 Research Protocol
Research Protocol
Tumor Lysis Syndrome Associated with Urothelial Cancer: A Case Series
Razelle Hernandez1, Jill Cassaday2, Jeffrey Wang3, Anne Tang4 and Jue Wang*2,4,5
1Midwestern University College of Pharmacy Glendale, USA
2University of Arizona Cancer Center at Dignity Health St. Joseph’s Hospital and Medical Center, USA
3Baylor College of Medicine, USA
4Creighton School of Medicine, USA
5University of Arizona College of Medicine, USA
Jue Wang, Professor of Medicine, Head of Genitourinary Oncology, University of Arizona Cancer Center at Dignity Health St. Joseph’s, Phoenix, USA.
Received Date: March 27, 2019; Published Date: April 29, 2019
Abstract
Background: Tumor lysis syndrome (TLS) is an oncologic emergency with limited treatment options. Although the vast majority of TLS is associated with rapidly proliferating hematologic malignancies, it is often overlooked in solid tumor cases and has only been rarely associated with urothelial carcinomas. The objective of this review is to investigate the clinical characteristics and outcomes of TLS in patients with urothelial carcinoma.
Methods: Retrospective literature and case review and pooled analysis.
Results: Four cases of TLS in urothelial carcinoma were identified in over 180 published cases of TLS in solid tumors; one additional case was included from our patient database. The median age of these patients was 72 years (range: 63-77) and 60% were female. Two patients had urothelial carcinoma of the renal pelvis and three had bladder cancer with metastases. All of these cases were associated with therapy-induced TLS with a median time to event of 14 days (range: 8-22 days) after receiving chemotherapy. Laboratory parameters were consistent among all cases with elevations in uric acid and LDH, hypocalcemia, hyperphosphatemia, and hyperkalemia. Treatment for TLS included aggressive supportive measures with intravenous hydration. One patient received hemodialysis; four of the five patients received urate oxidase, while one patient received allopurinol as treatment support. The mortality in this case series was 60%.
Conclusion: TLS in urothelial cancer is associated with very high mortality. TLS should be considered as a differential diagnosis when evaluating acute kidney injury and electrolyte abnormalities in patients with urothelial cancer especially in cases of high tumor volume and rapid growth.
Keywords: Urothelial carcinoma; Transitional cell carcinoma; Tumor lysis syndrome; Oncologic emergency
Introduction
Tumor lysis syndrome (TLS) is one of the major oncologic emergencies that can cause metabolic and electrolyte derangements which in turn result in acute renal dysfunction, arrhythmias, seizures, and eventually death [1]. Cellular death mediated by cancer therapy or spontaneous cellular lysis in rapidly dividing tumors leads to the release of intracellular contents comprised of potassium, phosphorous and uric acid. Although the vast majority of TLS cases are associated with rapidly proliferating hematologic malignancies [2], there are increasing reports of TLS in solid tumor with the availability of more effective treatment during last two decades [3-13]. Prior to 2017, only one case of TLS in urothelial carcinoma had been reported in literature [3,4]. Subsequently, our team reported two cases of TLS in urothelial carcinoma [5,6]. The incidence of TLS in urothelial cancers is currently unknown and we believe these clinical observations deserve further investigation. The objective of this study is to examine the available published case reports of TLS in urothelial cancer, and to assess clinical characteristics, management and outcomes of TLS in patients with urothelial carcinoma.
Materials and Methods
Study design and literature search strategy
This is a retrospective systematic review of published case reports in patients with urothelial cancer who were diagnosed with TLS with the addition of one more patient in our institution’s database. PubMed/Medline and Cochrane Central were searched for articles focused on TLS in patients with urothelial carcinoma published from January 1950 to December 2018. Search terms included: “tumor lysis syndrome”, “solid tumor”, “urothelial carcinoma” or “transitional cell carcinoma”.
Data collection and statistical analysis
Information regarding the patients, including age at diagnosis, gender, primary cancer, and co-morbidities (such as renal function); tumor characteristics, laboratory results, treatment, and the outcome were recorded when available. Descriptive statistics such as frequency counts, mean, median and ranges were used to characterize the pooled sample.
