 Review Article
Review Article
Natural Products Derived from Plants and Seaweeds Against Clinical Importance Respiratory Viruses
Max Willian Lisboa Gomes1,2, Caroline de Souza Barros1,3, Priscilla Oliveira Esteves1,4, Rafaela dos S.P Gomes1,2, Claudio Cesar Cirne-Santos1,2, Valeria Laneuville Teixeira1,5, Izabel Christina Palmer Paixao1,2*
1Postgraduate Program in Science and Biotechnology, Institute of Biology, Fluminense Federal University, Postgraduate Program in Neurology, Brazil
2Laboratory of Molecular Virology and Biotechnology, Department of Cellular and Molecular Biology, Institute of Biology, Fluminense Federal University, Brazil
3Laboratory of Immunovirology, Department of Immunobiology, Institute of Biology, Fluminense Federal University, Brazil
4Marine Algae Natural Products Laboratory (ALGAMAR), Department of Marine Biology, Institute of Biology, Fluminense Federal University, Brazil
5Algae Biology and Taxonomy Laboratory (LABIOTAL), Postgraduate Program in Neotropical Biodiversity, Institute of Biosciences, Federal University of the State of Rio de Janeiro, Brazil
Postgraduate Program in Science and Biotechnology, Institute of Biology, Fluminense Federal University, Postgraduate Program in Neurology, 24020-141, Niteroi, RJ, Brazil.
Received Date: August 11, 2021; Published Date: November 29, 2021
Abstract
Acute respiratory tract infections are the most common diseases in all individuals, regardless of age or sex. Epidemiological research and community-based studies conducted since the early 20th century have determined the rates of disease and the pathogens involved in such infections. More recently, advances in diagnostic techniques have enabled a more complete identification of the viruses involved in respiratory infections, which has aided in the ability to target specific therapeutic agents to the causative pathogens. Natural products have been used since earliest times as a treatment for various diseases. In this review, we show that natural products derived from plants and seaweed present promising results for several respiratory viruses such as human metapneumovirus (HPMV), Influenza, Respiratory Syncytial Virus (RSV), Rhinovirus, Paramyxovirus, Hantavirus, Adenovirus, Feline, Murine and other Human Coronaviruses, in addition to new therapeutic perspectives against COVID-19.
Keywords: Natural products; Plant; Algae; Antiviral; Respiratory; COVID-19
Introduction
Many viral species are of clinical importance and affect the world causing diseases in a large number of people. Among these, many studies mention different viruses such as Adenovirus [1], Coronavirus [2], Hantavirus [3], Orthomyxovirus [4], Paramyxovirus [5], Measles Virus [6], Rhinovirus [7] and the group that most affects people worldwide, Influenza Virus [8,9]. Natural products are widely used as alternative treatments for many diseases, whether viral, bacterial, parasitic or fungal. Many pathogens are resistant to synthetic and specific drugs for different targets. The study with natural products substances is important to increase the spectrum of treatment and reduce the incidence of microbial resistance to treatment acquired by pathogens with a smaller range of drugs [10].
Respiratory viruses have been a major public health problem since earlier times. Viral infections that directly affect the respiratory tract today include infections with the highest transmissible capacity and, such as Influenza, one of the highest mortality and morbidity rates worldwide [11]. Its characteristic of easy transmissibility occurs because the virus is found in droplets and secretions expelled by the respiratory system, justifying in most cases, the number of cases configured in pandemics [12,13]. Respiratory infections represent one of the highest case incidence rates among diseases that affect humans. The mortality rate of respiratory infections in developed countries is much lower than that of developing countries, precisely because of hygiene, sanitation and culture measures [14,15].
Respiratory viruses have a distribution based on seasonality, as they have a specific predominance in certain seasons. We can see Influenza and RSV infections especially in winter. Picornaviruses cause infections throughout the year, while Enteroviruses are more common in summer, and Rhinoviruses in winter [16]. Diseases that are caused by respiratory viruses can be classified as common colds, pharyngitis, laryngitis, acute bronchitis, pneumonia, among other complications commonly observed [17]. The immunosuppression factor can also cause openness to infections in the respiratory tract involving Cytomegalovirus, Echovirus, Coxsackie virus, that can cause severe conditions involving acute pharyngitis [18]. Globalization and ease of movement among people in different countries were factors that enabled the violent transmission of this respiratory virus. Today, we need government measures from Health authorities, the work of science and health professionals to control global infection [16].
