 Research Article
Research Article
Simulation of Bifurcation in Brain Illness/ Remediation. Enzyme Reactions of the Neurocycle
Ibrahim Mustafa1,2, Asmaa Awad1, Elkamel A1 and Said Elnashaie3*
1Department of Chemical Engineering, Waterloo University, Canada
2Department of Biomedical Engineering, Helwan University, Egypt
3Department of Chemical and Biological Engineering, University of British Columbia (UBC), Canada
Said Elnashaie, Department of Chemical and Biological Engineering, University of British Columbia (UBC), Vancouver, Canada.
Received Date: November 06, 2019; Published Date: December 06, 2019
Abstract
Enzymes-Reactions Model is used to study: dynamics, bifurcation, and chaos of the acetylcholine neurocycle (ACNC). The effects of feed choline concentrations, Acetylcholinesterase (AChE) activity as bifurcation parameters are studied. It was found that feed choline concentrations are important and have direct effect on ACNC through certain important range of parameters. Detailed bifurcation analysis is carried out in order to uncover important features of the system, such as static/dynamic bifurcation and chaos. These findings are related to the real phenomena occurring in the neurons, like periodic stimulation of neural cells and non-regular functioning of acetylcholine receptors. Results are compared to the results of physiological experiments and other published models. As there is strong evidence that cholinergic brain diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD) are related to the concentration of acetylcholine, the present findings are useful for uncovering some of the characteristics of these illnesses/remediation.
Keywords: Acetylcholinesterase; Acetylcholine; Choline; Acetate; Parkinson’s disease (PD); Alzheimer’s disease (AD); Dynamic behavior; Bifurcation; Chaos
Notation:
Abbreviations: LMICs: Low-And Middle-Income Countries; IDF: International Diabetes Federation; NEHEP: National Eye Health EducationProgram; BDHS: Bangladesh Demographic and Health Survey; BIRDEM: Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders; IPH: Institute of Public Health
Introduction
A complete neurocycle of Acetylcholine (ACh) involves a coupled two-enzyme system with the following two simultaneous events [1]:
Synthesis event: ACh is synthesized from choline and acetyl- CoA bio catalyzed by the enzyme choline acetyltransferase (ChAT) and immediately stored in small vesicular compartments closely attached to the cytoplasmic side of the presynaptic membranes [1,2].
Degradation event: Once ACh has completed its activation duty, the synaptic cleft degradation begins removing the remaining ACh. This occurs when the ACh is consumed by the acetylcholinesterase (AChE) to form choline and acetic acid [3-5].
It has been found that the synthesis of ACh in the nerve endings is based on the preferential utilization of the choline which is supplied by the high affinity carrier from extra cellular fluid. Choline in the extra cellular fluid of the brain comes either directly from the free choline of the blood plasma, or from the brain cells, where it has been released from choline containing compounds [4,5] found that using the steam pulse sequence (one of the main techniques commonly used for the diagnosis of imaged brain abnormalities), concentrations of choline in human brain frontal lobe white matter are found to be 1.9±0.5 μmol/g wet wt. In the thalamus, the concentrations are 2.0±0.4 mol/g wet wt. Once in the extracellular space of the brain, choline can be taken up by all cells and used as a precursor of structural membrane phospholipids such as phosphatidylcholine and sphingomyelin and uniquely by cholinergic neurons for the conversion to ACh [6]. Phosphatidylcholine in these neurons also comprises a storage pool of choline, and the hydrolysis of phosphatidylcholine catalyzed by phospholipase Deliberates choline from this reservoir for the conversion to ACh by ChAT [7].
Several studies [8,9] indicate that neurotransmitter external choline (modified) availability is an important factor regulating the dynamics of ACh metabolism and it appears that choline may be the rate-limiting substrate for ACh synthesis in vivo. Although a controversy exists [10] concerning the form of choline that is transported from the periphery to the brain, it is generally agreed that brain choline available for ACh synthesis is derived from sources outside the cholinergic neuron and include: (I) choline in the form of phospholipids, (2) free choline in plasma, and (3) choline generated from the hydrolysis of ACh.(Kewitz et al.,1975 Ansell and Spanner ,1975) If a significant fraction of the choline used for ACh synthesis is derived from ACh hydrolysis, then under conditions of AChE inhibition, the size of this choline pool may be the limiting factor regulating the magnitude of the increase in ACh levels [2].
Several studies have suggested that acetate may affect the central nervous system (CNS) [Carmichael et al., (1991)]. The main entry point for acetate into metabolism in vertebrates is its conversion to acetyl-CoA by acetyl-CoA synthetase. The acetyl-CoA which is formed from acetate can be used for energy generation; on the other hand, acetyl-CoA is utilized in the brain’s cholinergic neurons for ACh synthesis. It has been shown that extracellular acetate is accumulated by cholinergic nerve terminals for ACh formation and release [11].
There are some factors other than choline availability to ChAT that regulates ACh synthesis such as choline uptake to the presynaptic neuron and acetate to prevent expansion of the tissue ACh store [12-14] showed that the effect of AChE inhibition experimentally, on ACh labeling from acetate offers little support to the idea [15] that reuptake of acetate derived from hydrolyzed transmitter plays a role in maintaining ACh synthesis. In this way, acetate differs from the other product of transmitter hydrolysis, choline, which has been shown to be recaptured and used for ACh synthesis [15].
[16] investigated the neurocycle of the ACh utilizing a twocompartment model with AChE as the only enzyme. The investigations unveiled complex static and dynamic behavior including bifurcation, instability, and chaos. [4] investigated a complete but simplified neurocycle for the ACh as a neurotransmitter in an AChE/ChAT system and found that complex dynamic bifurcations, hysteresis, multiplicity, period doubling and period halving, as well as period adding, and period subtracting dominated the dynamics of the system. [5] presented the formulation of a diffusion-reaction model utilizing available biokinetic information to simulate the in-vivo behavior of AChE and ChAT coupled enzymes system using a novel two-enzyme/two-compartment model to explore the bifurcation and chaotic behavior of this enzyme system simulating the ACh neurocycle in the brain. They carried out a detailed bifurcation analysis over a wide range of parameters in order to uncover some important information related to the phenomena occurring in the physiological experiments, like periodic stimulation of neural cells and non-regular functioning of ACh receptors. [5,16].
In this work we try to investigate the effect of choline, and AChE activity using two kinetic mechanisms one for the synthesis of ACh by the enzyme ChAT and the other for hydrolysis of ACh by the enzyme AChE. We attempt to analyze the synthesis of ACh at the level of single cells, rather than the whole nervous system and try to investigate the role of feed (external) choline and acetyl CoA on the ACh processes. The present work extends up on our earlier investigation [17-19]. Here we still employ a novel diffusionreaction model but improve upon our previous investigation by considering realistic kinetic schemes and data for ChAT synthesis reaction, and account for the recycle effects of choline.
