
1Department of Medical Specialties, Gaston Berger University of Saint Louis, Senegal
2Department of Medical Specialties, Thies University, Senegal/p>
3Department of Neurological, Fann National University Hospital, Senegal 4Department of Medical Specialties, Cheikh Anta Diop University, Senegal
Seck Lala Bouna, Department of Medical Specialties, Gaston Berger University of Saint Louis, Saint Louis, Senegal.
Received Date: January 27, 2020; Published Date: February 05, 2020
Abstract
Introduction: Continuous spikes and waves during slow sleep (CSWS), are characterized by marked activation of epileptic activity on electroencephalographic (EEG) recording during slow sleep. Their topographic distribution is not homogeneous, and this could be not random. The aim of our study is to describe topographic aspects of CSWS and look for correlation with patients age.
Patients and methods: We conducted a descriptive retrospective study in the neurophysiological department of the neurological service of Fan national university hospital in Dakar (Senegal), over a 2 years period. We studied topographical aspects of EEG anomalies of children whose recordings presented CSWS, then looked for correlation between the predominant diffusion region of these and the age of patients.
Results: The CSWS were found on 1.02% of the global sample EEG and 2.60% of the children EEG. The sex-ratio was 1.42. The age varied from 3 to 14 years at the time of the first recording with a mean age of 6.65 +/- 2.63 years. The predominant age group was 3 to 8 years (81%). The main indications were generalized tonico-clonic seizures, focal seizures and benign epilepsy with centro-temporal spikes. CSWS were predominant on the left hemisphere on awake as well as sleep EEG. During waking state, the basal activity was normal on 96.97% of the recordings, anomalies were found on 96.97%, and the major diffusion location were the peri-sylvian regions. Sleep architecture was altered for 63.49% of patients. Topographically, CSWS diffused predominantly in the temporal, central and frontal regions. Children with anterior predominant diffusion of CSWS were older than those with posterior predominant diffusion and the difference was statistically significant (p = 0,031).
Conclusion: CSWS topographic distribution is variable and likely age-related. Given that brain areas have functionnal specificities, this could orient neuropsychological tests in cognitive deficits.
Keywords: Fann; Continuous; Spikes; Waves; Sleep; Topographic; Age
Abbreviations: CSWS: Continuous Spikes and Waves During Slow Sleep; EEG: Electroencephalographic, Electroencephalogram; Or: Odds Ratio; CI: Confidence Interval
Introduction
Continuous spikes and waves during slow sleep (CSWS), are characterized by marked activation of epileptic activity on electroencephalographic (EEG) recording during slow sleep [1], acom panied by the occurrence of or increase of pre-existing spikes and waves, these becoming bilateral and continuous/subcontinuous [2]. The anomalies can occur during awakness, realizing isolated spikes and waves or bursts of spikes and waves [1-3]. They can be due to epileptic encephalopathy [4,5], but sometimes to some focal epilepsies, wether structural or not [6,7]. Thus, the syndrom (epileptic encephalopathy with CSWS) must be differentiated from the EEG pattern that can be seen in different clinical contexts [8]. Many aspects have been described regarding this EEG pattern. Indeed, considering topographic feature, many studies reported a non-homogeneous topographic distribution of the anomalies on EEG, with an either anterior or posterior predominant diffusion [9- 14]. This distribution is not random.
The aim of our study is to describe topographic aspects of EEG of patients presenting CSWS on their EEG recordings and to look for correlation between the topographic distribution and the age of patients.
Patients and Methods
We conducted a descriptive and retrospective study in the neurological department of the Fann national university hospital in Dakar, from June 1st, 2017 to May 31th 2019. The study population was all patients admitted at the neurophysiological department to undergo an EEG recording. We reviewed the recordings of all the children (age from 3 to 16 years) during this period. We included in the study, all EEG on which CSWS were identified, based on quantitative criteria consistent with spikes and waves index showing occupation by the anomalies of at least 50% of the period. We collected the personal data of patients, the EEG indications, the data related to EEG recording during waking state when it was done (basal activity aspects, other anomalies), then sleep EEG recording (sleep architecture aspects, other anomalies). Regarding anomalies, we precised their type, the spikes and waves index during sleep, their topographic characteristics (major diffusion : anterior if the predominant topography was fronto-central, fronto-temporal, centro-temporal or fronto-centro-temporal; posterior if the predominant topography was temporal posterior, pariéto-temporal, temporo-occipital or temporo-pariéto-occipital). Then we looked for correlation between the location of predominant diffusion and the age of the patients, using chi2 test. Data were analyzed with Microsoft Excel and described anonymously with respect for confidentiality for all patients.