Contingency plans are necessary to control the growth curve of these respiratory viruses. Contagion from infected droplets is concomitant with the overcrowding of public services, environments associated with urban transport. One of the most important measures to control the cases and growth of individuals infected with SARS-CoV-2 is social isolation. At-risk groups need to stay away from agglomerations precisely so that they are not exposed to infected patients [19].
Influenza
The influenza A, influenza B and influenza C viruses were isolated in 1933, 1940 and 1951 respectively. Both influenza A and B belong to the family Orthomyxoviridae and to the genus Influenzavirus [20]. Influenza C is in a specific genus since it is the only species of the genus, affecting humans and pigs. Influenza viruses are enveloped by a lipid bilayer containing their single-stranded negative sense RNA genome. The capsid of these viruses is of helical symmetry, with Influenza A and B composed of eight RNA segments and Influenza C by seven RNA segments. As for their characteristics of clinical importance, we can only categorize Influenza A and B, as they are significantly pathogenic to human hosts. Influenza C, on the other hand, causes a very rare infection and is usually associated with mild pathogenesis in the respiratory tract [9].
Adenovirus
The first isolation of adenovirus occurred from primary adenoid cell culture of children in the early 1950s. The icosahedral symmetry capsid that surrounds the linear stranded DNA genome of this enveloped virus has 12 vertices. Its infection is prevalent worldwide and the rate of infected people always varies between sporadic cases and epidemic periods. Adenoviruses have great stability in the environment, so they are easily transmitted indoors. Its clinical manifestation involves infections and diseases in the upper and lower respiratory tract, being considered a public health problem [1].
Respiratory Syncytial Virus (RSV)
Respiratory syncytial virus (RSV) was isolated in 1956 where an experimental chimpanzee displayed a disease similar to a common cold, demonstrating a human pathogen. RSV infection can cause severe manifestations in children at the lower respiratory tract. The most common afflictions are bronchiolitis and pneumonia. Patients with comorbidities and immunodeficiency have high risk factors for infection. These viruses belong to the Paramyxoviridae family and to the Pneumovirus genus, as well as they are single-stranded negative sense RNA viruses, not segmented, encoding about 10 viral proteins. Regular RSV epidemics follow seasonality, being common in winter and in temperate climates [9].
Rhinoviruses
Rhinoviruses have more than 100 serotypes and can cause respiratory diseases in the upper respiratory tract. These viruses are more common in children and are generally associated with many colds and febrile illnesses. Following the line of the other respiratory viruses, there are conditions in which the infection gets worse and becomes more serious. Patients with previous lung problems may experience pneumonia due to Rhinovirus infection. Rhinoviruses belong to the Picornaviridae family and usually grows at lower rates compared to other respiratory viruses [7].
Human Metapneumoviruses (hPMV)
HPMV or Human Metapneumovirus are responsible for approximately 2-12% of respiratory diseases in the pediatric lower respiratory tract. The virus is a member of the Paramyxoviridae family and the only one in the Metapneumovirus genus. Since its isolation in 2001, the biggest challenge for medicine and virology research is to understand the pathogenic mechanism of these viruses, precisely to develop effective antivirals and vaccines [21].
Hantaviruses
Hantaviruses are known to cause two distinct syndromes with hemorrhagic fever and hantavirus pulmonary syndrome (HPS). Consequently, Hantavirus infection should be considered one of the causes of respiratory diseases in areas where Hantaviruses are endemic. Hantaviruses are RNA viruses belonging to the Bunyaviridae family. Its most common reservoirs are rodents, moles, bats. And in contrast, the more these rodent carriers experience population imbalances, the greater the chances of an outbreak due to Hantavirus [3].
Measles Viruses
Measles viruses have an RNA genome, and they belong to the Morbilivirus genus and the Paramyxoviridae family. The Measles virus most often infects human hosts and non-human primates. Clinically, the Measles virus after the incubation period of 8-12 days can be observed with fever, cough, runny nose and in some cases, conjunctivitis. It can also be observed in patients as skin rashes, usually as erythematous spots on the face and neck. Measles viruses are capable of causing serious illness in children; however, we have a vaccine today for the major circulating strain [6].