Proposed New Diffusion Reaction Two-Enzyme / Two Compartment Model
The (AChE/ChAT) enzymes system inside the neural synaptic cleft can be schematically described in a simplified manner as shown in (Figure 1). The complete neurocycle of the ACh as a neurotransmitter is simulated as a simplified two-enzymes/twocompartment model. Each compartment is defined as a constant flow; constant volume, isothermal, continuous stirred tank reactor (CSTR) and the two compartments are separated by a permeable membrane. The behavior for a single synaptic vesicle is described by this simple two compartment model, assuming that all the events are homogeneous in all vesicles and using the proper dimensionless groups. From an enzyme kinetics point of view, the most general case is when the kinetics of both enzyme reactions has a double non-monotonic (dependence upon both state variables of substrate and hydrogen ions concentration). ACh is assumed to be synthesized in compartment 1 by ChAT due to the activation reaction as follows [5,12]:
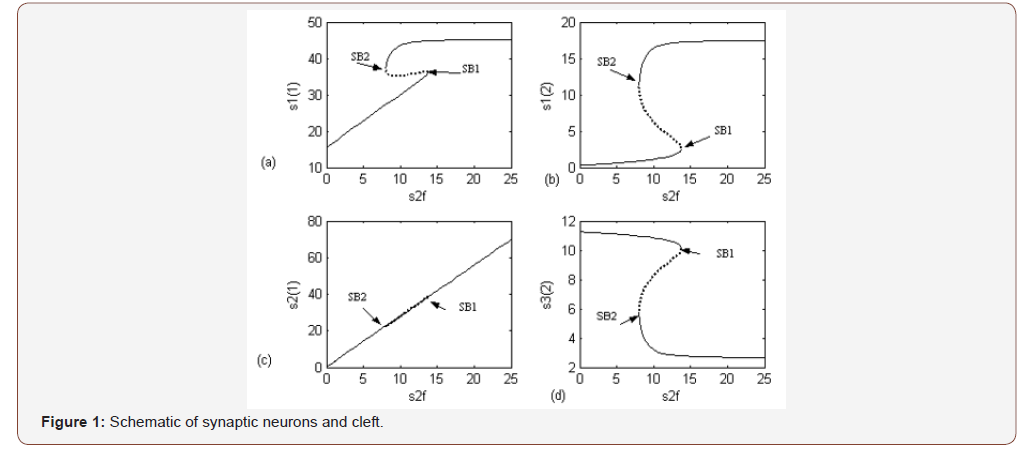
Both reactions R(1) and R(2)are considered to be substrate inhibited and hydrogen ions affected. This leads to a non-monotonic dependence of the reaction rates on the substrates concentrations and pH. The rates can be formulated by employing certain assumptions and basic biokinetics knowledge as explained in the following section. The details of the derivation are given in our previous work (Mustafa et al., 2008). The final dimensionless forms of the ordinary differential equations of the eight state variables are summarized in (Table 1). The model equations are in terms of eight state variables S1(1) S1(2) S2(1) S2(2) S3(1) AND S3(2) and twentyfive parameters (Tables 2&3). All values of the parameters (with respective references) used in this investigation are given in (Table 1-3).
Table 1: Dimensionless forms of the ordinary differential equations of the eight state variables [17].
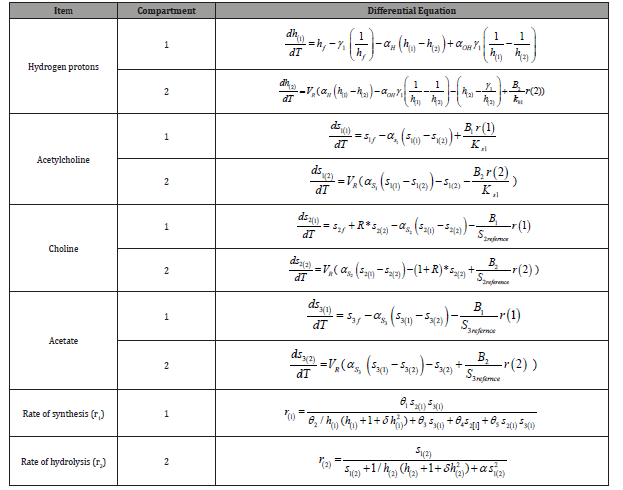
Table 2: Dimensionless state variables, parameters and other terms [17].
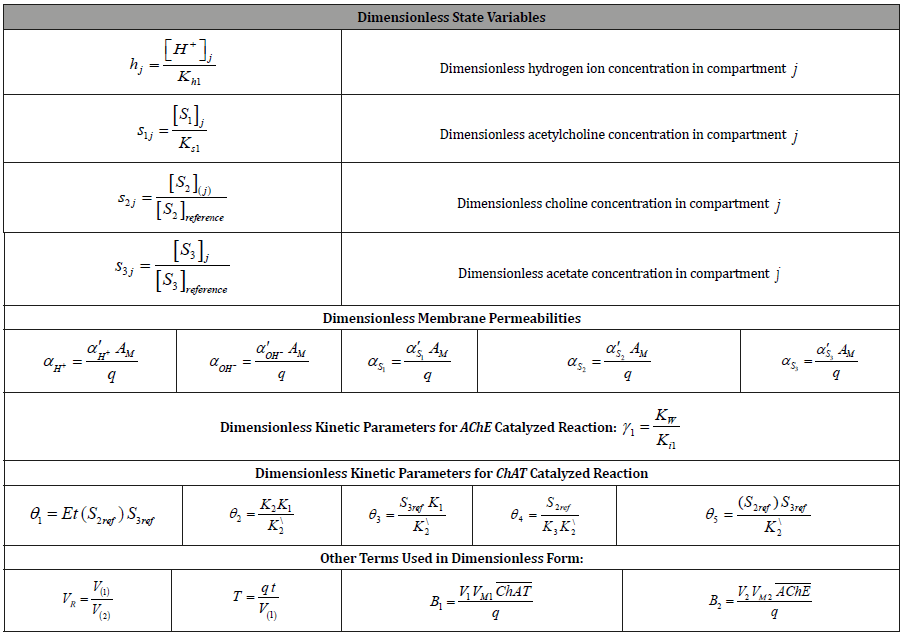
Table 3: Values of the kinetic parameters.