Results
Epidemiological aspects
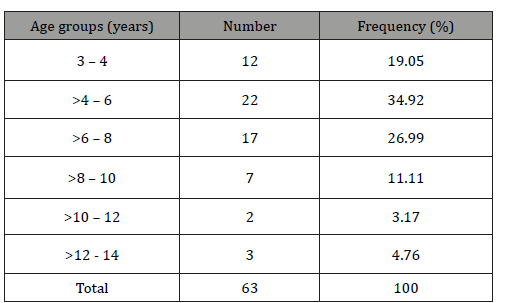
Six thousand sixty-eight patients have had an EEG recording between June 1st 2017 and May 31th 2019, of whom 2424 were 3 to 16 years old. During this period, 97 EEG showed CSWS, corresponding to 63 patients of whom 18 benefited from control EEG. The CSWS were found on 1.02% of the global sample and 2.60% of the children EEG. They were 37 (58,73%) male and 26 females (41,27%), with a sex-ratio of 1.42. The patients’ age at the time of the first recording varied from 3 to 14 years (Table 1), with a mean age of 6.65 +/- 2.63 years and a median of 6 years. On the whole sample including the 18 control EEG, the mean age was 7,39 +/- 1.41 years. The predominant age group was 3 to 8 years (81% of the total group) with a peak frequency at >4 to 6 years (34.92%) (Table 1).
Table 1:Patients distribution according to age.
Clinical characteristics
EEG indications were as follows : 33,33% of patients suffered from generalized tonico-clonic seizures (with one having psychomotor retardation, another one behavioral disorder); 25.39% had focal seizures (of whom one had global cognitive deficit and another one alternating seizures and psychomotor retardation); 14.28% suffered from benign epilepsy with centrotemporal spikes (of whom one had learning disabilities); 12.70% had already diagnosed CSWS; 7.93% had unprecised seizures (one of them with cognitive deficit). For the remaining 6.36% (4 patients) indications were respectively: myoclonic epilepsy, encephalopathy, psychomotor retardation, no diagnosis for the last. Thus, 8 patients (12.70%) had cognitive disorder on their EEG indication.
Electroencephalographic characteristics
Thirty-three patients (52.40%) benefited from awake EEG recording.
Awake EEG aspects
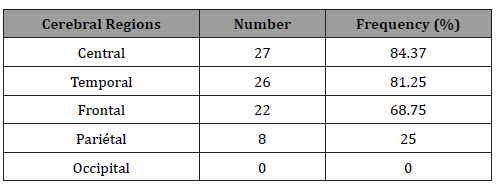
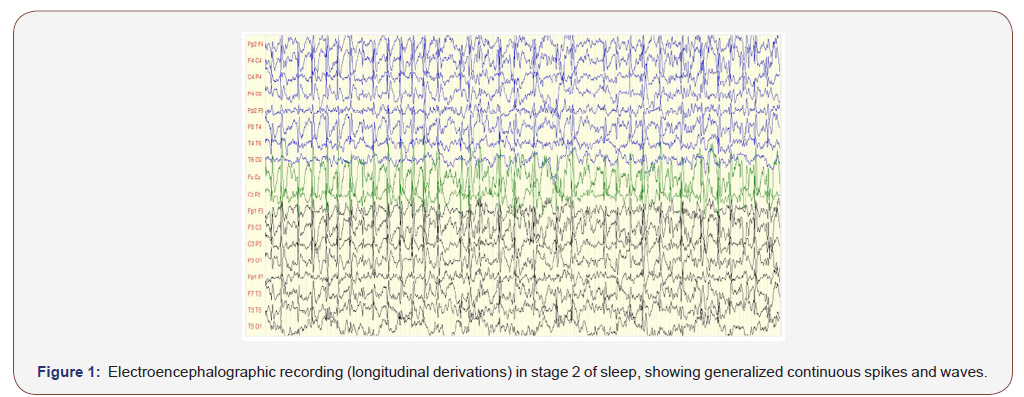
On awake EEGs, the basal activity was normal in 96.97% of cases, not well modulated in one patient (3.03%). Anomalies were found on 96.97% of the recordings (Figure 1): it was diphasic spikes or spikes and waves, occuring on a paroxysmal way. They were predominant on the left hemisphere in half of the sample, on the right one for 34.37%, were symmetrical for 12.5% and maximal on the midline for 3.13%. The major diffusion region was fronto-centro-temporal for 37.5% of children, centro-temporal for 15.15%, fronto-central and fronto-centro-parieto-temporal for 9.37% each, then centro-parieto-temporal and fronto-temporal for 6.25% each, fronto-centro-parietal, frontal, temporal, centro-parietal and temporo- parietal for 3.16% each. Thus the frequency in which brain lobes were involved in the major diffusion of paroxysmal anomalies was higher for peri-sylvian regions, respectively: central, temporal, frontal (Figure 1 and Table 2).
