 Research Article
Research Article
Prognostic Implication of Positive Area on Bone Scans (%PAB- Quantitative Parameter) in Bones of Patients with metastatic Prostate cancer
Nayab Mustansar, Assistant Professor, Consultant Nuclear Physician, Women Medical College, Abbottabad, Pakistan.
Received Date: June 26 , 2019; Published Date: July 10, 2019
Abstract
Prostate Cancer is one of the common cancers in the world. It could primarily disseminate to the bone and can lead to death. In order to address its life-threatening distant metastasis, it is important to diagnose it earlier for timely treatment. Bone metastasis is usually diagnosed deploying bone scan imaging. However, interpretation of the bone scans is a tedious procedure for the physicians and often leads to misinterpretation either as overestimation or underestimation of the metastasis. To minimize the risk of misinterpretation, one of the accurate methods is quantitative analysis of the bone scans in order to ascertain, whether a metastatic lesion is present or not. There are several methods to-date which can be used to analyze the extent of such lesions. The aim of this study is to use %PAB (quantitative parameter) on a baseline bone scans. Moreover, an improved methodology is introduced by comparing the results with PSA levels. 141 patients with histopathologically proved prostate cancer were chosen to implement the % PAB on individual baseline bone scans. After which, for the calculation of risk of progression or regression of disease and survival rate, 40 patients were chosen from the same dataset. A serial follows up scan was performed to calculate 2-years survival rate. The dataset was again analyzed using the same quantitative parameter and the cut off were calculated as % PAB: 0.5. It was found out that the % PAB method is a good prognostic indicator in baseline scans. Moreover, the prostate cancer patients with the cut off % PAB > 0.5 showed increase risk of disease progression and less survival.
Keywords: Prostate cancer; Tumours; Cancer; PAB; Carcinoma
Introduction
The skeleton
The adult human skeleton has a total of 213 bones, excluding the sesamoid bones. The appendicular skeleton has 126 bones, axial skeleton 74 bones, and auditory ossicles six bones. Each bone constantly undergoes modelling during life to help it adapt to changing biomechanical forces, as well as remodeling to remove old, microdamage bone and replace it with new, mechanically stronger bone to help preserve bone strength [1]. The four general categories of bones are long bones, short bones, flat bones, and irregular bones. Long bones include the clavicles, humeri, radii, ulnae, metacarpals, femurs, tibiae, fibulae, metatarsals, and phalanges. Short bones include the carpal and tarsal bones, patellae, and sesamoid bones. Flat bones include the skull, mandible, scapulae, sternum, and ribs. Irregular bones include the vertebrae, sacrum, coccyx, and hyoid bone. Flat bones form by membranous bone formation, whereas long bones are formed by a combination of endochondral and membranous bone formation. The skeleton serves a variety of functions. The bones of the skeleton provide structural support for the rest of the body, permit movement and locomotion by providing levers for the muscles, protect vital internal organs and structures, provide maintenance of mineral homeostasis and acid-base balance, serve as a reservoir of growth factors and cytokines, and provide the environment for haematopoiesis within the marrow spaces [2]. The long bones are composed of a hollow shaft, or diaphysis; flared, cone-shaped metaphyses below the growth plates; and rounded epiphyses above the growth plates. The diaphysis is composed primarily of dense cortical bone, whereas the metaphysis and epiphysis are composed of trabecular meshwork bone surrounded by a relatively thin shell of dense cortical bone. The adult human skeleton is composed of 80% cortical bone and 20% trabecular bone overall. Different bones and skeletal sites within bones have different ratios of cortical to trabecular bone. The vertebra is composed of cortical to trabecular bone in a ratio of 25:75. This ratio is 50:50 in the femoral head and 95:5 in the radial diaphysis.
Bone tumors
Bone tumors develop when cell in the bone divide without control, forming a mass of tissue. Most bone tissues are benign and they don’t spread. However, they may still weaken bone and can lead to fracture and cause other problems. Bone cancers may destroy normal bone tissues and can spread to other parts of the body called as metastasis.
Benign bone tumors
They are more common than the malignant tumors. Following are the most common benign tumors.