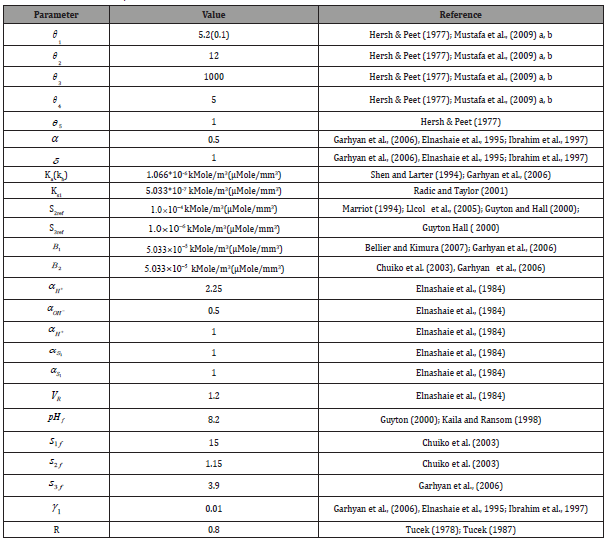
Techniques and Numerical Tools
The bifurcation diagrams are obtained using the software package XPP-AUT 5.0 (Ermentrout, 2002) which utilizes the wellknown continuation software AUTO97 [15]. This package is able to perform both steady state and dynamic bifurcation analysis, including the determination of entire periodic solution branches using the efficient continuation techniques.
The classical time trace and phase plane for the dynamics are used. However, for high periodicity and chaotic attractors these techniques are not sufficient. Therefore, other presentation techniques based upon the plotting of discrete points of intersection (return points) between the trajectories and a hypersurface Poincaré surface, [13,20] are also employed [1]. These discrete points of intersection are taken such that the trajectories intersect the hyperplane transversally and cross it in the same direction. They are used to construct the Poincaré one parameter bifurcation diagram. A stiff solver with automatic step size to ensure accuracy is used for the numerical simulation of the periodic as well as the chaotic attractors. The program employed by [15] was used to plot the Poincare diagram.
Physiological Values of the Parameters
In order to compare the behavior of our model with experimental investigations (Weckler (1988), Morel (1976), Schwartz et al., (1975), Lynn Wecker et al., (1979)) [5], and with the models of prior researchers [4, 5] it is necessary to use the same range of the variables. An extensive literature review leads us to the following ranges:
• pH in the range of 6.95 to 7.15 was measured in human brain [21].
• pH in the range of 6.95 to 7.35 was reported in a feline model, at which the cat is assessed as a model of experiments [15].
• ACh in a rat brain was found to be in the range of kmol/m3. (Free ACh) to (total ACh) [2]. While, in guinea pig cerebral cortex the range was (free ACh) to kmol/m3. (total ACh) [2,12]. These values represent content of free and total ACh in the brain.
• ACh concentration in human placenta was reported to be in the range of to kmol/m3. (Wessler et al., 2001).
• ACh in the isolated rings of rat pulmonary artery was measured to be in the range of to kmol/m3 produced concentration-dependent relaxations (Kysela and Torok, 1996).
• Choline concentration in mouse rat brain is about - kmol/ m3. [2,12] and [2].
• Choline concentration in human plasma was reported to be in the range of to kmol/m3. (Chay and Rinzel, 1981).
The real concentration of ACh in cholinergic neurons of the brain is not known [2,12]. The content of ACh in the rat brain will be taken as 1.2 x10-5 kmol/m3 [2] On the assumption that the neurons represent 1/3 of the weight of the brain ( The rest being attributable to glial cells and extracellular fluid), that the ACh is confined to cholinergic neurons , and that cholinergic neurons represent 10% of the total volume of all neurons, the concentration of ACh in the cholinergic neurons will be equal to 1.2 x10-5 kmol/m33. Despite the uncertainties associated with this estimate (the main being the proportion of cholinergic neurons in the total neuronal population of the brain), it is evident that, in the light of the present knowledge, the estimated equilibrium concentration of ACh (12 x10-5 kmol/m3) and the estimated concentration of ACh in cholinergic neurons (36 x10-5 kmol/m3) do not appear vastly different incompatible values. A higher concentration of ACh in presynaptic nerve endings might be achieved in two ways: by the accumulation of ACh in synaptic vesicles, and by higher concentration substrates in this part of neuron.
Results and Discussion
Feed choline concentrations
We investigate static and dynamic bifurcation due to the change of feed choline concentration. We studied the static bifurcation at higher value of feed ACh concentrations s1f =15 corresponding to 0.755 x10-5 kmol/m3 as a medium value in the range of ACh in rat brain given by Tucek 1978.and the dynamic bifurcation at s1f =2.4 corresponding to 0.12 x10-5 kmol/mm3 which is the lowest value in the range given by Tucek 1978. The range given by Tucek 1978 is [ 0.12×10−5 to 1.77×10−5 ] kmol/m3.
• Case (1): Static Bifurcation at s1f =15 (corresponding to 0.755 x10-5 kmol/m3)
Figures 2 (a, b, c, d and e) show the bifurcation diagrams with as the bifurcation parameter for a very wide range of values using fixed values of and h1f = 0.0062682 equivalent to pH=8.2. Only a static bifurcation in the form of hysteresis-controlled system is found in this case. Figures 2(a, b, c, d and e) respectively show the static bifurcation through investigating the effect of changing the feed choline concentration (s2f) on the ACh concentrations (s11, and s12) in compartments 1 and 2, choline concentration in compartment 1 (s21) and acetate concentration in compartment 2(s32). The figures show that s11, and s12 are increasing with increasing s2f until certain value of s2f then s11, and s12 remain constant with further increase of s2f. This is compatible with the experimental results done by Tucek 1990 and Lefresne 1973 who indicated that the content of ACh increases until it reaches a certain limiting value and then remains stable when the choline substrate concentration increases. However; s21 increases continuously as a function of s2f. If we investigated the effect of s2f on s22, s22 will behave like s21. The ACh concentrations synthesized in both compartments 1 and 2 (s11, s12) are increasing with high rate at, however; at high feed choline concentration corresponding to, ACh was synthesized less efficiently from the feed choline concentration which accumulated in nervous tissue. This is in agreement with the results obtained by Schwartz et al., (1975) who showed that at low concentration of feed choline, the fraction of the choline converted to ACh approached 60-75 %; this fraction decreased as the external choline concentrations were increased (Figure 2).