Coronaviruses
Over the past 12 years, two novel β-CoVs, the severe acute respiratory syndrome CoV (SARS- CoV) and the Middle East respiratory syndrome CoV (MERS-CoV) have emerged, and these viruses can cause severe human diseases. The lack of effective drug treatment and the associated high morbidity and mortality rate of these two CoVs, as well as their potential to cause epidemics, highlight the need for novel drug discovery for the treatment of CoV infection [22]. In view of the current pandemic scenario for the new Coronavirus (SARS-CoV-2), the objective of this review is to show the therapeutic strategies involving natural products from plants and algae for respiratory viruses of clinical importance.
Coronaviruses have a wide diversity between hosts, in addition to a great variance in their cell tropism. The vast majority of alphacoronaviruses and beta coronaviruses infect mammals. At the same time, gamma and delta coronaviruses infect birds and some mammals [23]. By 2019, 6 different types of Coronaviruses capable of infecting humans have been reported: HCoV-229E; HCoV-OC43; HCoV-NL63 and HKU1 capable of inducing mild respiratory syndrome in humans. The most vulnerable hosts were babies and the elderly. Both SARS-CoV and MERS-CoV showed a more aggressive profile regarding infection, causing severe acute respiratory syndrome in human patients, presenting a mortality rate much higher than other Coronaviruses [24].
In January 2020, China concluded in laboratory tests that the unknown pathogen was a Coronavirus, confirmed by a test where the sample showed 95% homology with bat coronavirus and 70% similarity with SARS-CoV. As with all respiratory viruses, exponential growth was observed in a short time, even in people who had not had contact with the live animal market, immediately suggesting that community transmission was taking place [25]. SARS-CoV-2, characterized during the outbreak in China, that until 16/03/2020 was responsible for infecting more than 80,000 people (China) and more than 153,000 worldwide causes the disease now called COVID-19 [26].
