 Research Article
Research Article
Biostatistical Analysis on Hookworm Infection in Humans
Bin Zhao1*, Xia Jiang2, Jinming Cao3 and Kuiyun Huang1
1School of Science, Hubei University of Technology, China
2Hospital, Hubei University of Technology, China
3School of Information and Mathematics, Yangtze University, China
Zhao, School of Science, Hubei University of Technology, Wuhan, Hubei, China.
Received Date: April 23, 2019; Published Date: May 06, 2019
Abstract
Hookworm infections are one of the classified NTDs that burden many areas of the world. In order to ensure that the most effective measures are being taken to address the hookworm epidemic in SSA, the control programs must be reevaluated. In particular countries where programs are yielding the best results (in terms of lowering the incidence of infections and reinfections) should be evaluated to determine the components of their program that stand out and can be beneficial in other areas where programs have shown some lapses. The goal of this program evaluation is to recommend amendments to programs in countries with the greatest burden of disease, such as Kenya, in order to facilitate a reduction and possible elimination of new cases and reinfections of hookworm in the most vulnerable populations. These amendments may prove to be useful in other countries where programs are lacking essential components for successful outcomes and also in reducing the burden in the general population. In order to accomplish this goal, the extent of the burden of hookworm infection in SSA, this burden’s impact on the health of the most vulnerable populations, and recommended strategies to control and prevent future hookworm infections must first be determined. From there, country specific control programs in Kenya will be evaluated by comparing it to recommendations made by WHO and to a successful program in Bangladesh. The integrated results also provide some information into the management to improve the quality of these programs.
Keywords: Fagopyrum tataricum gaertn; Nitrogen; Starch properties
Introduction
Neglected Tropical Diseases (NTDs) are a group of diseases that affect many people, especially those of lower socioeconomic status [1]. These diseases do not account for high mortality rates but their morbidity rates far outweigh any other disease in the world [2]. Strategies to address many of these conditions are therefore mainly based around morbidity control through treatment rather than prevention. Hookworm infections are one of the classified NTDs that burden many areas of the world. It also holds the record as the leading cause of chronic anemia [3], with the highest incidence seen in Sub-Saharan Africa (SSA) [1]. This infection is complicated by coinfections with other diseases that plague this region of the world. The co-infection of hookworm and malaria has led to high morbidity rates especially among the most vulnerable populations: women of child bearing ages and school-aged children [4]. These infections contribute to the substantially high rate of anemia in this cluster of individuals due to their higher need for hemoglobin production [5] combined with increased hemoglobin loss (seen in hookworm infection) or destruction of red blood cells (seen in malaria). Furthermore, these infections lead to many other health disparities in children born to infected mothers such as low birth weights and preterm deliveries [6]. The additive effects of these factors may be the reason for these countries decreased livelihood and productivity [2] and, therefore, reevaluation of treatment programs should be considered to ensure that these burdens are being appropriately managed.
Programs that are currently in progress allocate many of its resources into treatment of Hookworm infection through Mass Drug Administration (MDA) with anthelminthic medications that are donated by two major drug companies: GlaxoSmithKline and Johnson & Johnson [7]. Although MDA may seem like a good idea to help alleviate the morbidity of these diseases some studies have shown that the treatment with anthelminthic drugs do not significantly increase hemoglobin levels or decrease the prevalence of anemia during pregnancy [8]. Other prevention measures are focused on educating the people of SSA on proper sanitation to eliminate person to person transmission and reinfections [9]. Although the appropriate measures are theoretically being implemented there is still a major burden of severe anemia among pregnant women due to many cases of reinfections and new infections [10]. These problems place an economical strain on the resources and thus make the efforts towards hookworm eradication near impossible. Additionally, recent studies have shown possible uprising of drug resistance in hookworm infections which could indicate a greater problem that will need to be addressed in the near future [3].