Table 2:Paroxysmal anomalies dominant diffusion on awake electroencephalogram.
On awake EEGs, the basal activity was normal in 96.97% of cases, not well modulated in one patient (3.03%). Anomalies were found on 96.97% of the recordings (Figure 1): it was diphasic spikes or spikes and waves, occuring on a paroxysmal way. They were predominant on the left hemisphere in half of the sample, on the right one for 34.37%, were symmetrical for 12.5% and maximal on the midline for 3.13%. The major diffusion region was fronto-centro-temporal for 37.5% of children, centro-temporal for 15.15%, fronto-central and fronto-centro-parieto-temporal for 9.37% each, then centro-parieto-temporal and fronto-temporal for 6.25% each, fronto-centro-parietal, frontal, temporal, centro-parietal and temporo- parietal for 3.16% each. Thus the frequency in which brain lobes were involved in the major diffusion of paroxysmal anomalies was higher for peri-sylvian regions, respectively: central, temporal, frontal (Figure 1 and Table 2).
Sleep EEG aspects
a) Sleep architecture
For thirty-six point fifty-one per cent of the sleep EEG recordings, sleep micro-architecture was normally organized, while it was altered for 63.49%. On the whole sample (including the 18 control recordings) these rates were respectively 45.36% and 54.64%.
The continuous spikes and waves during slow sleep
Topographically, CSWS predominated in the left hemisphere in 49.20% of patients, in the right one for 41.27%, sometimes on the left sometimes on the right on the same recording for 7.94%, on the midline for 1.59%. The predominating diffusion region was variously distributed : fronto-centro-temporal (23.81%) centro-parieto- temporal (14.30%) fronto-temporal (12.70%), fronto-central (11.11%), centro-temporal (9.52%), frontal (7.94%) parieto-temporal (4.76%), fronto-centro-parietal / parieto-temporo-occipital / centro-parietal (3.17% for each) then temporal / fronto-parito- temporal / fronto-centro-parieto-temporal /fronto-parieto-temporo- occipital (1.59% for each). So, the frequency in which brain lobes were involved in major diffusion of the paroxysmal anomalies was 74.60% for temporal regions, 66.67% for central regions, 63.50% for frontal regions, 33.33% for parietal regions, 4.76% for occipital regions (Table 3 & Figure 2).