All ages are susceptible to SARS-CoV-2 infection. Droplets, generated during coughing where this infected material is exposed to another individual’s respiratory tract, are responsible for transmission. Asymptomatic people and those who have not yet manifested the symptoms are also able to transmit the virus. As much as all ages are susceptible to infection, there are individuals at a greater risk, such as elderly, patients living with HIV; Diabetics; Hypertensive and individuals who already had influenza infection [27] (Figure 1).
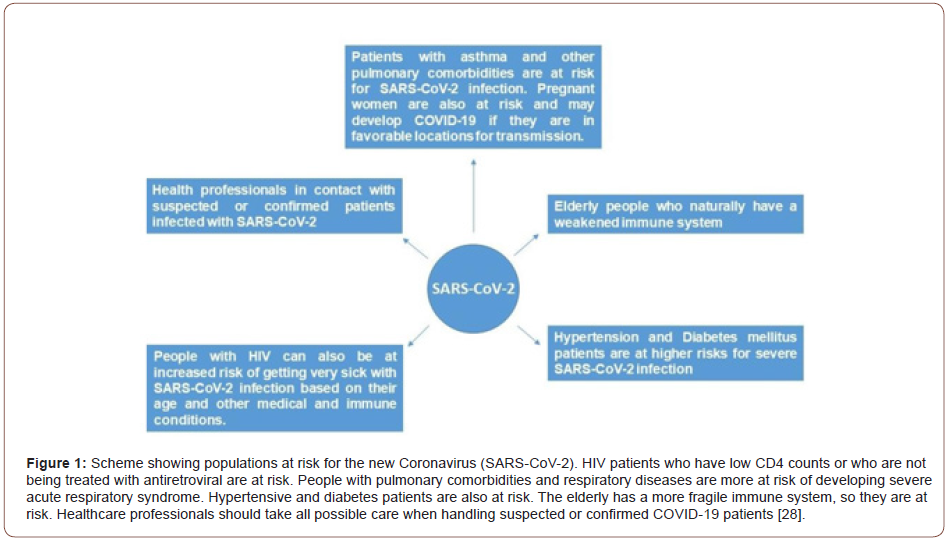
New perspectives involving treatment against respiratory viruses using natural products
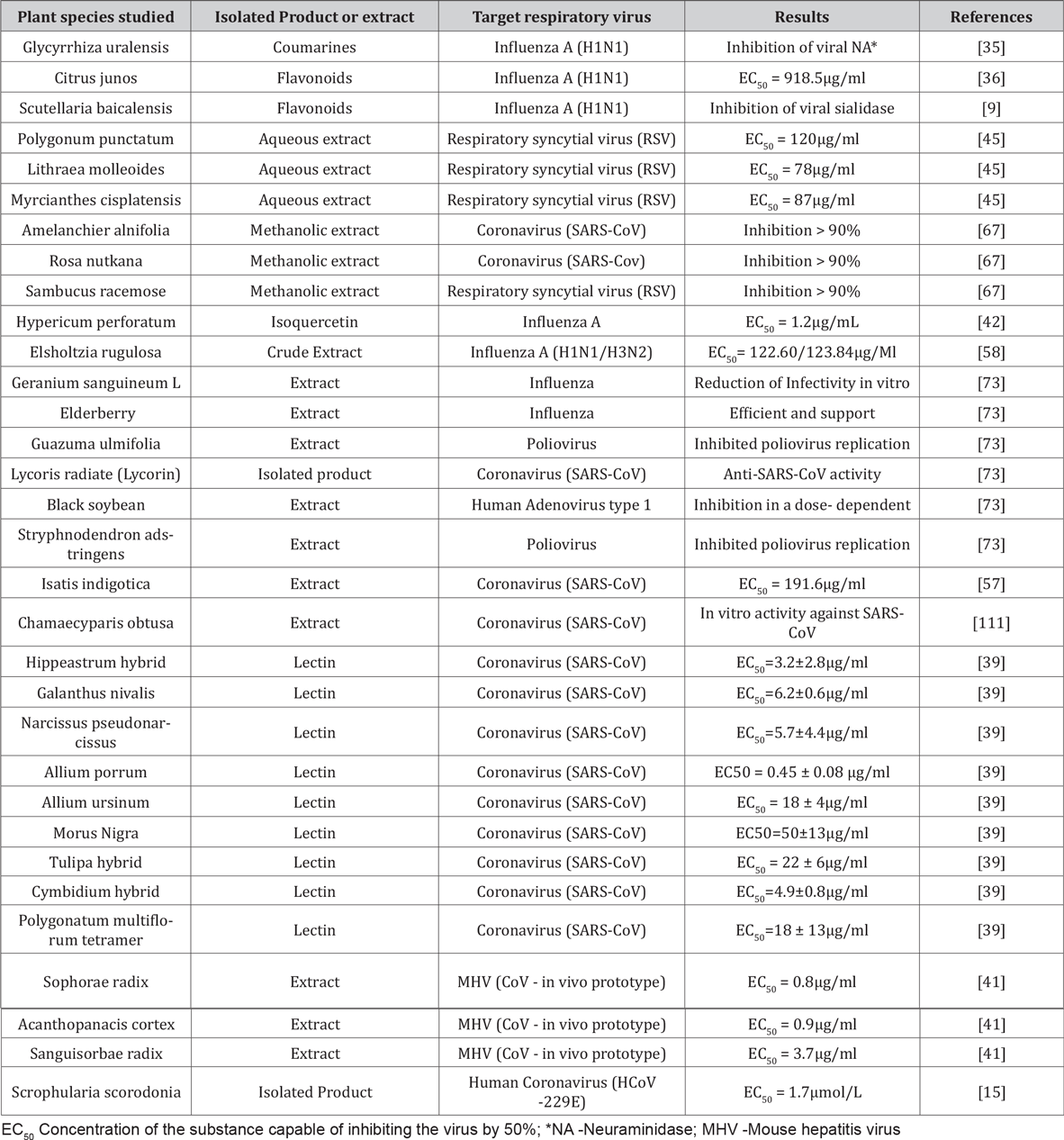
Human coronavirus infections have been a public health problem since the 2002 SARS epidemic in China, where it affected thousands of patients. Viral respiratory diseases are responsible for a large number of deaths worldwide. With the absence of effective vaccines and effective antiviral therapies, natural products have been an excellent alternative against these pathogens [29,30]. Plant studies show many activities against respiratory viruses. The products produced by the most varied species of plants demonstrate potent antimicrobial inhibitory activities [31]. The products from plants can interact by inhibiting the neuraminidase present in the Influenza group viruses, a crucial enzyme in the replication process of these viruses in their target cell. The literature shows a series of activities derived from the products of these plants, such as the biflavonoids isolated from Ginkgo biloba and Glycyrrhiza uralensis that exhibits inhibitory activity against H1N1, being a potential inhibitor of neuraminidases (73). A list of studies done with plants that showed inhibitory activities of respiratory viruses is shown in table 1. Many Brazilian plants have medicinal activities and are already used by the population as medicines for several comorbidities [32]. Brazilian plants show different antiviral activities against different groups of pathogens such as HSV-1 (herpes simplex virus type 1), HSV-2 (herpes simplex virus type 2) and Poliovirus. Plants like Trixis praestans and Cunila spicata showed results against Adenovirus [33,34] (Table 1).