Results
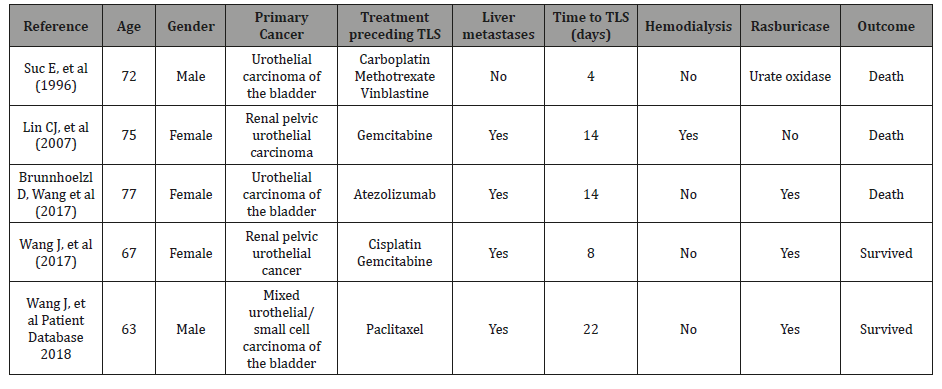
Four published case reports of TLS in urothelial carcinoma were identified among 180 cases of’ TLS in solid tumor reported literature. In addition, we included one patient identified in our database. Patient demographics and disease characteristics, as well as time onset of TLS from cancer treatment, management and outcome of TLS, are summarized in (Table 1). The median age of the patients in this cohort was 72 years (range: 63-77), and 3 of the 5 (60%) were women. Only one patient had chronic kidney disease prior to chemotherapy. The primary cancer included urothelial carcinoma of the renal pelvis and bladder cancer. Four of the five cases had liver metastases with greater than 10centimeter lesions and one case with right iliac fossa involvement. Clinical presentation of TLS included symptoms of fatigue, abdominal pain and progressive anorexia prior to admission. Laboratory parameters were consistent among all cases with elevations in uric acid and LDH, hypocalcemia, hyperphosphatemia, and hyperkalemia. Treatment preceding TLS varied among the case reports and included combination carboplatin, methotrexate and vinblastine, gemcitabine monotherapy, gemcitabine in combination with cisplatin, paclitaxel monotherapy, and monotherapy with a PD-L1 inhibitor, atezolizumab. All of these cases were associated with chemotherapy-induced TLS with a median time to TLS of 14 days (range: 8-22 days). Treatment for TLS included aggressive supportive measures with intravenous hydration. One patient received hemodialysis; four of the five patients received urate oxidase (three received the recombinant form of rasburicase and one patient received urate oxidase), while one patient received allopurinol as treatment support. None of these patients received TLS prophylaxis. The mortality in this case series was 60%.
Table 1: Summary of published reports on tumor lysis syndrome in patients with urothelial carcinoma.
Discussion
In this review, a total of five cases of TLS were identified after treatment in patients with urothelial carcinoma. It is noteworthy that three of the five cases were treated in our institution during last 24 months. We speculate that cases of TLS in urothelial carcinoma are largely under-recognized and underdiagnosed in community hospitals. There is a misguided clinical perception within the oncology community that TLS rarely occurs in solid tumor based on out-of-date TLS risk information. However, the incidence of TLS in solid tumors is clearly rising; this is supported by clinical observation and increasing reports of TLS in solid tumor in literature during last two decades [7-8]. The rising trend of TLS is largely thought to be due to the availability of more effective therapies and increased awareness of TLS [9-10]. In our recent internal audit of TLS in our 300-bed hospital, TLS in solid tumors now accounts for 15% of all TLS diagnosed in last three years. In a population based the study, tumor lysis syndrome contributed to 5% of the acute renal failure cases. In a routine clinical environment and clinical trial setting, TLS can easily be misclassified as electrolyte abnormalities or acute kidney injury [11-14]. Several other observations from this review are worth mentioning. First, TLS was reported in cases of urothelial carcinoma that involved both the upper and lower urinary tract. Second, time to TLS after treatment resulted in a median duration to TLS of 11 days, with a range of 4 to 22 days. Third, a high mortality rate of 60% was observed in urothelial carcinomas with TLS, as compared to the 20% mortality rate in hematologic malignancies [7,8]. The high mortality rate in this case series is concerning and clinicians should be made aware that patients with TLS in solid tumors, particularly urothelial carcinoma can result in a potentially fatal prognosis. Finally, we identified one case of primary urothelial carcinoma that later transformed into small cell histology in metastatic liver lesion. Small cell carcinoma of the bladder is a rare pathology associated with high-grade malignant behavior and poor prognosis. It accounts for less than 1% of bladder cancers and clinical findings and treatment are still inconclusive [8]. There is currently no standard of treatment for small cell carcinoma and treatment options have only been extrapolated from small cell carcinoma of the lung. The majority of tumor lysis syndrome cases reported so far in the literature occurred after first-line therapy [5-13]. For the first time, we reported a case of TLS occurred after third line treatment in a patient with mixed urothelial carcinoma/ small cell carcinoma of bladder. The onset of TLS was 2 weeks after his third line treatment with the paclitaxel for progressive disease with the lung liver and lymph node metastasis. Interestingly, this patient previously received 4 doses of atezolizumab as second line treatment. Similar to small cell lung cancer, small cell variant of bladder cancer represents one of the most aggressive subtype of bladder cancer with a rapid proliferation rate [15]. The current standard treatment is platinum-based chemotherapy. Currently, there is no effective second line or third line therapy for small cell bladder cancer. A recent investigation demonstrated that atezolizumab in combination with chemotherapy significantly improved overall and the progression free survival in patients with extensive stage small cell lung cancer [16]. The extensive tumor burden, rapid proliferation of small cell component of bladder cancer and the potential synergistic effects of chemotherapy and PD1 blocking immunotherapy may explain the occurrence of TLS in this case after third line treatment. Despite the limitations of retrospective studies, this review provides the most up-to-date information regarding the diagnosis and outcomes of TLS in patients with urothelial carcinoma. Based on the presented results, TLS in urothelial carcinoma displays high mortality rates in patients with high tumor burden, visceral metastases, with or without preexisting renal dysfunction or baseline elevations in uric acid and therefore warrants urgent education, increased awareness and risk-stratified prophylaxis and management. Further investigation is necessary to define the true incidence of TLS in solid tumors such as urothelial cancer to provide clear recommendations for standards in prophylaxis.