1. Osteochondroma
2. Osteoid Osteoma
3. Giant cell Tumor
4. Osteoblastoma
5. Enchondroma
Metastatic cancer :The metastatic bone cancer is the one in which primary is present somewhere else in the body whereas it metastasizes to bone. Even though it spreads to the bone it is not considered as the bone tumor because the primary is present elsewhere. Cancers that commonly spread to the bones are:
1. Breast Cancer
2. Prostate Cancer
3. Lung Cancer
The axial skeleton, the primary site of active marrow, is the most common distribution of metastatic spread for patients with prostate cancer. At this time, there is no standard means by which osseous lesions can be directly visualized or quantified; thus, there is no qualified imaging biomarker for prostate cancer. Bone scintigraphy is commonly used to assess disease burden and treatment effects, but it is an imperfect modality for quantifying disease or for demonstrating treatment effects. Bone scans do not specifically identify cancer, can paradoxically worsen in the face of response (“flare”), and frequently improve only slowly if at all, despite patients ‘receiving active treatments [3].
The skeleton is the most common organ to be affected by metastatic cancer and the site of disease that produces the greatest morbidity. Skeletal morbidity includes pain that requires radiotherapy, hypercalcemia, pathological fracture, and spinal cord or even nerve root compression. From randomized trials in advanced cancer, it can be seen that one of these major skeletal events occur on an average every 3-6 months. Additionally, metastatic disease may remain confined to the skeleton with the decline in quality of life and eventual death almost entirely due to skeletal complication and their treatment. The prognosis of metastatic bone disease is dependent on the primary site with the breast and prostate cancer associated with a survival measured in years compared with lung cancer, where average survival is only a matter of months. Additionally, the presence of extraosseous disease and the extent and tempo of the bone disease are powerful predictors of outcome. The latter is best estimated by measurement of bone-specific-markers, and recent studies have shown a strong correlation between the rate of bone resorption and clinical outcome, both in terms of skeletal morbidity and progression of the underlying disease [4]. Bone is the third most common site for the metastatic cancer after lung and liver cancer .It is estimated that skeletal metastasis develops in 14-70% of all the tumor patients and autopsy-based studies report the occurrence in 70% patients with carcinoma Breast and Prostate. In addition to ca prostate and ca prostate, many other tumors like lung, thyroid, kidney and melanoma have predilection for skeletal metastasis. From all the randomized trials in advanced cancer, it is estimated that one of these major skeletal events occurs on average every 3-6 months [5] (Table 1).
Table 1: Incidence of bone metastasis, Prevalence and Survival [6].
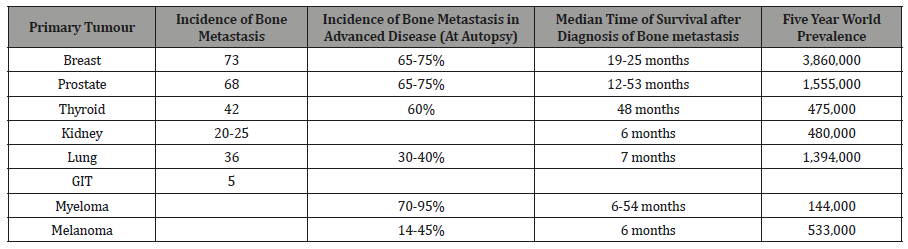
Vertebrae are most frequently involved (L>T>S>C). 38% of the metastatic disease involves the Thoraco-lumbar spine. Other bones involved in the order of the decreasing frequent are Pelvis, Ribs ,Sternum ,femur, humerus, Skull and hands. Ca Prostate specifically involves spine, femur, pelvis, skull, ribs and sternum while the one in breast carcinoma involves spine, pelvis, proximal femur, skull, ribs and mid-humerus Bone metastasis is clinically very important in prostate and breast cancer because of the prevalence of these diseases. By worldwide screening used worldwide e-g PSA levels for the prostate cancer and mammography for the breast cancer [7].
Aims and Objectives
To determine Prognostic Implication of Positive Area on Bone Scan (%PAB- Quantitative Parameter) in Bones of Patients with metastatic Prostate cancer.
Materials and Methods
The study was conducted at N.O.R.I (Nuclear Medicine Oncology and Radiotherapy Institute, Islamabad) from October 2013 to April 2014. The synopsis was approved by P.I.E.A.S, Ethical Review Committee N.O.R.I hospital.