Table 1: List of studied species, target respiratory virus, results obtained and bibliographic references.
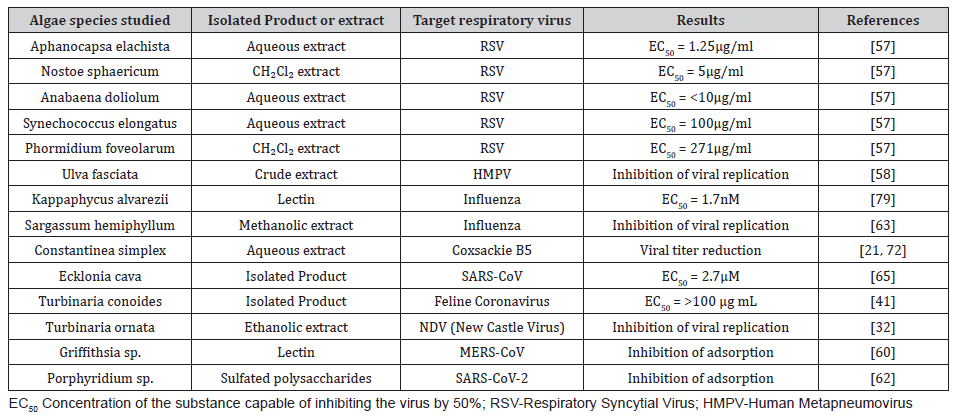
Research on natural products has already shown activity of plants inhibiting the replication of Coronaviruses, which are viruses of clinical importance for human health [37]. Native plants from Asia, already used by oriental medicine, tested for HSV-1 and HIV-1, were tested against SARS-CoV, and obtained promising results as protease inhibitors, interfering in the replicative cycle of these viruses [38,39]. The natural products isolated from marine organisms have many described activities. Algae can have antibacterial, antimalarial, antifungal, antiparasitic and antiviral activities. Many studies obtained by various groups against viruses in general (HSV-1, HIV-1, ZIKV and CHIKV) the antiviral activity of red, green and brown algae. Whether from their extracts or from isolated products, for example Caulerpin, which is an isolate from the green alga Caulerpa racemosa [40-45]. Researches on antiviral activity of algae products against respiratory viruses show activity of green algae such as Ulva fasciata (table 2) acting on the replication of Human Metapneumovirus (HMPV) [21]. Blue green Cyanophyta algae activity against Paramyxovirus is also observed [46]. The natural products from algae are also able to act against the replication of viruses such as Echovirus, Rhinovirus, Adenovirus and Vaccinia virus [47,48]. The extracts of the algae Constantinea simplex and Farlowia mollis demonstrate potent activities against the Vaccinia virus, that causes serious infections in the respiratory tract of both humans and animal hosts [49,50] (Table 2).
Table 2:List of studied species, target respiratory virus, results obtained and bibliographic references.
Marine natural products also show inhibitory activity against human Coronaviruses. Tests with extracts of red algae such as those of the Rhodophyceae genus showed anti-SARS-CoV and anti- Influenza activities [51]. Betting on the wide spectrum of inhibitory activities of marine products, in silico studies are also carried out to look for promising combinations of potential SARS-CoV inhibitors [52]. The sulfated polysaccharides of the Porphyridium sp. are shown to inhibit Coronavirus replication and the study by Mahadev et al; showed that the products from this algae have great biocompatibility with products used in hygiene and prophylaxis against Coronaviruses, including SARS-CoV-2, which causes COVID-19 [53].