Conclusion
Our review highlights the life-threatening outcome of TLS in the setting of urothelial cancers. Along with other solid tumors, clinicians should consider TLS as a differential diagnosis when evaluating acute renal failure and electrolyte abnormalities in patients with urothelial cancers. The findings of this review can hopefully increase the oncology community’s awareness of TLS and facilitate prompt recognition and appropriate management of this silent killer.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Gemici C (2006) Tumour lysis syndrome in solid tumours. Clin Oncol (R Coll Radiol) 18(10): 773-780.
- Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel (2010) Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol 149(4): 578-586.
- Lin CJ, Lim KH, Cheng YC, Chen HH, Wu CJ (2007) Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol 24(4): 455-457.
- Suc E, Leandri P, Moussion F, Puech JL, Suc A (1996) Cell lysis syndrome after chemotherapy for cancer of the bladder. Presse Med 25(40): 2044.
- Wang J, Ruvinov K, Cassaday J, Richard Trepeta (2017) Acute tumor lysis syndrome after cisplatin and gemcitabine for treatment of urothelial carcinoma of the renal pelvis: case report and review of literature. Ann Clin Lab Res 5(2): 165-168.
- Brunnhoelzl D, Weed M, Trepet R, Wang J (2017) Tumor lysis syndrome following a single alemtuzumab infusion for metastatic urothelial carcinoma involving both upper and lower tract. Archives in Cancer Research 5(1): 127.
- Wang JF, Harmon J, Allen S, Cassaday J, Ruvinov K, et al. Older age and liver metastases predict poor prognosis of Tumor Lysis Syndrome in Solid Tumor. In: Wang J (edt.), eBook “Top 25 Contributions on Cancer Research”. Berlin, Germany.
- Harmon J, Allen S, Cassaday J, Ruvinov K, Stoyanova D, et al. (2018) Liver Metastasis is an Independent Predictor for Mortality in Patients Who Developed Tumor Lysis Syndrome: Analysis of 132 Patients with Solid Tumors. J Clin Oncol 36(15).
- Wang J (2018) Tumor Lysis Syndrome Associated with Prostate Cancer: An Under-recognized Oncologic Emergency. J Emerg Crit Care Diagn Manag 1(1): 1-3.
- Leung B, Wang JF, Cassaday J, Wang J (2019) Clinical Features, Treatment, and Outcome of Tumor Lysis Syndrome in Germ Cell Tumors. J Urol Neph St 1(4):72-76.
- Cordrey E, Wang J (2018) Tumor Lysis Syndrome Associated with Immune Checkpoint Blockade in Solid Tumors. Jpn J of Cancer Oncol Res 1(1): 1005.
- Harmon J, Allen S, Stoyanova D, Ruvinov K, Cassaday J, et al. (2018) The Clinical Features, Treatment, Outcome and Prognosis of Spontaneous Tumor Lysis Syndrome in Solid Tumor. J Oncol Res Forecast 1(2): 1009.
- Allen S, William A, Cassaday J, Wang J (2018) Renal Cell Carcinoma with Extensive Rhabdoid Features: Case Report of Spontaneous Tumor Lysis Syndrome and Review of Literature. Urol Res Ther J 2(1): 119.
- Wilson FP, Berns JS (2014) Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis 21(1): 18-26.
- Bhatt VR, Loberiza FR Jr, Tandra P, Krishnamurthy J, Shrestha R, et al. (2014) Risk Factors, Therapy and Survival Outcomes of Small Cell and Large Cell Neuroendocrine Carcinoma of Urinary Bladder. Rare Tumors 6(1): 5043.
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, et al. (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small- Cell Lung Cancer. N Engl J Med 379(23): 2220-2229.v
-
Razelle Hernandez, Jill Cassaday, Jeffrey Wang, Anne Tang, Jue Wang. Tumor Lysis Syndrome Associated with Urothelial Cancer: A Case Series. Arch Clin Case Stud. 1(3): 2019. ACCS.MS.ID.000515.
-
Tumor cases, Patients, Hypocalcemia, Hyperkalemia, Urothelial cancer, Electrolyte abnormalities, Kidney injury, Urothelial carcinoma, Tumor lysis syndrome, Oncologic emergency.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