Patient’s demographic data
Total 141 patients with baseline bone scans were included in the study. The characteristics of the study population are depicted in the Table 2 (Table 2).
Table 2: Patient’s Demographic Data (Baseline bone scan).
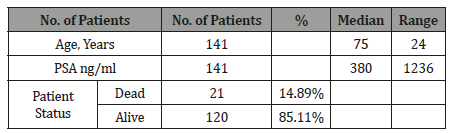
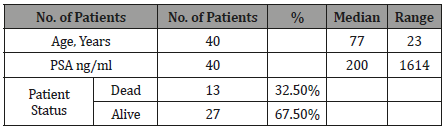
The patients with both baseline and follow up scans were 40 in total with median age of 77 and median PSA level of 200. The demographic data is given in the Table 3 (Table 3).
Table 3: Patient’s Demographic Data (Baseline and follow up scans).
1. Study design: Cross Sectional Study.
2. Place of study: Nuclear Medicine department of NORI
3. Duration: Six months.
4. Sampling Method: Nonprobability purposive sampling method
5. Sample size: 141 Patients with histopathologically proved Prostate Cancer.
(Baseline and Follow up scan of 40 patients with hormonal treatment).
Sample selection
Inclusion criterion:
1. Histological confirmed prostate cancer patients referred for evaluation of osseous metastasis within three months of diagnosis.
2. Written informed consent.
Exclusion criterion:
1. < 18 years.
2. Patients in which PSA levels was not available
3. Patients having other co-morbids.
Every patient included in our study underwent Bone Scintigraphy and later the quantitative parameter was applied on the bone scan. % BSI (Bone Scan Index) was applied to the scans for the evaluation of bone metastasis.
Patient preparation and procedure:Whole procedure was explained to the patients regarding bone scintigraphy and unless any contraindication patients were well hydrated and were instructed to drink two or 8 oz glasses of water between the time of injection and the time for delayed imaging. The patients were directed to micturate immediately before delayed image and were directed to drink plenty of water for at least 24 hours of radiopharmaceutical administration.
Radiation dosimetry in adults
The dose for the bone scintigraphy is given in the table 4 (Table 4).
Table 4: Radiation Dosimetry in Adults [8].

Then the following quantitative parameter was applied on the baseline bone scans.
%PAB (Positive area on bone scan): Positive area on bone scan is a quantitative method in which the osseous metastasis is considered as the positive area. The same method was applied to the dataset of patients using the formula given below:
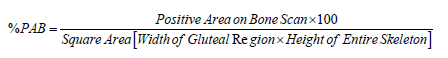
In this method the involved areas on the bone scan of a patient were measured using the computer software and are summed up; used as a numerator as mentioned in the above formula, whereas using the same software the width of the hip bone and the height of the skeleton was measured on the same bone scan; using as a denominator as per mentioned in formula and finally the percentage was calculated. The arbitrary cut off for %PAB method was taken as 0.5.The patients with %PAB above this cut off values were considered high risk as compared to the dataset of patients having % PAB values below this cut off. Nogouchi used the same method of %PAB for osseous metastasis quantitation and survival analysis [8].
% PAB Calculation was calculated using following steps using computer software
Step 1:
(Figure 1)
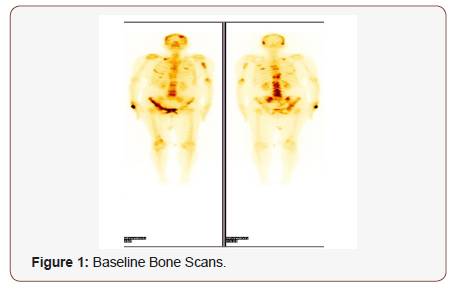
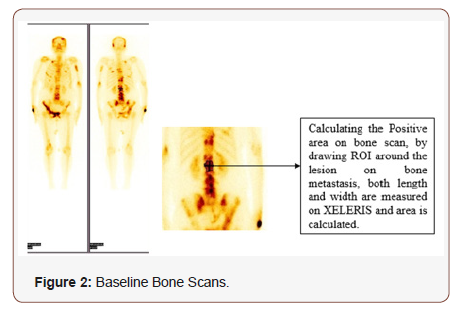
Step 2:
Calculating the Positive Area on Bone Scan:
(Figure 2)
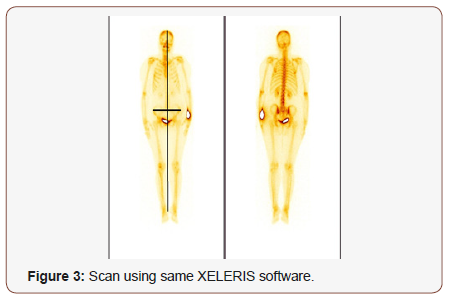
Step 3: Calculating the height of the Skeleton along with the width from the gluteal region on the Scan using same XELERIS software in mm units. (The Height is taken from vertex till heel of the skeleton whereas the width is calculated using the bilateral anterior superior ileac spine) (Figure 3).