Conclusion
Respiratory viruses are responsible for thousands of deaths of people every year and their pathogens infect children, adults and the elderly, making it a major public health problem. Although we have a flu vaccine, we still need more research and development of prophylactic alternatives and specific antiviral treatments for many diseases that end up creating chaotic situations around the world. Many studies are needed to develop and approve a reference drug in treatment for these diseases. In vitro and in vivo studies need to be carried out to ensure the safety and proven efficacy of the drug so that it is directed to humans in the clinical phase. The same process is categorized in vaccines, and this takes time and study.
Natural products from plants and algae are a very effective alternative in studies aimed at the development of new antiviral drugs. Studies involving the mechanism of action of these drugs against respiratory viruses and the question of the bioavailability of these products for large-scale production are very important issues for the future of antivirals derived from natural products.
Compliance with Ethical Standards
This article does not contain any studies with human participants performed by any of the authors.
Conflicts of Interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this article.
Acknowledgment
The graphical abstract was created with BioRender®.
References
- Scott MK, Christina Chommanard, Xiaoyan Lu, Dianna Appelgate, LaDonna Grenz, et al. (2016) Human adenovirus associated with severe respiratory infection, Oregon, USA, 2013–2014. Emerging infectious diseases 22(6): 1044-1051.
- Hand J, Erica Billig Rose, Andrea Salinas, Xiaoyan Lu, Senthilkumar K Sakthivel, et al. (2018) Severe Respiratory Illness Outbreak Associated with Human Coronavirus NL63 in a Long-Term Care Facility. Emerging infectious diseases 24(10): 1964-1966.
- Van Hook CJ (2018) Hantavirus Pulmonary Syndrome-The 25th Anniversary of the Four Corners Outbreak. Emerging infectious diseases 24(11): 2056.
- Peiris J (2017) Orthomyxovirus, coronavirus and other respiratory viruses. in TAPPIN Workshop, Shanghai.
- Liu WK, De-Hui Chen, Wei-Ping Tan, Shu-Yan Qiu, Duo Xu, et al. (2019) Paramyxoviruses respiratory syncytial virus, parainfluenza virus, and human metapneumovirus infection in pediatric hospitalized patients and climate correlation in a subtropical region of southern China: a 7-year survey. European Journal of Clinical Microbiology & Infectious Diseases 38(12): 2355-2364.
- Leung A, KL Hon, KF Leong, C M Sergi (2018) Measles: a disease often forgotten but not gone. Hong Kong Med J 24(5): 512-520.
- Anzueto A, MS Niederman (2003) Diagnosis and treatment of rhinovirus respiratory infections. Chest 123(5): 1664-1672.
- Mhimbira F, H Hiza, E Mbuba, J Hella, L Kamwela , et al. (2019) Prevalence and clinical significance of respiratory viruses and bacteria detected in tuberculosis patients compared to household contact controls in Tanzania: a cohort study. Clinical microbiology and infection 25(1): 107. e1-107.e7.
- Bruning AH, Mariska MG Leeflang, Johanna MBW Vos, Rene Spijker, Menno D de Jong, et al. (2017) Rapid tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review and meta-analysis. Clinical Infectious Diseases 65(6): 1026-1032.
- Kernan MR, A Sendl, JL Chen, SD Jolad, P Blanc, et al. (1997) Two new lignans with activity against influenza virus from the medicinal plant Rhinacanthus nasutus. Journal of natural products 60(6): 635-637.
- Silva DR, Infeccoes Virais Do Trato Respiratorio.
- Martin-Loeches I, Ana Sanchez-Corral, Emili Diaz, Rosa Maria Granada, Rafael Zaragoza, et al. (2011) Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A (H1N1) virus. Chest, 139(3): 555-562.
- Bauch CT, T Oraby (2013) Assessing the pandemic potential of MERS-CoV. The Lancet 382(9893): 662-664.
- Garibaldi RA (1985) Epidemiology of community-acquired respiratory tract infections in adults: incidence, etiology, and impact. The American journal of medicine 78(6B): 32-37.
- Shi T, David A McAllister, Katherine L O Brien, Eric A F Simoes, Shabir A Madhi, et al. (2017) Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. The Lancet 390(10098): 946-958.
- Yoo JH (2019) The fight against the 2019-nCoV outbreak: an arduous march has just begun. Journal of Korean Medical Science 35(4): e56.