In order to ensure that the most effective measures are being taken to address the hookworm epidemic in SSA, the control programs must be reevaluated. In particular, countries where programs are yielding the best results (in terms of lowering the incidence of infections and reinfections) should be evaluated to determine the components of their program that stand out and can be beneficial in other areas where programs have shown some lapses. The goal of this program evaluation is to recommend amendments to programs in countries with the greatest burden of disease, such as Kenya, in order to facilitate a reduction and possible elimination of new cases and reinfections of hookworm in the most vulnerable populations. These amendments may prove to be useful in other countries where programs are lacking essential components for successful outcomes and also in reducing the burden in the general population. In order to accomplish this goal, the extent of the burden of hookworm infection in SSA, this burden’s impact on the health of the most vulnerable populations, and recommended strategies to control and prevent future hookworm infections must first be determined. From there, country specific control programs in Kenya will be evaluated by comparing it to recommendations made by WHO and to a successful program in Bangladesh. Then recommendations will be made to improve the quality of these programs.
Materials and Methods
Research articles were taken from Google, Google Scholar, PubMed and Medline Plus using key words (Hookworm infection, anemia, pregnancy outcomes, Sub Saharan Africa, soil transmitted helminthes, Neglected tropical diseases, Western African Centre for International Parasite Control, Eastern and Southern African Centre of International Parasite Control (ESACIPAC), Global Parasite Control Initiative (GPCI), Centre for neglected tropical diseases, sanitation in Sub-Saharan Africa, the WASH program, Bangladesh STH control program, and Save the children Bangladesh). Research articles included were published between 2004 and 2014 and available in full text versions. Current Policies on programs in effect in specific countries (i.e., Kenya and Bangladesh) were taken from the World Health Organization, USAIDS, government sources or specific funding organizations (i.e., the End Fund and Save the Children USA).
The WHOs recommended control program guidelines from 2006 [11] and updated recommendations from 2012 [12] were used as the gold standard to compare the compliance of programs in Kenya and Bangladesh and to make recommendations for improvement.
Results
The extent of the burden of hookworm infection in SSA and the impact of hookworm infection on the most vulnerable populations Hookworm infection in SSA affects more than 198 million people, more than any other NTD [1]. The leading cause of iron-deficiency anemia in pregnant women of SSA can be attributed to hookworm infections [1]. The burden of anemia during pregnancy has been proven to be associated with many poor outcomes such as low birth weights, pre mature labor and a higher maternal mortality rate [8]. The severity of the anemia can be further exacerbated by the co-infection of Malaria with Hookworm infection. Oddly enough, some studies have shown that infection with Hookworms can increase the individual’s risk of malaria infection and this is seen in the geographical overlap of both diseases [4]. The burden of hookworm infection in SSA is not equally distributed. Higher prevalence is seen along the coastal regions and areas of extremely high temperatures, with the greatest number of cases in Nigeria and Congo [1]. School-aged children in SSA are the demographic that are disproportionately affected by STHs with nearly half being affected in 2009. These infections lead to many disadvantages for this population such as mental and physical deficits and greatly affect school attendance and performance [1].
Recommended strategies to control and prevent future hookworm infections
World Health Organization 2006: The WHO developed a manual for health professionals and program managers in 2006 called “Preventive Chemotherapy in Human Helminthiasis: Coordinated use of anthelminthic drugs in control intervention.” This manual was issued to help countries achieve the 2010 goal set by WHO of regular administration of preventative chemotherapy to at least 75% and up to 100% of school-aged children at risk of soil transmitted helminthes (Hookworm, Whipworm (Trichuris), and Roundworm (Ascariasis) and schistosomiasis [11].