Step 4:Calculating the % PAB of the Scan by Applying the Formula:
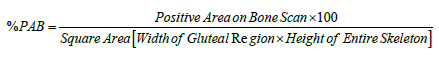
Results
Descriptive statistics
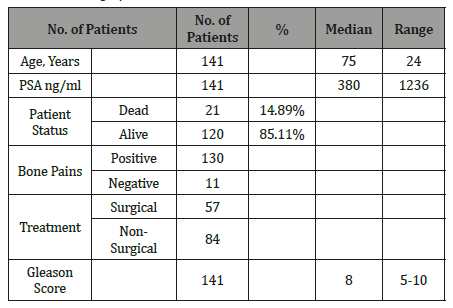
Total 141 patients having bone scans within three months of diagnosis were included. The mean age of the data set was 75 years(Table 5).
Table 5: Demographic data of Patients with baseline bone scans.
Similarly, the demographic data of patients with both baseline and follow scans is represented in the table below (Table 6).
Table 6: Demographic data of baseline and follow up scans.

The patient’s age ranges between 60 years -85 years in our study population with the mean age of 75 years. It can be represented via Bar chart as follows: (Figure 4).
Comparing %PAB quantitation method with PSA levels
Similarly, correlation statistics was also applied on the %PAB quantitation method and PSA levels to see the relationship between the two variables (Figure 5).
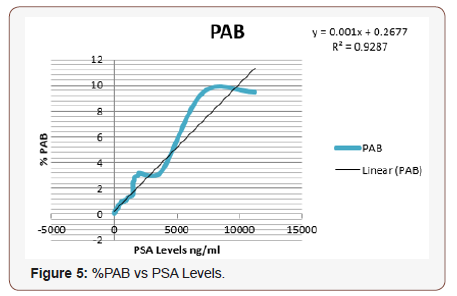
Equation derived from the graph:
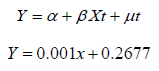
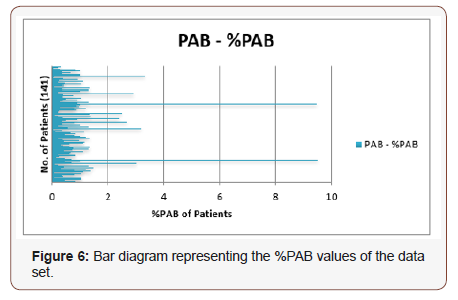
Here R2 value is 0.9287 which shows excellent linear correlation between %PAB and PSA levels , concluding the linear increase in quantitative values of the %PAB method with PSA levels. Bar diagram representing the %PAB values of the data set is shown below: (Figure 6)
Analysis of baseline and follow up scan in dead v/s alive patients using quantitative parameter
The baseline and follow up scans were analyzed by using quantitative parameter as follows:
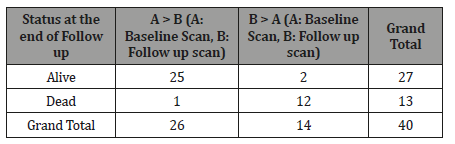
%PAB in baseline and follow up scan and its effect on survival: Similarly, the %PAB quantitative parameter was applied on the baseline and follow up scans of 40 patients. It was observed that increase in the tumor burden % PAB on the follow up scans after treatment is a bad prognostic factor (Table 7).