- Ritchie AI, P Mallia, SL Johnston (2017) Viral syndrome infections in humans. of the The respiratory disease syndromes tract constitute caused by the respiratory commonest viruses infectious can vary disease from mild, predominantly upper respiratory syndromes, such as the common cold, to more severe lower respiratory disease (pneumonia, bronchiolitis), and are influenced by both the virus type and host factors, such as age. Although the severity of clinical disease varies, the vast majority of viral respiratory tract infections in healthy individuals result in mild symptoms lasting a few days and are self-limiting. However, when respiratory viruses infect individuals with chronic lung diseases, the consequences of infection are more severe. Respiratory infections in patients. Acute Exacerbations of Pulmonary Diseases 77: 76.
- Chow AW, S Doron (2018) Evaluation of acute pharyngitis in adults. UpToDate.
- Freitas CMd, et al. (2020) Fiocruz's contingency plan for the disease pandemic caused by SARS-CoV-2 (COVID-19).
- Thomas JK, J Noppenberger (2007) Avian influenza: A review. American Journal of Health-System Pharmacy 64(2): 149-165.
- Mendes GdS, Angelica Ribeiro Soares, Fernanda Otaviano Martins, Maria Carolina Maciel de Albuquerque, Sonia Soares Costa, et al. (2010) Antiviral activity of the green marine alga Ulva fasciata on the replication of human metapneumovirus. Journal of the Institute of Tropical Medicine of Sao Paulo 52(1): 03-10.
- Assiri A, Allison McGeer, Trish M Perl, Connie S Price, Abdullah A Al Rabeeah, et al. (2013) Hospital outbreak of Middle East respiratory syndrome coronavirus. 369(5): 407-416.
- Chan JFW, Kin-Hang Kok, Zheng Zhu, Hin Chu, Kelvin Kai-Wang To, et al. (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections 9(1): 221-236.
- Van der Hoek L, Krzysztof Pyrc, Maarten F Jebbink, Wilma Vermeulen-Oost, Ron J M Berkhout, et al. (2004) Identification of a new human coronavirus. Nature medicine10(4): 368-373.
- Liu Y, Albert A Gayle, Annelies Wilder-Smith, Joacim Rocklov (2020) The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of travel medicine 27(2).
- Ruan Q, Kun Yang, Wenxia Wang, Lingyu Jiang, Jianxin Song (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 46(5):846-848.
- Rothan HA, SN Byrareddy (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of Autoimmunity 109: 102433.
- Li B, Jing Yang, Faming Zhao, Lili Zhi, Xiqian Wang, et al. (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clinical Research in Cardiology 109(5):531-538.
- Chen JL, P Blanc, CA Stoddart, M Bogan, EJ Rozhon, et al. (1998) New iridoids from the medicinal plant Barleria prionitis with potent activity against respiratory syncytial virus. Journal of natural products 61(10): 1295-1297.
- Tsuchiya Y, M Shimizu, Y Hiyama, K Itoh, Y Hashimoto, et al. (1985) Antiviral activity of natural occurring flavonoids in vitro. Chemical and pharmaceutical bulletin 33(9): 3881-3886.
- Wang X, Wei Jia, Aihua Zhao, Xiaorong Wang (2006) Anti‐influenza agents from plants and traditional Chinese medicine. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 20(5): 335-341.
- Andrighetti-Frohner C, TCM Sincero, AC da Silva, LA Savi, CM Gaido, et al. (2005) Antiviral evaluation of plants from Brazilian atlantic tropical forest. Fitoterapia 76(3-4): 374-378.
- Simoes C, M Falkenberg, LA Mentz, EP Schenkel, M Amoros, et al. (1999) Antiviral activity of south Brazilian medicinal plant extracts. Phytomedicine 6(3): 205-214.
- Lemos ICS, Gyllyandeson de Araujo Delmondes, Ana Deyva Ferreira dos Santos, Enaide Soares Santos, Dayanne Rakelly de Oliveira, et al. (2016) Ethnobiological survey of plants and animals used for the treatment of acute respiratory infections in children of a traditional community in the municipality of barbalha, ceará, Brazil. African Journal of Traditional, Complementary and Alternative Medicines 13(4): 166-175.
- Mishra S, A Pandey, S Manvati (2020) Coumarin: An emerging antiviral agent. Heliyon 6(1): e03217.
- Bylka W, I Matlawska, N Pilewski (2004) Natural flavonoids as antimicrobial agents. Jana 7(2): 24-31.