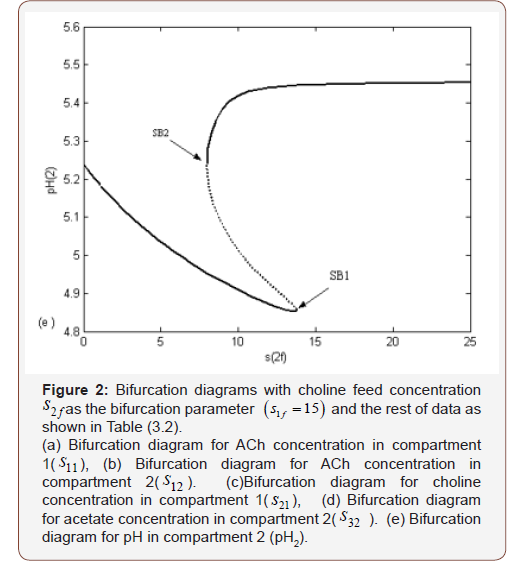
From the constancy of ACh levels in both compartments in the presence of choline concentrations higher than the critical value, i.e. we can conclude that the release of ACh varied in parallel to the incorporation rate of the feed choline (s2f) to be catalyzed by the enzyme ChAT to produce ACh in compartment 1. The released transmitter in compartment 2 is compensated by the ACh synthesized in compartment 1. Therefore, the rate of ACh synthesis must be equal to the rate of transmitter release.
• Case (2): Dynamic Bifurcation at s1f =2.4
Figures 3 (a, b, c, d and e) show the dynamic bifurcation diagrams
using feed choline concentrations (s2f) as the bifurcation parameter
but with a different value of feed substrate concentrations which
represent a very low feed ACh concentrations. The figures show
different stages in the neurocycle for a narrow range of the
bifurcation parameter kmol/m3. It 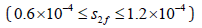 is clear that the system has rich dynamics phenomena at low
concentration of S2f where the feed choline concentrations are too
small to start the synthesis reaction catalyzed by ChAT (Figure 3).
is clear that the system has rich dynamics phenomena at low
concentration of S2f where the feed choline concentrations are too
small to start the synthesis reaction catalyzed by ChAT (Figure 3).
Three main regions in the bifurcation diagram are observed, each one corresponding to a different form of qualitative behavior. There are two Hopf bifurcations (HBs). The first HB1 appears at S2f = 0.69and the other HB2 at S2f = 1.14085.
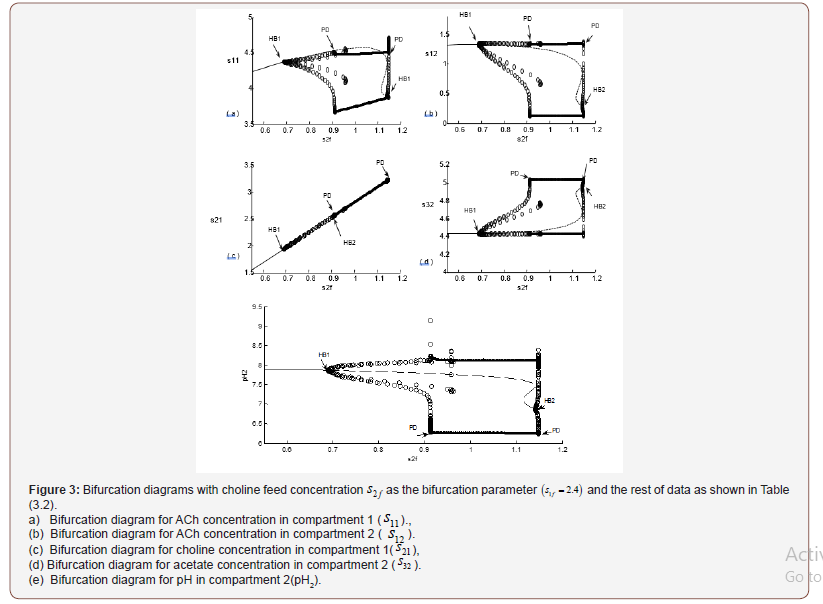
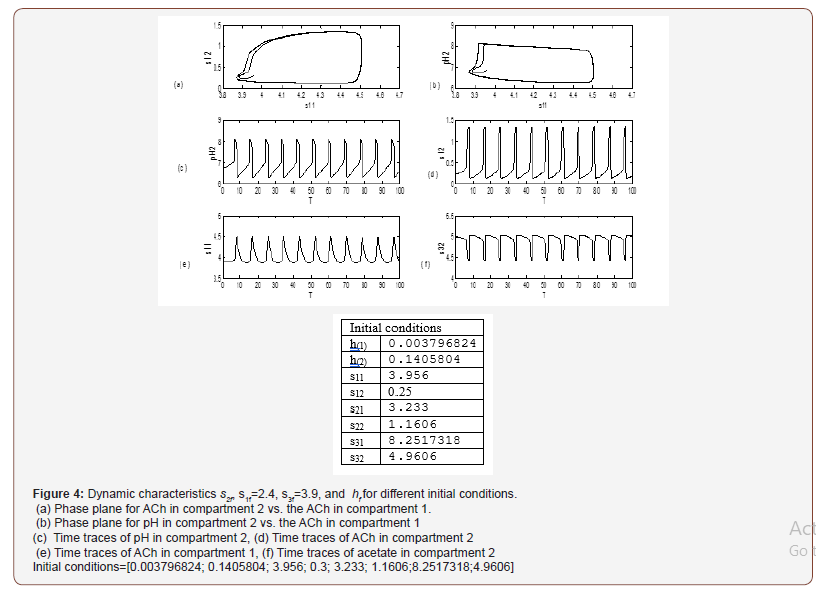
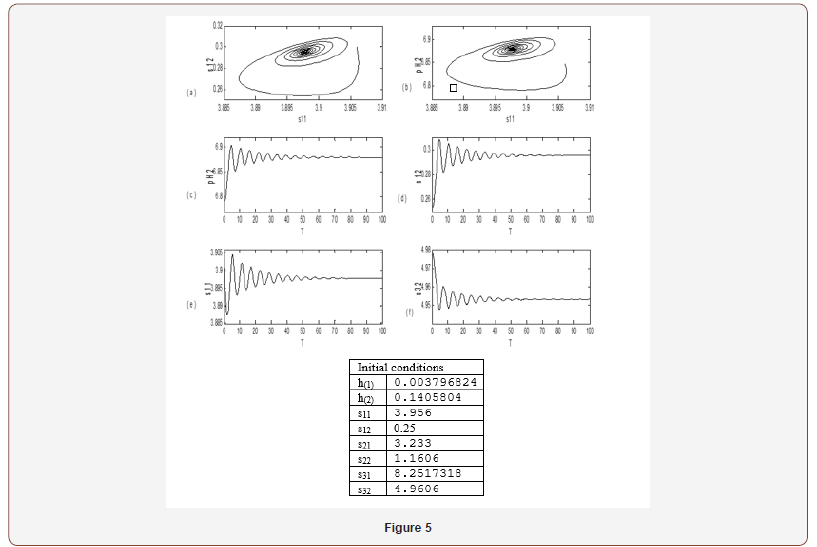
The physiological values correspond to a range of feed choline concentration between 11.41×10−5 and 11.4676×10−5 . The pH in compartment 2 (PH2) is inside the physiological expected range where it is between 6.75 and 8.2. This region with low choline concentration is thus characterized by the presence of bistability. The period doubling (PD) points occur at s2f = 0.913 and s2f =1.147. The period doubling is one of the routes leading to chaos. The first initial conditions (Figures 4) lead to a periodic attractor, while the second initial conditions (Figures 5) lead to a point attractor. In addition, when initial conditions are changed in certain range, chaos will appear as will be shown in (Figures 4-9).