Table 7: Effect of % PAB calculation in Baseline and follow up scans.
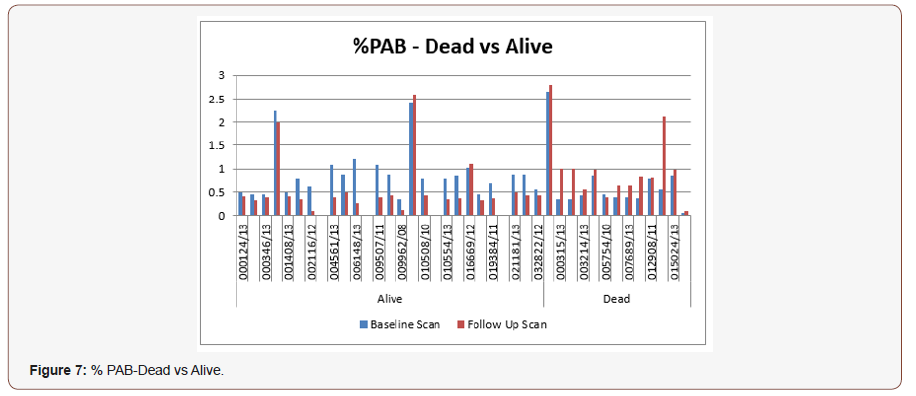
The ‘A’ represents the tumor burden on baseline bone scan whereas the ‘B’ represents the tumor burden on the follow up bone scan. By comparing the tumor burden on baseline and follow up scan with the %PAB( Positive area on bone scan) it has been observed that as the tumor burden increases in follow up scans there is less chances of the survival of a patient. By this method we conclude from above table that out of 13 dead patients this method significantly tells the rate of decrease survival i-e 92.3 %. Furthermore, by taking the cut off of 0.5, it was observed that the patients with tumor burden > 0.5 in follow up bone scans showed treatment failure and disease progression as compared to the patients with the less tumor burden %PAB value of <0.5. It can be represented via bar diagram as: (Figure 7)
It has been observed from the bar diagram that in data of alive patients (shown on the left extreme side) the tumor burden as calculated via %PAB method, was more in the baseline scan (represented as blue bars) which later after treatment, decreases in the follow up scan (represented as red bars) suggesting increase in survival of these patients. Whereas the extreme right of the bar diagram shows dead patients data suggesting that the baseline scan calculated tumor burden (represented as blue bars) did not improve after treatment showing increase in tumor burden >%PAB >0.5 (represented as red bars) thus showing the treatment failure and less survival.
Quantitative parameter and survival curve
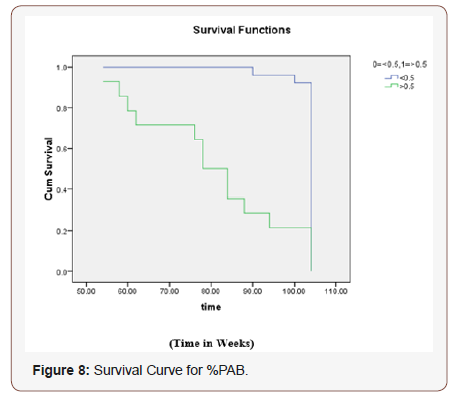
The survival curves were generated by using the respective cut off values to the data set of 40 patients for a period of two years (Figure 8).
Ho: There is no difference regarding survival among two levels.
H1: There is difference regarding survival among two levels.
The Chi-squared statistic of log rank test is 28.257 with associated P-value 0.000 less than 0.05 rejects null hypothesis. The conclusion therefore is that, statistically, the two survival curves differ significantly, or that the grouping variable has a significant influence on survival time. Rejection of null hypothesis shows that two levels <0.5 and > 0.5 are not identical regarding survival. The conclusion is that the curve representing the patients with the decrease tumor burden (<0.5) has low risk and better survival then the patients with %PAB values >0.5.