- Yuan H, Qianqian Ma, Li Ye, Guangchun Piao (2016) The traditional medicine and modern medicine from natural products. Molecules 21(5): 559.
- Chiow K, MC Phoon, Thomas Putti, Benny KH Tan, Vincent T Chow (2016) Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pacific journal of tropical medicine 9(1): 1-7.
- Yang JL, TKQ Ha, WK Oh (2016) Discovery of inhibitory materials against PEDV corona virus from medicinal plants. Japanese Journal of Veterinary Research, 64(Supplement 1): S53-S63.
- Cirne-Santos CC, Valeria L Teixeira, Luiz Roberto Castello-Branco, Izabel CPP Frugulhetti, Dumith Chequer Bou-Habib (2006) Inhibition of HIV-1 replication in human primary cells by a dolabellane diterpene isolated from the marine algae Dictyota pfaffii. Planta medica 72(04): 295- 299.
- Cirne-Santos CC (2018) Antiviral effect of the seaweed Osmundaria obtusiloba against the Zika virus. Journal of Medicinal Plants Research 12: 387-395.
- Cirne-Santos CC, Caroline de Souza Barros, Caio Cesar Richter Nogueira, Renata Campos Azevedo, Kristie Aimi Yamamoto, et al. (2019) Inhibition by marine algae of chikungunya virus isolated from patients in a recent disease outbreak in Rio de Janeiro. Frontiers in microbiology 10: 2426.
- De Souza Barros C (2017) Therapeutic efficacy in BALB/C mice of extract from marine alga Canistrocarpus cervicornis (Phaeophyceae) against herpes simplex virus type 1. Journal of applied phycology 29(2): 769-773.
- Garrido V, Caroline Barros, Vanessa A Melchiades, Rainomar Raimundo Fonseca, Sergio Pinheiro, et al. (2017) Subchronic toxicity and anti-HSV-1 activity in experimental animal of dolabelladienetriol from the seaweed, Dictyota pfaffii. Regulatory Toxicology and Pharmacology 86: 193-198.
- Esteves PO (2019) Antiviral Effect of Caulerpin Against Chikungunya. Natural Product Communications 14(10).
- Patterson GM (1993) Antiviral Activity of Cultured Blue‐Green Algae (Cyanophyta). Journal of phycology 29(1): 125-130.
- Abdo SM, et al. (2012) Antiviral activity of freshwater algae. Journal of Applied Pharmaceutical Science2(2): 21.
- Rey P et al. (2018) Evaluation of the antiviral activity of the brown alga Sargassum fluitans against Echovirus 9. Cuban Journal of Tropical Medicine 70(2): 1-10.
- Richards JT, ER Kern, LA Glasgow, JC Overall, EF Deign, et al. (1978) Antiviral activity of extracts from marine algae. Antimicrobial agents and chemotherapy 14(1): 24-30.
- Ehresmann D, Soheil Zorofchian Moghadamtousi, Sazaly Abubakar, Keivan Zandi (1977) Antiviral substances from California marine algae 1. Journal of phycology 13(1): 37-40.
- Singh RS, AK Walia (2018) Lectins from red algae and their biomedical potential. Journal of Applied Phycology 30(3): 1833-1858.
- Li Sy, Cong Chen, Hai-Qing Zhang, Hai-Yan Guo, Hui Wang, et al. (2005) Identification of natural compounds with antiviral activities against SARS- associated coronavirus. Antiviral research 67(1): 18-23.
- Nagle V, et al. (2020) Marine red alga Porphyridium sp. as a source of sulfated polysaccharides (SPs) for combating against COVID-19.
-
Claudio Cesar Cirne-Santos, Valeria Laneuville Teixeira, Izabel Christina Palmer Paixao etc al.. Natural Products Derived from Plants and Seaweeds Against Clinical Importance Respiratory Viruses. Arch Biomed Eng & Biotechnol. 6(2): 2021. ABEB.MS.ID.000635. DOI: 10.33552/ABEB.2021.06.000635.
-
Natural products, Plant, Algae, Antiviral, Respiratory, COVID-19, Adenoviru, Coronavirus, Hantavirus, Orthomyxovirus, Paramyxovirus, Measles Virus, Rhinovirus, Microbial resistance, Hygiene, sanitation
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