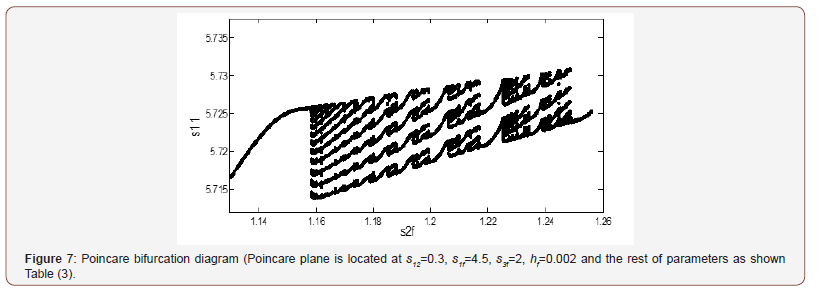
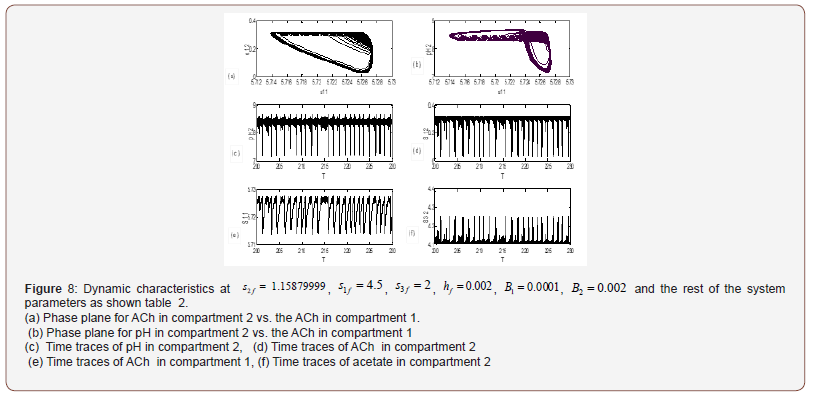
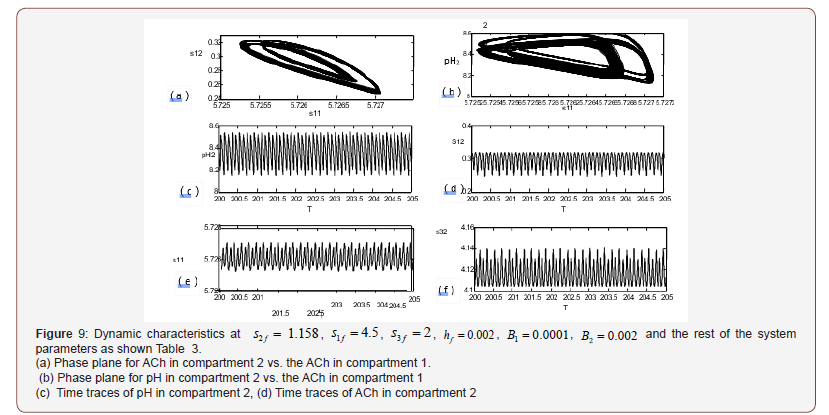
AChE enzyme activity
AChE enzyme activity (B2) is chosen in order to gain some insight on the possible consequences of the change in the activity of the enzyme to hydrolyze the neurotransmitters. Wecker et al (1978) investigated the effect of AChE inhibition on the synthesis of acetylcholine in discrete brain regions in animals treated with the organophosphorus cholinesterase inhibitor paraoxon. They found that administration of paraoxon (0.23 mg/kg) inhibited acetylcholinesterase activity by nearly 90% in the striatum, hippocampus and cerebral cortex and acetylcholine levels increased to 149%, 124% and 152% of control values, respectively. Free choline levels were unaltered by paraoxon in the hippocampus and cerebral cortex, but significantly decreased in the striatum to 74% of control.
Imbalances in activity of AChE enzyme can be linked to
devastating diseases like Alzheimer’s and Parkinson’s [5]. This
bifurcation parameter(B2) incorporates the enzyme activity and
three constants (AChE concentration in compartment 2, volume of
compartment 2 and the flow rate): 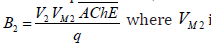 the maximum rate of ACh hydrolysis that contains kinetic constant that dominates the final reaction step.
the maximum rate of ACh hydrolysis that contains kinetic constant that dominates the final reaction step.
Therefore, variation of the bifurcation parameter B 2 gives the effect of the variation of the enzymatic activity for the AChE catalyzed reaction. Two cases are analyzed for this particular bifurcation parameter. Case (1) which illustrates the important hysteresis (or short-term memory) phenomenon involved with the enzymatic activity (this phenomenon is extremely important for the possible link of this physiological system with the cholinergic diseases [4]. The second case discusses the dynamic bifurcation associated with the enzyme activity. These cases are discussed in detail below.