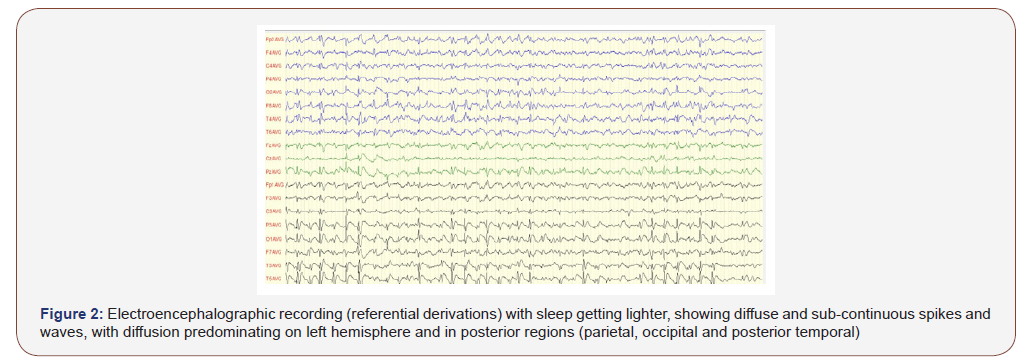
Thus it appears that most of the patients (90.47%) had anterior major diffusion of the CSWS, while it was posterior for 7.94%. For one patient (1.59%) it was bipolar: antero-posterior.
b) Correlation between CSWS and age
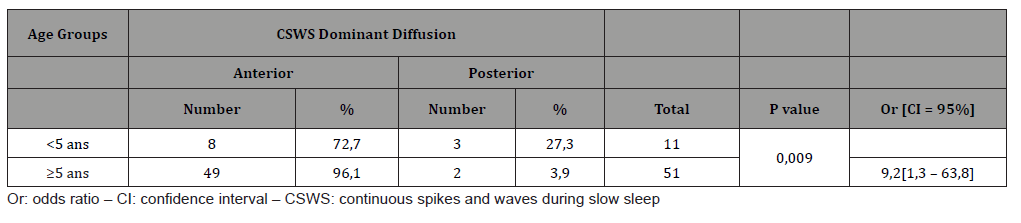
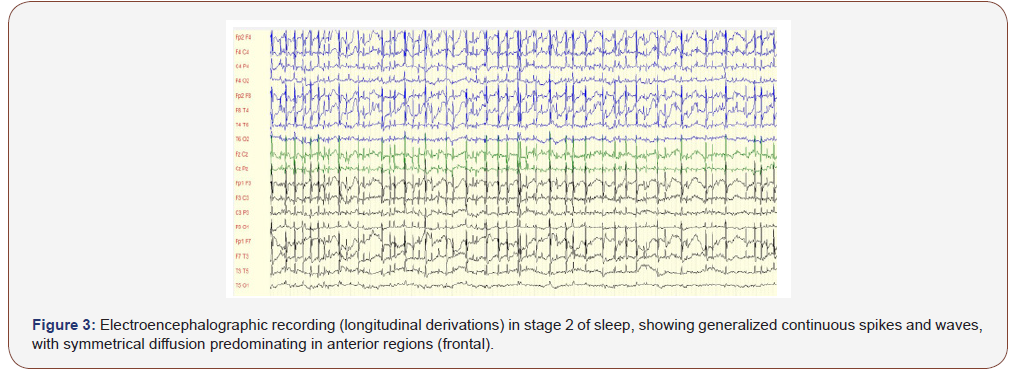
Children with an anterior predominant diffusion of the CSWS were 3 to 14 years old with a mean age of 6,9 ± 2,6 years; those with a posterior predominant diffusion were 3 to 8 years old (respectively 3 years for 3 of them, 5 years, 8 years) with a mean age of 4,4 ± 2,2 years. The difference was statistically significant (p =0,031). After distribution into 2 age groups, we notice that for children 5 or more years old, anterior predominant diffusion was found in 96,1% against 72,7% for the second group. The difference was statistically significant, as children of the first group were 9,2 times more likely to have anterior predominant diffusion of the CSWS (Table 4 & Figure 3).
Table 3:Continuous spikes and waves dominant diffusion on sleep electroencephalogram.