Discussion
The bone metastasis is one of the commonest complications of few tumors. The tumors that most commonly metastasize to bone are the tumors from the prostate in men and breast in women. Among the complication of bone metastasis, bone pain, is the worst consequence, affecting 66% of the patients who have the bone metastasis [9]. The patients with prostate cancer usually don’t have any clinically measurable or evaluable method for the quantification of their tumor burden by conventional criterion. Cutanoeus or subcutaneous nodule or palpable lymphadenopathies are uncommon. Pulmonary or Visceral metastases are also infrequent. Moreover 80% of the patients have skeletal metastases which are the only clinically obvious areas of tumor. These lesions are usually osteoblastic or represent a mixed pattern on plain radiograph but are best defined on the Bone scans. Metastasis to the bone is the most serious complication of solid malignant neoplasm, and by far the most common malignant tumors involving the skeleton [10].
The present knowledge of quantifying the metastatic bone disease is still not sufficient. A lot of work has been done to quantify the bone metastasis using bone scans. Radionuclide bone scans are strongly positive in cases of bone involvement of prostate cancer patients irrespective of whether the lesions are lytic, mixed or pure plastic. The conventional method of calculating the bone metastasis is by combining qualitative assessment of all the sequential bone scans and bony films with tumor markers (Such as PSA and Alkaline Phosphatase levels). Although serum acid phosphatase may present the progression and regression of disease, approximately 25% -30% of patients with metastatic disease have a normal acid phosphatase. Thus, it cannot be used to tell about the tumor status [11].
Interpretation of bone scan is a subjective process, dependent on the experience and knowledge of the nuclear medicine physician is a question. Reports of bone scans often express uncertainty e-g ‘may be degenerative’ or ‘possible metastasis, and the tumor burden can be expressed as ‘extensive metastatic disease’. Reporting like this has a disadvantage as it can be perceived differently by the nuclear physicians and the referring physicians. Such type of communication usually leads to inappropriate and incorrect treatment in patients with severe disease and an effective medication may be stopped as a result of a misconception [12]. Therefore, a method is needed to define prognostic strata and to assess response to treatment in the majority of patients, that is, those with predominantly bone metastasis [13]. To quantitate all bone metastasis in patients is a time-consuming task, since the patients with metastatic involvement usually have more than one disease site. Several studies have evaluated different ways to quantify the extent of bone involvement during therapy. Interpretation of the bone scans is a tedious procedure for the physicians and often leads to misinterpretation either as overestimation or underestimation of the metastasis. To minimize the risk of misinterpretation, one of the careful methods is quantitative analysis of the bone scans in order to ascertain, whether a metastatic lesion is present or not. There are several methods to-date which can be used to analyze the extent of such lesions. For example, quantitation of the bone scan i-e % BSI (Bone scan index) [14], % PAB (Positive area on bone scans) [15], EOD (extent of disease) [16] and BLS (Bone lesion scoring) [17]. Among these, %BSI has most frequently been used and validated in various studies (140-141). Novel automated software based on %BSI quantitative calculations has been developed and is in clinical use in many nuclear medicine departments. However, automated %BSI calculator (EXINI bone TM & BONENAVITM) have not been extensively employed in routine nuclear medicine practice because of its high cost. Another potential risk of inaccuracy in using this automated quantitation software’s is their limited training databases as these software’s use either Swedish or Japanese patient’s data [18,19]. Despite all these shortcomings there is body of evidence that these quantitative bone scan parameters not only increase interpretation accuracy but can also serve as image biomarkers. Based on this premise this study was designed to evaluate the accuracy of % PAB.
In our study we applied quantitative parameter %PAB on baseline bone scans to assess the tumor burden of 141 histopathologically proved carcinoma prostate patients. Results of all fours quantitation methods were individually compared with PSA levels (ng/ml), to evaluate the efficacy of % PAB as effective disease status indicator. For evaluation as a prognostic indicator, 40 patients having a serial follow up scans were chosen. The follow up datasets were again analyzed using the same quantitative parameter. For purpose of quantification arbitrary cut off were applied for it. Cut offs were % PAB: <0.5 low risk and >0.5 high risk. Age range of our study population was between 60 years -85 years in our study population with the mean age of 75 years. Most of the published data showed similar age range of age as seen in our patient cohort (140-141).