A. Case (1): Static Hysteresis Behavior
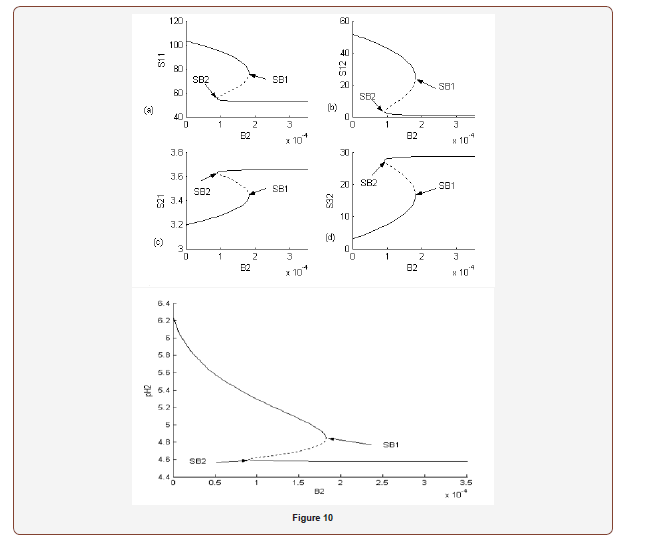
Figure 10 show the bifurcation diagrams with as the bifurcation
parameter for a very wide range of values using fixed values of 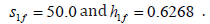 Only a static bifurcation in the form of
hysteresis-controlled system is found in this case. As the value of
the bifurcation parameter is decreased (equivalent to a decrease
in AChE enzyme activity) while all other parameters are kept
constant, different regions in the neurocycle are observed as shown
in Figures 10 (a, b, c, d and e) as follows:
Only a static bifurcation in the form of
hysteresis-controlled system is found in this case. As the value of
the bifurcation parameter is decreased (equivalent to a decrease
in AChE enzyme activity) while all other parameters are kept
constant, different regions in the neurocycle are observed as shown
in Figures 10 (a, b, c, d and e) as follows:
• Region 1: High enzyme activity in the region 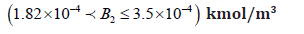
In this region the system exhibits a unique stable steady state. In this region the AChE catalyzed reaction operates at its maximum capacity, consuming almost all the ACh in compartment(2)(S1(2)). Figure 10(b) shows that the dimensionless (S1(2)) is kept in the range (S1(2))=0.003379 − 2.94.Figure 10(a) shows that dimensionless (S1(1))is kept in the range (S1(1))=54.74 − 51.92 . Figures 10(c) and 10(d) show that (S21(1))and (S1(1)) and (S3(2)) (products of AChE catalyzed reactions) have their maximum values in this region. The acetate in compartment (2) maximum value is (S1(1)) and (S3(2))=28.9, and the choline in compartment (1) maximum value is (S2(1))=3.66074 Figure. 10(e) shows that pH(2) has its lowest value which is pH(2) (maximum hydrogen concentration) because hydrogen ions are also produced in compartment 2 as the acetic acid disassociates into acetate and hydrogen ions.
This region corresponds to a very low value of ACh concentration in compartment(1) between and kmol/m3while in compartment (2) between s 2(1) = 3.66074 and 2.6125×10−5 kmol/m3 The pH in compartment 2 is also low ranging between 4.6 and 4.57, while the choline concentration in compartment (1) is almost constant varying between (3.63433 -3.66074) x 10-4 kmol/m3 In comparison to the results of Mahecha Andres [4], ACh in compartment (1) and (2), and choline concentration in compartment (1) and acetate concentration in compartment (2) , here, are higher than that in Mahecha Andres et al. This is because the choline recycle principle was not taken into consideration by Mahecha Andres et al., (2004) where the choline produced from the postsynaptic neuron is the main source for the choline required for ACh synthesis in the presynaptic neuron or compartment (1). In addition; the rate of synthesis of ACh which is proposed as shown in the Appendices is more efficient than used by Mahecha Andres et al, (2004).
• Region 2: 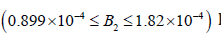
kmol/m3
As the enzyme activity is decreased to, a hysteresis phenomenon occurs, and a multiplicity of steady states is observed between the two static bifurcation points (SB1 and SB2) in this region. This phenomenon is dominating the behavior of the system (it occurs for a wide range of the bifurcation parameter 2 B ). Hysteresis causes the state variables to be very sensitive in the neighborhood of the static bifurcation points. For example, in Figure 10(b) the dimensionless (S1(2)) jumps from (S1(2)) = 2.94345 to (S1(2)) = 43.4 with a slight decrease in enzyme activity near the static bifurcation point SB2 (Figure 10).
This region fits reasonably well to the expected physiological
behavior. Figure 10(a) shows that 1(1) s varies in the range 96.4 and
54.74 corresponding to 48.52×10−6 and 27.55×10−6 kmol/m3 while
the dimensionless varies in the range 43.4 and 0.83 corresponding
to 21.9×10−6 and 0.418×10−6 kmol/m3. Figure 10 (e) shows that
pH(2) is out of the expected physiological range and is varying
between 4.64 and 5.57. Figure 10(c) shows that 21 (1) s is within the
expected range between 3.264×10−4 and 3.66×10−4 kmol/m3. In the
model of Mahecha Andres et al (2004) the hysteresis phenomena
appeared in the 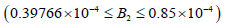 range 3
which is smaller than our range. In addition, the ACh concentration
in compartment (2) ( ) ( ) 1 2 s in their model was in the range and
kmol/m3 which is closer to ours and the pH(2) which is out of the
expected physiological range and it was varying between 4.6 and
5.35 so it is also close to the range of ours.
range 3
which is smaller than our range. In addition, the ACh concentration
in compartment (2) ( ) ( ) 1 2 s in their model was in the range and
kmol/m3 which is closer to ours and the pH(2) which is out of the
expected physiological range and it was varying between 4.6 and
5.35 so it is also close to the range of ours.
Region 2: 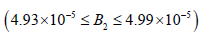 kmol/m3.
kmol/m3.
In this region chaos may develop via the Feigenbaum (1980)
Period doubling (PD) route which appears at 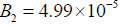 as shown in Figs 11(a, b, c, d and e). In comparison to Mahecha Andres’s model, Period doubling at
as shown in Figs 11(a, b, c, d and e). In comparison to Mahecha Andres’s model, Period doubling at 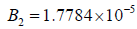 which is
smaller than the previous range.
which is
smaller than the previous range.
• Region 3: 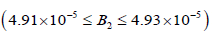 kmol/m3.
kmol/m3.
As the enzyme activity decreases to 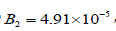 Hopf
bifurcation occurs (HB1). Bistability behavior is observed where
periodic and point attractors coexist with an unstable periodic
orbit as the separatrix separating the domains of attraction of
the periodic and point attractors. This type of bistability can
lead to different types of attractors even at the same value of B2
if the initial conditions are different.Figure 11(a) shows that s1(1)
oscillates between 3.897 and 4.74 corresponding to 1.961×10−6 and 2.39×10−6 kmol/m3. pH(2)oscillates between 6.3 and 7.4, which
is a region near to the expected physiological pH values (Figure
11(e)). Figure 11(b) shows that s1(2) oscillates between and kmol/
m3 corresponding to a low ACh concentration. Figure 11(c) shows
that s2(1) is close to the expected physiological range with soft
oscillations between and kmol/m3.
Hopf
bifurcation occurs (HB1). Bistability behavior is observed where
periodic and point attractors coexist with an unstable periodic
orbit as the separatrix separating the domains of attraction of
the periodic and point attractors. This type of bistability can
lead to different types of attractors even at the same value of B2
if the initial conditions are different.Figure 11(a) shows that s1(1)
oscillates between 3.897 and 4.74 corresponding to 1.961×10−6 and 2.39×10−6 kmol/m3. pH(2)oscillates between 6.3 and 7.4, which
is a region near to the expected physiological pH values (Figure
11(e)). Figure 11(b) shows that s1(2) oscillates between and kmol/
m3 corresponding to a low ACh concentration. Figure 11(c) shows
that s2(1) is close to the expected physiological range with soft
oscillations between and kmol/m3.