The manual outlines two steps for control programs: “Rapid assessment” of the prevalence of these infections and “Decision charts” for recommended treatment options. Step 1 (Rapid Assessment) focused on conducting an epidemiological survey to map the distribution of STHs. This was suggested to be carried out by dividing up areas where STHs were expected to be and collecting stool samples from 50 children in at least 5-10 schools in each area. Step 2 (Decision charts) involved taking the data from step 1 and developing a plan for MDA for each area depending on estimated prevalence of the infections. “Low risk communities” were determined to have a prevalence less than 50% but greater than 20% of the school-age children being infected and “High risk communities” were determined to have a prevalence more than 50%. Low risk communities were recommended to receive MDA one time a year and two times a year for high risk communities. Treatment (with either albendazole or mebendazole) was directed to all pre-school children, school-age children, women of child bearing ages, pregnant women in their second and third trimester, and adults in high risk occupations (i.e., tea pickers and miners). According to WHO, the aim of the program was “morbidity control” with the idea that “periodic treatment of atrisk populations [would] reduce the intensity of infected individuals from morbidity due to soil-transmitted helminthes” [11].
World Health Organization 2012: An analysis of data collected from SSA control programs in 2009 (only about 31.1% of the 883 million people targeted received treatment) suggested that the 2010 goal of 75% treatment coverage was not going to be met [12]. In the WHO new 2012 manual, “Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases,” it was hypothesized that the reason for these short comings was either due to inaccurate reporting of actual drug administration or an insufficient quantity of drugs available. The issue regarding the lack of drug supply was addressed in 2012 by the pharmaceutical industry and other organizations (i.e., USAID, UK department for International Development, etc.) with the initiation of drug donations and this subsequently allowed some countries to reach the 75% treatment coverage goal after 2010 [12]. A new goal to be achieved by 2020 was created by the WHO in 2012:
• 75% of preschool aged and school aged children in need of treatment are to be treated.
• 75% coverage with preventive chemotherapy will be achieved in preschool and school aged children in 100% of countries.
In order to achieve these goals for STH control, large scale MDA was the main intervention recommended by WHO. According to the report, “preventive chemotherapy interventions, if sustained with high coverage for 5-8 years, will decrease morbidity and halt its recurrence from five targeted NTDs;” STH being one of the five NTDs. The WHO also mentioned the clause that if sanitation conditions in SSA did not improve then NTD would not be eradicated [12].
According to the WHO, the most successful NTD control programs were those where there was national awareness of the burden of these diseases, incorporation of a control program into the national healthcare plan, adequate financial support, and collaborations between various stakeholders (i.e., ministries, communities, non-governmental organizations, and international partners, etc.) [12]. This may be the reason why additional interventions were suggested in 2012 that involved collaborations between many sectors of public health in order to achieve these goals. These additional recommendations included vector and intermediate host control, veterinary public health involvement, implementation of health awareness and education, and capacity building [12]. Although most, if not all, of the recommended control program interventions were developed to address morbidity control, the WHO also mentioned that “significant” prevention and control of NTDs could be achieved with the development and access of a vaccination [12].
Discussion
Comparing the WHO recommendations to current programs in Kenya
Mass drug administration: According to the World Health Organization’s 2012 report on how to control NTDs the main intervention recommended was mass drug administration (World Health Organization, 2012a) [12]. This intervention also seems to be the main intervention being carried out in Kenya with a relatively small focus on education. The WHO stated that 16.7 million Kenyan children from ages 1-15 years old were at risk of STH in 2012 [13]. Of the over 16 million at risk about 28% received STH treatment [14]. Through the addition of more funding and donations that sprouted after realization that the 2010 WHO goals were not met, the treatment coverage of at-risk individuals for STHs greatly increased [15].
Community capacity: According to the WHO, the most successful NTD control programs are those where there is national awareness of the burden of the diseases, incorporation of a control program into the national healthcare plan, adequate financial support, and collaborations between various stakeholders (i.e., ministries, communities, non-governmental organizations, and international partners, etc.) [12]. These components were addressed in Africa at the Sixth Conference of African Union Ministries of Health in April 2012 when many African leaders recognized the need to support strong NTD control and elimination programs [15].