Bone quantitative parameter (%PAB) as prognostic indicators and treatment response evaluator
In subset of our patient population we did serial scanning to evaluate the efficacy of the four-bone quantitative parameter as prognostic and survival indicator and a guide for treatment response evaluation. Utility of bone scan quantitative parameters as bio-imaging markers is most extensively explored. However, only BSI has been utilized for this purpose. Especially after the availability of the commercial BSI software there is growing research and clinical use of this Bone scan quantitation parameter in prognostic and survival analysis. Its utility is not only focused in prostate cancer patients but also there have been research studies utilizing it in all epithelial cell tumors leading to bone metastasis. In our study population of 40 patients we did serial scanning and predicted survival based on these parameters. In our study, 40 patients underwent serial scanning and quantitation on both the baseline and follow up bone scans was done and its correlation with the PSA levels was calculated. PSA levels were taken before baseline and after following up bone scans. It was concluded that the quantitative parameter was good in explaining tumor burden as regression or progression of disease, with decrease survival in patients having increased tumor burden in follow up scans. The patients who had tumor burden (More numerical values) of the baseline bone scan quantitative parameter then the follow up bone scan showed decrease risk of disease progression and good survival. It was further observed that the after some specific cut off value of the quantitative parameter there is increasing risk of disease progression. (% PAB: 0.5). In %PAB cut off was taken at 0.5. Below that patient were considered low risk and above that it was high risk. . In table 7 it is shown that those patient whose %PAB values were decreased in follow up scans as compared to the baseline scan showed better survival as compared to those who had increased in %PAB on subsequent scan (7.6 % vs 92.3 %). In Figure 8 it is noticeable that in alive patients’ group most of the patients were showing decline in %PAB (N=21). While in dead patients group 11 patients showed increase in the %PAB and 1 patient showed static %PAB. Out of these 11 patients 4 were already in high risk group but 7 patients were in low risk group i.e. %PAB <0.5. However, if we make our cut off point lower than many of the dead patient will shift into the high-risk group. PAB was initially used by Noguchi et al [15] and they used to cut off point at 0.46 (141). This data shows that PAB can predict outcome and in patients having initial high-risk grade or positive change in grade (from lower to higher) carries poor prognosis. The Chi-squared statistic of log rank test is 28.257 with associated P-value 0.000 less than 0.05 rejects null hypothesis. The conclusion therefore is that, statistically, the two survival curves differ significantly, or that the grouping variable has a significant influence on survival time. The conclusion is that the curve representing the patients with the decrease tumor burden (<0.5) has low risk and better survival then the patients with %PAB values >0.5. Similar findings were noted by Noguchi as well in 56 patients which they analyzed. Kaplan–Meier plot shows diseasespecific survival after treatment of metastatic prostate cancer for those with %PAB <0.5 and greater than 0.5 (P 0.00).
An overall trend seen in all serial scanning patients was that, there was decline in quantitative parameter numerical values or it remained stable in comparison with the patient which died where quantitative parameter numerical values were mostly increased. So %PAB quantitative parameter is strong predictors of tumor burden and is equally good in risk stratification too. The changes seen on serial bone scans reflected metastatic activity in the skeleton. Deterioration on the bone scan indicated disease progression or poor prognosis. Improvement on scan reflects regression of metastatic disease and usually implied a favorable survival. Consistent stabilization on the scan correlated with clinical stable disease and was associated with better survival than for the progressing patients.
Limitations
The limitation of our project is that we have included all the baseline bone scans of Carcinoma Prostate Patients irrespective of the treatment (Hormonal- Non-Hormonal). Due to less time tenure of the project it was not possible to collect both the Baseline and the follow up scans of all the patients, though we have included 40 patients with both baseline and follow up scan (on hormonal treatment only) data. But in such small group of patients we cannot comment on the prognostic value and survival of the patients accurately.
The results are analyzed irrespective of the ‘Flare Phenomenon’ and that’s why a lot of variation is observed.
Conclusion
Quantitative Parameter (% PAB) is good in quantifying the tumor burden and is a good indicator in determining the disease status. The disease prediction as progression or regression can easily be determined by using it. Our present study suggests that %PAB method is one of the accurate methods in quantifying as a simple and reproducible estimate of the percentage of the skeleton involved in metastasis. It may also be very constructive to stratify patients in clinical trials. Large-scaled trials and further studies with more statistical power is required to assess the utility of serial % PAB in monitoring the treatment effects and its worth as significant predictor of survival after hormonal treatment. The prostate cancer patients with the %PAB > 0.5 showed disease progression and less survival.