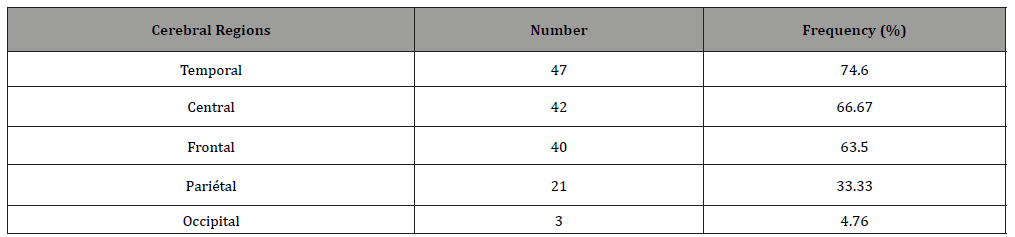
Table 4:Continuous spikes and waves during slow sleep dominant diffusion according to age.
Discussion
Epidemiological aspects
We collected over a period of 2 years, data of 63 patients EEG recording having CSWS, corresponding to 1.02% of all the patients that have benefit from EEG during this period and 2.60% of the children. Demographic characteristics of the sample, as we describe them in a former study [14], are consistent with most of the littérature data. This is the case for the sex-ratio in favour of male gender [15-18,8], the mean age of 7,39 years +/- 1.41, the predominant age groups from 3 to 8 years (81% of the population) with a frequency peak at >4-6 years (34.92%) [19,20,8], even if some authors have found higher mean age [17] or higher age frequency peak [21].
Electroencephalographic aspects
a. Electroencephalographic aspects of awake EEG
Regarding paroxysmal anomalies, their topography was similar to what is classically reported in the litterature, particularly by Giorgis et al. [6] and Tassinari et al. [22,23]. The same applies to the asymmetrical distribution of the anomalies [6,20,12].
b. Electroencephalographic aspects of sleep EEG
We analyzed topographically, the first EEG recording of the 63 patients. The CSWS predominated on the left hemisphere for 49.20% of patients, the right one for 41.27%. This left predominance, although slight in our series, has been reported by Saltik et al. [20] and Aeby et al. [21], with higher frequency rates, respectively 62,5% and 66,6% of their cases.
In our series, predominant diffusion of anomalies included more often temporal (74.60%), central (66.67%) and frontal regions (63.50%), which probably explains that most of the subjects (90.47%) had anterior major diffusion of CSWS, as defined by some authors [24,22]. Thus, rolandic and peri-rolandic regions were the most frequently involved in the major diffusion zone of the CSWS. This also support our idea that most of the patients, mainly those with tonico-clonic generalized seizures might have had a focal component in their clinical manifestations, particularly compatible with benign epilepsy with centro-temporal spikes. Indeed, focal anomalies have been described in CSWS with clinical correlations, frontal predominance on EEG being correlated to global or specific nonlinguistic deficits [9,12,13], and temporal predominance to linguistic deficits [10,11]. Some patients of ours ample presented cognitive deficit but didn’t benefit from neuropsychological tests in order to discriminate specifically the nature of the disorder.
c. Correlation between CSWS predominant diffusion
The mean age of children with major anterior diffusion was higher than that of those with posterior major diffusion: respectively 6,9 ± 2,6 years and 4,4 ± 2,2 years; with a statistically significant difference (p = 0,031). Many authors [25], described a relationship between age and the preponderant location of epileptic foci in childhood epilepsies. In general, it has been shown that the younger the patients are (preschool age), the more posterior the anomalies foci are (occipital); by contrast, the older they are, the more anterior the epileptic foci are (frontal, temporal) [25-28].
This dichotomous aspect is confirmed when we divide the sample into 2 groups: under 5 years of age on one hand, and five years old or more on the other hand, major posterior diffusion being more frequent in the first group. The opposite is true when we consider major anterior diffusion, with a statistically significant difference.
According to some authors, epileptic foci in focal seizures, particularly in idiopathic ones, might have an anterio-posterior migration [26,27]. The process might be related to brain cortical maturation [25], the occipital regions developing first and frontal areas last [27]. But other studies did not demonstrate it [26]. As the pathology in CSWS is linked to slow wave activity during sleep, this later being more pronounced at the age of onset of CSWS, it explains the risk for hyper synchronization and then evolution into spikes and waves [25].