• Region 4: 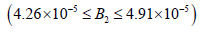 kmol/m3.
kmol/m3.
This region is dominated by unique stable periodic attractors
showing sustained oscillations with similar qualitative behavior
to the one discussed in Case I. This region corresponds to a range
of pH(2) between 6.15 and 8.2 (Figure 11(e)) which is close to the
expected physiological pH values. In Figure 11(b) s1(2)oscillates
between 5.033×10−7 and 8.81×10−7 kmol/m3 corresponding to
low ACh concentration. The choline concentration in compartment
1 is in the expected physiological range with soft oscillations around 3.223×10−4 and 3.233×10−4 kmol/m3 as shown in Figure
11(c). The range of this region is smaller that of Andres which is 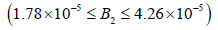 kmol/m3.
kmol/m3.
• Region 5: 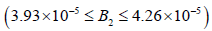 kmol/m3.
kmol/m3.
In this region, as the enzyme activity decreases to 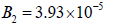 , the second Hopf bifurcation occurs (HB2). Bistability behavioris observed where periodic and point attractors coexist with an
unstable periodic orbit as the separatrix separating the domains
of attraction of the periodic and point attractors. The second Hopf
bifurcation occurs (HB2) in Mahecha Andres is at
, the second Hopf bifurcation occurs (HB2). Bistability behavioris observed where periodic and point attractors coexist with an
unstable periodic orbit as the separatrix separating the domains
of attraction of the periodic and point attractors. The second Hopf
bifurcation occurs (HB2) in Mahecha Andres is at 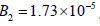
• Region 6: 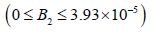 kmol/m3.
kmol/m3.
In this region there is a unique stable steady state (point
attractor). The physiological values in this region correspond to
range of pH(2) from 7.87 to 8.2 which is near to the range of Mahecha
Andres results where pH(2) is between 7.83 and 8.23 (Figure 11(e)).
In Figure 11(a), s1(1) reaches the maximum value at very low values
of B2, where s1(1) = 6.62 corresponding to (3.33×10−6 kmol/m3)
which is the highest value in Figures 11(a and b).Figure 11(b)
shows that s1(2) reaches its maximum value (1.66×10−6 kmol/m3) as
the reaction that consumes it almost stops in this region. However, in the model of Mahecha Andres et al., the maximum value of s1(2)
reaches its (1.258×10−6 kmol/m3). In Figure 11(c) s2(1) reaches its s
its lowest value (3.275×10−4 kmol/m3) as the reaction that produces
it almost stops. This value of s2 (1) is higher than that in the model
of Mahecha Andres et al., (2004), as discussed before because the choline consumed in the presynaptic neuron which is compensated
by recycling the choline produced by hydrolysis reactions was not
taken into consideration by Mahecha Andres. Therefore, the range
of the dynamics of the results of Mahecha Andres et al (2004)
is larger than ours where HB1 and HB2 appear in the results of Mahecha Andres in the range 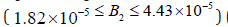 (kmol/
m3) and HB1 and HB2 appear at B2= 1.82×10−5 , and 4.43×10−5
respectively; however our model show that HB1 and HB2 appear in
the range
(kmol/
m3) and HB1 and HB2 appear at B2= 1.82×10−5 , and 4.43×10−5
respectively; however our model show that HB1 and HB2 appear in
the range 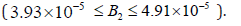
Summary and Conclusion
In this work, the effects of both feed choline and acetate
concentrations on a coupled AChE/ChAT enzymes system have
been investigated considering the choline reuptake into the
presynaptic neuron. It has been found that as the feed choline
concentrations increase, ACh levels in both compartments increase gradually until 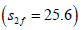 where ACh is synthesized less efficiently
where ACh is synthesized less efficiently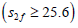 when. So that the release of ACh in compartment 2 varies
in parallel to the incorporation of the choline in compartment 1 to
produce ACh. The released transmitter can be compensated by the
ACh synthesized in compartment 1 (s11). Therefore, the rate of ACh
synthesis must be equal to the rate of transmitter release. This is
in agreement with the results obtained by Schwartz et al., (1975).
when. So that the release of ACh in compartment 2 varies
in parallel to the incorporation of the choline in compartment 1 to
produce ACh. The released transmitter can be compensated by the
ACh synthesized in compartment 1 (s11). Therefore, the rate of ACh
synthesis must be equal to the rate of transmitter release. This is
in agreement with the results obtained by Schwartz et al., (1975).
At low concentrations of the feed choline concentrations, it has
been found that the system exhibits complex dynamics bifurcation
including chaotic behavior via a period doubling sequence in the range 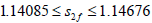 A bistability behavior is observed
where periodic and point attractors coexist with an unstable
periodic orbit as the separatrix separating the domains of attraction
of the periodic and point attractors. This bistability leads to the
condition, that at the same value of s2f slightly different initial
conditions lead to different types of attractors. Both periodic orbits
and steady stationery states are co-existing together as shown
in Figures 4 and 5. Then periodic orbits cease to exist, at a PLP
bifurcation, and the system recovers its stability. The biochemical
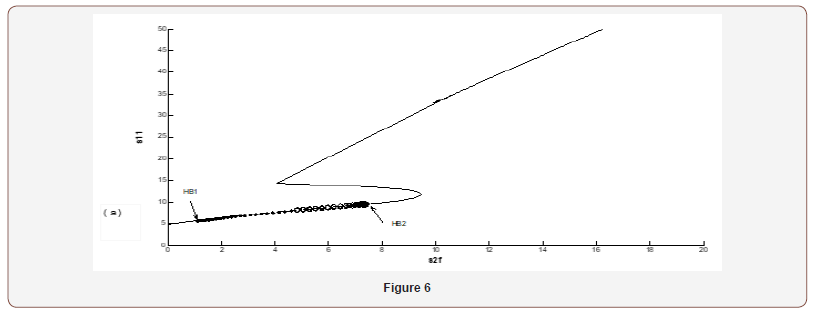
interpretation for the unstable waves in Figures 4 and 6 is that they
occur as a consequence of the competition between diffusion and
enzyme reaction(s). It can be concluded that the uptake of choline
into the nervous system at low external concentrations plays a
determining role in synthesis of the transmitter. In addition, ACh
was synthesized considerably less efficiently from the excess choline
which accumulated in nervous tissue at external concentrations
greater than about 30 *10-4 kmole/m3.