Comparing WHO guidelines to a successful STH program in Bangladesh
Mass drug administration: MDA with albendazole was one of the key components in the Bangladesh program that was implemented towards STH and other intestinal worm control. In addition to MDA with albendazole, iron and vitamin A mass administration was also carried out in the school systems. Organization and planning of these events was conducted at the National level with the cooperation between the Ministry of Health and the Ministry of Education as well as by an outside planning organization (Save the Children Organization which is also the implementing organization) for review. A “Detail Implementation Plan” was required by each program manager to track the programs progress and to ensure that the program was following a timeline. Monthly orientation for school teachers on how to efficiently administer the mediations were implemented to “ensure maximum participation from teachers and to mobilize the community and parents to maximize school attendance on National Deworming Days.” [16]. Although many avenues were used disseminate information on these National Deworming Days, school attendance may have still received suboptimal turnouts due to many factors such as child illnesses, environmental factors (i.e., flooding), or the need of the children to work in their family’s field [17].
Community capacity building: Community capacity building contributed to a major component of the program. Starting at the school level, student brigades (now called “little Doctors”), consisting of students from grades 4 and 5, were formed each school year. Each of these brigades were designated with specific tasks in the community and in the school that were developed by each program manager before the school year started. Some of these tasks included assisting in hygiene and sanitation maintenance in the school and community, educating the community and school on proper sanitation and hygienic practices, assisting with the national deworming day, and conducting community surveys and awareness [16].
At the community level, in addition to community education and surveying tasks performed by the student brigades, each community held a “community courtyard session” and a “mother’s gathering” multiple times a year to address the concerns in the community and to further educate the community on the necessity of sanitation, hygiene, and their participation in the National Deworming Days at school. National deworming Days that took place at the schools were also broadcasted with the assistance of mosques, newspapers and other publication outlets to maximize school attendance on these days in order to successfully treat the most children possible [16].
Comparing the Kenya and Bangladesh programs
Mass drug administration: Both the Kenya and Bangladesh programs administered an anthelminthic drug (either albendazole or mebendazole) once or twice a year, depending on the severity of the infections and prevalence of worms. Both programs also offered training to individuals who are administering the medications (mainly school teachers). Both programs also received the medications as donations from either the pharmaceutical companies directly or indirectly through funds donated by other organizations (i.e., USAIDS, etc.).
Community capacity building: The idea of a communitybased intervention seems to be common in both the Kenya and the Bangladesh Programs. This is especially true when it comes to urging parents to allow their children to participate in the national deworming programs by sending their child to school. Also, community education on sanitation and hygiene is another commonality that was seen in both programs. On the contrary, efforts aimed towards community involvement and building community capacity seemed greater in Bangladesh where more activities were aimed at getting the community involved (i.e., student brigades, mother gatherings, and community courtyard sessions).
Other recommended program components by WHO 2012
According to the WHO 2012 guidelines, other components of the treatment programs should have been considered such as vector and intermediate host control, and veterinary public health involvement. Vector control programs and veterinary public health involvement would not be of any significant use in controlling STH which are mainly due to ingestion of food and water that are contaminated with egg infested feces or through direct inoculation of hookworms in infested soils. Intermediate host control can be achieved by increasing sanitation and hygiene measures to avoid contact with infected fecal matter through proper hand washing, avoiding open defecation, increasing the use of shoes outdoors, and maintaining cleanliness of latrines [14]. The implementation of these interventions were seen in both of these control programs and are a major part of the initiatives recommended by the WASH program for STH control. WASH and Neglected Tropical Diseases [14] Vitamin A and Iron mass distributions in Bangladesh proved to be a successful component of their program. A similar intervention was seen in Kenya through marketing and sale of a vitamin and mineral supplementation named Sprinkles Micronutrient powder which was to be used with daily food intake. This program was also successful in reducing iron deficiency anemia and vitamin A deficiency and increasing the recovery rate of anemia in children in Kenya [18]. This intervention, although proven to be successful and sustainable in research studies, was not sustainable in practice since marketing efforts decreased and anemia rates went back up [19].
Effectiveness comparison
The effectiveness of national STH control programs for both Bangladesh and Kenya can be standardized and compared by viewing anemia rates in pregnant women and by comparing the percent of the population with improved sanitation facilities from data obtained by the World Bank (Figure 1).