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Algra PR, Tissing H, Falke TH, Arndt JW, Verboom LJ (1991) Detection of vertebral metastases: comparison between MR imaging and bone scintigraphy. Radiographics 11(2): 219-232.
- Parfitt AM (1994) Osteonal and hemiosteonal remodeling: The spatial and temporal framework for signal traffic in adult bone. J Cell Biochem 55(3): 273-286.
- Pollen JJ, Witztum K, Ashburn WL (1984) The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. Am J Roentgenol 142(4): 773–776.
- Powell GJea (1991) Localization of parathyroid hormone related protein in Bone seeking cancer metastases: increased incidence in bone compared with other sites. Cancer Res 51: 3059–3061.
- Susan S (2004) Musculoskeletal system. In: Gray’s Anatomy. New York, Elsevier 39: 83-135.
- Schneider A, Kalikin L, Mattos AC, Keller ET, Allen MJ, et al. (2005) Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology 146(4): 1727–1736.
- Taoka T, Mayr N, Lee HJ, Yuh WT, Simonson TM, et al. (2001) Factors influencing visualization of vertebral metastases on MR imaging versus bone scintigraphy. Am J Roentgenol 176: 1525– 1530.
- Morris MJ, Akhurst T, Larson S, Ditullio M, Chu E, et al. (2005) Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubular chemotherapy. Clin Cancer Res 11(9): 3210–3216.
- American Cancer Society (2007) Cancer facts and figures.
- Sodeman TM. Batsakis JG (1977) Acid phosphatase. In: Tannenbaum M(ed.) Urologic Pathology: The Prostate. Philadelphia: Lea and Feibiger 2: 179-139.
- Citrin DL, Cohen AI, Harberg J, Schlise S, Hougen C, et al. (1981) Systemic treatment of advanced prostatic cancer: development of a new system for defining response. J Urol 125: 224–227.
- Kaboteh R, Gjertsson P, Leek H, Lomsky M, Ohlsson M, et al. (2013) Progression of bone metastasis in prostate cancer patients- automated detection of new lesions and calculation of bone scan index EJNMI research 3(1): 1-6.
- Yagoda A, Watson RC, Natale RRD (1979) A critical analysis of response criteria in patients with prostatic cancer treated with cisdiamine dichloride platinum 11. Cancer 44: 1553-1560.
- Dennis ER, Jia X, Mezheritskiy IS, Stephenson RD, Schoder H, et al. (2012) Bone scan index: A quantitative a treatment response biomarker for castration resistant prostate cancer patients. J Clin Oncol 30: 519-523.
- Noguchi M, Kikuchi H, Ishibashi M, Noda S. (2003) Percentage of the positive area of bone metastasis is an independent predictor of disease death in advanced prostate cancer. Br J Cancer 88(2):195–201.
- Soloway MS, Hardeman SW, Hickey D, Barbara Todd RN, Scott S, et al. (1988) Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 61(1): 195-202.
- Mariana R, Anders Bl, Mattias O, Lars E, Elin T (2014) Increase in bone scan index during abiraterone treatment in relation to reduced survival in mCRPC patients. J Clin Oncol 4: 32.
- Nakajima K, Nakajima Y, Horikoshi H, Munehisa U, Hiroshi W, et al. (2013) Enhanced diagnostic accuracy for quantitative bone scan using an artificial neural network system: a Japanese multi-center database project. EJNMMI Res. 3(1): 83.
- Osamu T, Yuko H, Yona O, Naofumi M, Lars E (2014) Investigation of computer-aided diagnosis system for bone scans: a retrospective analysis in 406 patients. Annals of Nuclear Medicine 28: 329-339.
-
Nayab Mustansar. Prognostic Implication of Positive Area on Bone Scans (%PAB- Quantitative Parameter) in Bones of Patients with metastatic Prostate cancer. Adv Can Res & Clinical Imag. 2(1): 2019. ACRCI.MS.ID.000527.
-
Prostate cancer , Tumors , Cancer , PAB , Carcinoma, Osteochondroma Osteoid osteoma, Giant cell tumor, Osteoblastoma, Enchondroma
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