A bistability behavior is observed
where periodic and point attractors coexist with an unstable
periodic orbit as the separatrix separating the domains of attraction
of the periodic and point attractors. This bistability leads to the
condition, that at the same value of s2f slightly different initial
conditions lead to different types of attractors. Both periodic orbits
and steady stationery states are co-existing together as shown
in Figures 4 and 5. Then periodic orbits cease to exist, at a PLP
bifurcation, and the system recovers its stability. The biochemical
interpretation for the unstable waves in Figures 4 and 6 is that they
occur as a consequence of the competition between diffusion and
enzyme reaction(s). It can be concluded that the uptake of choline
into the nervous system at low external concentrations plays a
determining role in synthesis of the transmitter. In addition, ACh
was synthesized considerably less efficiently from the excess choline
which accumulated in nervous tissue at external concentrations
greater than about 30 *10-4 kmole/m3.
The results are analyzed based on the physiological values in order to simulate the ACh hydrolysis in the synaptic cleft in compartment 2. The system in case of external disturbances such as the sudden change of feed choline concentration to the presynaptic neurons could be affected by the hysteresis with a sudden increase in ACh concentration in both compartments (especially in compartment 2 where ACh concentration increases 6 folds from c to9.1×10−6 kmol/m3 near SB1 as shown in Figure 2(b) with a small variation in the input conditions thus simulating the sudden neural transmission, this means that the gradual synthesis of ACh is not satisfactory for rapid synthesis of ACh to compensate for the released amount and that a simple regulatory mechanism of linear feedback of the ACh level can accomplish the ACh replenishment for maintenance of the transmission activity. It would follow from dynamic analysis and the importance activity of enzymes that in the presynaptic terminal, a feedback mechanism of neurotransmitter levels operates as a basic control apparatus to regulate the synthesis and supply of transmitters in response to the release.
It has been found that the feed acetate concentrations have less effect on ACh concentrations in both compartments in comparison to the feed choline concentrations. The system is rich with the dynamics at low concentration of s3f where the feed acetate concentrations are too small to start the synthesis reaction catalyzed by ChAT. These results are in agreement with the experimental results of Kwok et al., (1982) who showed that increased acetyl- CoA delivery alone is not enough for optimal AsCh synthesis, and it is possible that choline delivery is limiting to transmitter synthesis. Finally, it can be concluded that feed choline is the most important substrate to ACh synthesis.
Many state variables correspond to the physiological expected values in some regions. This can direct future experimental research in order to use this novel eight-dimensional model for simulating real physiological behavior. Availability of good experimental data for human brain in future can help to greatly improve this model for deeper understanding of the physiological behavior and can help in planning better brain experiments and linking the complex behavior investigated in this paper to the cholinergic disorders and diseases such as AD and PD.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Awad A, Fgaier H, Mustafa I, Elkamel A, Elnashaie S, (2019) Pharmacokinetic/ Pharmacodynamic modeling and simulation of the effect of medications on β-amyloid aggregates and cholinergic neurocycle, Computers & Chemical Engineering 126: 231-240
- Bielarczyk H, Szutowicz A (1989) Evidence for the regulatory function of synaptoplasmic acetyl- CoA in ACh synthesis in nerve endings. Biochemical journal 262: 377-380.
- Birks RI, Worsley KJ, Birks RI (1985) Activation of ACh synthesis in cat sympathetic Ganglia: dependence on external choline and sodium- pump rate. journal of Physiology 367: 401-417
- Brown Jon T, Kate L, Weatherall, Laura R, Corria, et al. (2010) Vesicular release of glutamate utilizes the proton gradient between the vesicle and synaptic cleft. Frontiers in Synaptic Neuroscience 2: (15).
- Damsma G, Lammerts van Bueren D, Westerink I BHC, Horn A (1987) Determination of ACh and choline femtomole range by means of HPLC, a post-column enzyme reactor, and electrochemical detection. Chromatographia. 24: 827-831.
- Elnashaie SSEH, El-Rifai MA, Ibrahim G (1983) The effect of hydrogen ion production on the steady-state multiplicity of substrate inhibited enzymatic reactions. II. Transient behavior. Applied Biochemistry and Biotechnology 8(6): 467.
- Elnashaie SSE, Uhlig F, Affane Cb (2007) Numerical techniques for chemical and biological engineers using MATLAB: a simple bifurcation approach, Springer. New York.
- Feigenbaum ML (1980) Universal behavior in nonlinear systems. Los Alamos Science 1: 4-36.
- Fgaier H, Mustafa IHI, Awad AAR, Elkamel A, (2015) Modeling the interaction between β-amyloid aggregates and choline acetyltransferase activity and its relationship with cholinergic dysfunction through two-enzyme/two-compartment model. Computational and mathematical methods in medicine.
- Glenn Wetzel, Joan Heller Brown (1983) Relationship between Choline uptake ACh synthesis and ACh release in isolated rat atria. Journal of pharmacology and Experimental Therapeutics 226(2): 343-348.
- Hindmarsh JL, Rose RM, (1984) Model of neuronal bursting using three coupled first order differential equations, Proceedings of the Royal Society of London. Series B Biological Sciences 221(1222): 87-102.
- Holden AV, Fan YS, (1992a) From simple to simple bursting oscillatory behavior via chaos in the Rose- Hindmarsh model for neuronal activity. Chaos Solitons & Fractals 2(3): 221–236.
- Holden AV, Fan YS (1992b) From simple to complex oscillatory behavior via intermittent chaos in the Rose-Hindmarsh model for neuronal activity. Chaos Solitons & Fractals 2(4): 349–369.
- Holden AV, Fan YS (1992c) Crisis-induced chaos in the Rose-Hindmarsh model for neuronal activity. Chaos, Solitons & Fractals 2(6): 583–595.
- Ibrahim G, Saleh O, Mustafa IH, El Ahwany AH, Elnashaie SSEH (2010) Modeling Periodic and Aperiodic Behavior of Acetylcholine Hydrolysis. International Review of Chemical Engineering (IRECHE) 2(6): 647-666.
- Ismail H Ulus, Richard J Wurtman, Charlotte Mauron, Blusztajn JK (1989) Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Research 484(1-2): 217-227.
- Mustafaa IH, Ibrahim G, Elkamel A, Elnashaie SSEH, Chen P (2009) Nonlinear Feedback Modeling and Bifurcation of the Acetylcholine Neurocycle and its Relation to Alzheimer’s and Parkinson’s Diseases. Journal of chemical engineering science 64(1): 69-90.
-
Said Elnashaie , Ibrahim Mustafa, Asmaa Awad1, Elkamel A. Simulation of Bifurcation in Brain Illness/Remediation. Enzyme Reactions of the Neurocycle. Arch Biomed Eng & Biotechnol. 3(3): 2019. ABEB.MS.ID.000564.
-
Acetylcholinesterase, Acetylcholine, Choline, Acetate, Parkinson’s disease (PD), Alzheimer’s disease (AD), Dynamic behavior, Bifurcation, Chaos, Illnesses
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