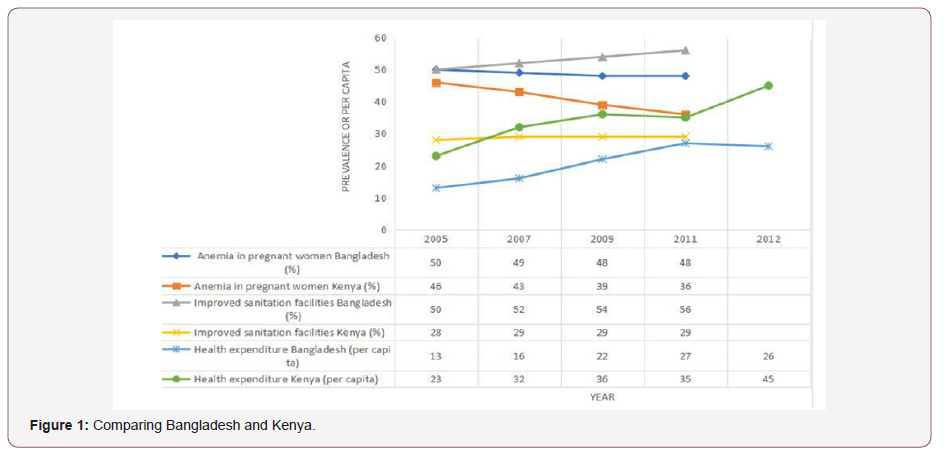
Based on the data we see that there is a greater decrease in anemia rates among pregnant women in Kenya compared to a gradual decrease in anemia rates seen in Bangladesh. Overall the Kenya rates are lower but this may be due to higher rates of prenatal care seen in Kenya. Data taken from the World Bank states that in 2009 92% of women in Kenya received prenatal care as opposed to 53% and 55% in 2010 and 2011, respectively, in Bangladesh (data for 2009 in Bangladesh was not available) [20]. Overall, it is apparent that Bangladesh spends less per capita on healthcare, has higher anemia rates among pregnant women with a significantly lower percentage of women receiving prenatal care, and has a greater percent of its population with access to improved sanitation facilities.
Funding comparison
According to WHO the estimated cost for MDA interventions in schools average US$ 0.06 (including cost of the drug and distribution). Most drugs are donated by GSK or J&J [12]. According to the WHO 2012 report, GSK donates up to 400 million doses or albendazole per year and J&J donates 200 million tablets of mebendazole per year for STH. The Bangladesh program received funding and donations primarily from GSK and private donations from Save the Children USA which assisted with financial costs of the program. The average annual cost of deworming, vitamin A, and Iron supplementation per child was 30 Taka (~ US$ 0.39). This covered Vitamin A supplementation and deworming twice per year and sixteen weeks of iron supplements (two times per week) per child [16].
Sustainability
Both programs have national support for program planning and implementation, have adequate funding provided by multiple donors and both encourage community involvement and education. The biggest issue for STH control is reinfection rates which can be controlled with improved sanitation, hygiene, and education. As seen in Bangladesh, once these elements are addressed the community can upkeep the program interventions to improve infection rates. This is more difficult for Kenyan communities where sanitation levels are not as advanced as seen Bangladesh with the installation of a large quantity of latrines. Current programs in place like Sanergy, funded by USAIDS in 2011 to increase the number of latrines in Kenya, may contribute significantly to gaining control over infection and reinfections with STHs [21].