Conclusion
CSWS are a rare EEG pattern which seriousness is due to their neuropsychological impact if undiagnosed. Their topographic distribution is variable. They likely predominate in the dominant cerebral hemisphere, more frequently in the peri-sylvian regions, leading to linguistic or not cognitive deficits, that have adverse effects on the neurobehavioral development of the child. The dominant location of the anomalies is topographically variable but seems to be age-related. Posterior dominant location is more willingly seen in preschool age, while the anterior dominant location in seen in older children. This topographic distinction is interesting, as it can be a guidance for neuropsychological assessment and allow more specific tests according to the dominant location. Thus, latent cognitive deficits could be diagnosed in apparently asymptomatic subjects.
Acknowledgement
None.
Conflict of Interest
No financial interest or conflict of interest.
References
- Scheltens-de Boer M (2009) Guidelines for EEG in encephalopathy related to ESES/ CSWS in children. Epilepsia 50(suppl 7): 13-17.
- Van Hirtum-Das M, Licht EA, Koh S, Wu JY, Shields WD (2006) Children with ESES: variability in the syndrome. Epilepsy Res 70(S1): 48-58.
- Jayakar PB, Seshia SS (1991) Electrical status epilepticus during slow-wave sleep: a review. J Clin Neurophysiol 8(3): 299-311.
- Galanopoulou AS, Bojko A, Lado F, Moshé SL (2000) The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev 22(5): 279-295.
- Praline J, Barthez MA, Castelnau P, Debiais S, Lucas B, et al. (2006) Atypical language impairment in two siblings: relationship with electrical status epilepticus during slow wave sleep. J Neurol Sci 249(2): 166-171.
- De Giorgis V, Filippini M, Macasaet JA, Masnada S, Veggiotti P (2017) Neurobehavioral consequences of continuous spike and waves during slow sleep (CSWS) in a pediatric population: A pattern of developmental hindrance. Epilepsy Behav 74: 1-9.
- Smith MC, Hoeppner TJ (2003) Epileptic encephalopathy of late childhood: Landau-Kleffner syndrome and the syndrome of continuous spikes and waves during slow-wave sleep. J Clin Neurophysiol 20(6): 462-472.
- Groppa S, Ciolac D, Chiosa V (2017) Diagnostic and Therapeutic Advances in Epileptic Encephalopathy with Continuous Spike-Wave of Sleep. Glob J Intellect Dev Disabil 1(3): 555565.
- Beaumanoir A (1995) EEG data. In: Beaumanoir A, Bureau M, Deonna T, Mira L, Tassinari CA (Eds.), Continuous Spikes and Waves During Slow Sleep. Electrical Status Epilepticus During Slow Sleep-Acquired Epileptic Aphasia and Related Conditions. Eds John Libbey, London, United Kondom, pp. 217-223.
- Morrell F, Whisler WW, Smith MC, Hoeppner TJ, de Toledo-Morrell L, et al. (1995) Landau–Kleffner syndrome. Treatment with subpial intracortical transection. Brain 118(6): 1529-1546.
- Paetau R, Granstrom ML, Blomstedt G, Jousmaki V, Korkman M, et al. (1999) Magnetoencephalography in presurgical evaluation of children with the Landau–Kleffner syndrome. Epilepsia 40(3): 326-335.
- Scholtes FB, Hendriks MP, Renier WO (2005) Cognitive deterioration and electrical status epilepticus during slow sleep. Epilepsy Behav 6(2): 167-173.
- Van Lierde A (1995) Therapeutic data. In: Beaumanoir A, Bureau M, Deonna T, Mira L, Tassinari CA (Eds.), Continuous Spikes and Waves During Slow Sleep. Electrical Status Epilepticus During Slow Sleep: Acquired Epileptic Aphasia and Related Conditions. Eds John Libbey, London, United Kondom: 225-227.
- Seck LB, Akrim H, Sarr MM, Diop-Sène MS, Ka M, et al. (2019) Les pointes-ondes continues du sommeil: aspects électro-encéphalographiques au service de neurologie du Centre Hospitalier National Universitaire de Fann de Dakar. AJNS 38(2): 89-97.
- Caraballo RH, Cersosimo RO, Fejerman N (2008) Childhood occipital epilepsy of Gastaut: a study of 33 patients. Epilepsia 49(2): 288-297.
- Chen J, Cai F, Jiang L, Hu Y, Feng C (2016) A prospective study of dexamethasone therapy in refractory epileptic encephalopathy with continuous spike-and-wave during sleep. Epilepsy Behav 55: 1-5.
- Gencpinar P, Dundar NO, Tekgul H (2016) Electrical status epilepticus in sleep (ESES)/continuous spikes and waves during slow sleep (CSWS) syndrome in children: An electroclinical evaluation according to the EEG patterns. Epilepsy Behav 61: 107–111.
- Kramer U, Sagi L, Goldberg-Stern H, Zelnik N, Nissenkorn A, et al. (2009) Clinical spectrum and medical treatment of children with electrical status epilepticus in sleep (ESES). Epilepsia 50(6): 1517-1524.
- Nieuwenhuis L, Nicolai J (2006) The pathophysiological mechanisms of cognitive and behavioral disturbances in children with Landau-Kleffner syndrome or epilepsy with continuious spike-waves during slow-waves sleep. Seizure 15(4): 249-258.
- Saltik S, Uluduz D, Cokar O, Demirbilek V, Dervent A (2005) A clinical and EEG study on idiopathic partial epilepsies with evolution into ESES spectrum disorders. Epilepsia 46(4): 524-533.
- Aeby A, Poznanski N, Verheulpen D, Wetzburger C, Van Bogaert P (2005) Levetiracetam efficacy in epileptic syndromes with continuous spikes and waves during slow sleep: experiences in 12 cases. Epilepsia 46(12): 1937-1942.
- Tassinari CA, Bureau M, Dravet C, Dalla Berndina B, Roger J (1992). Epilepsy with continuous spikes and waves during slow sleep-otherwise described as ESES (epilepsy with electrical status epilepticus during slow sleep). In: Roger J, Bureau M, Dravet C, Dreifuss FE, Perret A (Eds.), Epileptic Syndromes in Infancy, Childhood and Adolescence (2nd edn), John Libbey, London, United Kingdom, pp. 245-256.
- Tassinari CA, Rubboli G, Volpi L, Meletti S, d'Orsi G, et al. (2000) Encephalopathy with electrical status epilepticus during slow sleep or ESES syndrome including the acquired aphasia. Clin Neurophysiol 111(S2): 94–102.
- Patry G, Lyagoubi S, Tassinari CA (1971) Subclinical “electrical status epilepticus” induced by sleep in children. A clinical and electroencephalographic study of six cases. Arch Neurol 24(3): 242–252.
- Bölsterli Heinzle BK, Bast T, Critelli H, Huber R, Schmitt B (2017) Age-Dependency of Location of Epileptic Foci in "Continuous Spike-and-Waves during Sleep": A Parallel to the Posterior-Anterior Trajectory of Slow Wave Activity. Neuropediatrics 48(1): 36-41.
- Saito N, Kanazawa O, Tohyama J, Akasaka N, Kamimura T, et al (2008) Brain maturation- related spike localization in Panayiotopoulos syndrome: magneto-encephalographic study. Pediatr Neurol 38(2): 104-110.
- Yoshinaga H, Kobayashi K, Ishizaki Y, Wakai M, Tominaga Y, et al. (2009) Age-dependent spike localization in various epileptic syndromes. Pediatr Neurol 41(6): 440-444.
- Konishi T, Naganuma Y, Hongou K, Murakami M, Yamatani M, et al. (1994) Changes in EEG foci with age in childhood partial epilepsies. Clin Electroencephalogr 25(3): 104-109.
-
SECK Lala Bouna, SARR Mamadou Moustapha, AKRIM Hanane, KA Mamadou, DIOP Alassane Mamadou, et al. Continuous Spike and Waves During Slow Sleep in the Neurological Department of Fann National University Hospital of Dakar: Topographic Aspects and Age. Arch Neurol & Neurosci. 6(5): 2020. ANN.MS.ID.000650.
-
Sleep, Medical Specialties, Neurological, Continuous, Spikes, Waves, Sleep, Topographic, Age, cognitive.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