Conclusion
Mass drug administration is at the forefront of neglected tropical disease control, especially against STH. Though this may seem like the only way to overcome the burden of STHs it must not be forgotten that emphasis on other sectors such as education, sanitation and access to safe water are also key components that can prevent reinfection of STH [22]. The 2012 London declaration actually calls for greater integration between the WASH program, which addresses each of these key sectors and NTD programs [15]. The WASH program pinpointed specialized interventions to help control STHs which included increasing access to sufficient amounts of safe water (for hygienic, environmental, drinking, food preparation, and sanitation purposes), decreasing the occurrence of open defecation, disposing of infant and child feces properly, increasing improved sanitation coverage, promoting maintenance and cleaning of latrines, improving hand washing practices (before meals, after work, and after defecation), and increasing the usage of shoes outdoors [14]. All of these components were clearly implemented in the Bangladesh program. The implementation of the ISWMS in Kenya can begin to address some issues of sanitation regarding proper waste disposal but many other factors still need to be addresses. Suggestions have been made that there needs to be “a shift from morbidity control to transmission control through targeted health education, waste management and sanitation” [7]. The 2012 WHO Road Map for Implementation stated that “the development of and access to vaccines would make a significant contribution to prevention and control” [12]. A vaccination against hookworm infections is currently in clinical trials and may prove to be the first step towards “transmission control” and eradication of this particular NTD if implemented in a revised treatment program [5]. Once this becomes available for mass distribution many of the current MDA programs can be used to distribute the vaccination. One potential downfall to this idea would be funding of the vaccination. This parameter cannot be addresses until a known cost for the vaccine is available. The possible benefits of a vaccine would be prevention of reinfection from Hookworm disease which could lead to its overall eradication. This intervention would have the largest impact on the most at risk individuals: women of childbearing ages and school-age children and eventually may lead to better maternal and infant mortality outcomes and improve school absenteeism. Theoretically, implementation of similar interventions as seen in Bangladesh into a Kenya control program and in other SSA countries should yield similar results. Yet other factors still stand in the way of implementing such programs such as proper drug management for storage and distribution to prevent MDA delays, coordinated mapping for efficient intervention targeting, adequate local monitoring and evaluation capabilities and other downfalls. Ameliorating these issues, especially adequate drug management capacities, will be essential if the 2020 target for 75% coverage of all at risk individuals in 100% of the affected countries is to be met [15].
Specific Recommendations
Improvements on community capacity through collaborations between students, parents and teachers, education on STH prevention and control, and improved access to clean water for sanitation and hygiene in conjunction with appropriate adjuvant medication/vaccination to improve the overall general health of the SSA region should be implemented. The current programs in place can be utilized to implement the interventions seen in the Bangladesh program. Through improved program monitoring and planning these interventions should allow SSA to make strides toward better control and possibly eradication of Hookworm infection and other such NTDs.
Limitations
Difficulties in finding updated program guidelines or evaluations was a major limitation in this study. Many of the websites were outdated (Centre for neglected tropical diseases whose country reports were based on pre-2010 data, the West African Centre for International Parasite Control had very limited information which most was from before 2010). It was also noted that limited research on the actual burden of the disease might have been due to relatively inconsistent research and monitoring of NTDs. Also, the vaccine against hookworm would ideally be the major addendum to many eradication programs but it is still in clinical trials so the exact efficacy is still unknown. Also, cost analysis to determine program accessibility, implementation and sustainability cannot be determined until the vaccine is available for mass distribution.
Strengths
The issues surrounding NTDs have been gaining attention due the focus on obtaining the 2015 Millennium Development Goals. This research involves new technology (the hookworm vaccination) that shows potential for making a significant impact on the incidence of hookworm infection and it may possibly be the key to eradication. Also, this program evaluation may add to knowledge that would help achieve Africa’s Millennium Development goals that addresses maternal and child health. The deadline to meet these goals is 2015 and therefore this evaluation may have more of an impact toward the WHO 2020 goals for NTD control.
Acknowledgement
We would like to express my gratitude to all those who helped us during the writing of this article.
Conflict of interest
We have no conflict of interests to disclose and the manuscript has been read and approved by all named authors.
References
- Hotez P, Kamath A (2009) Neglected Tropical Diseases in Sub-Saharan Africa: Revview of Their Prevalence, Distribution, and Disease Burden. PLoS Neglected Tropical Diseases 3(8): e412.
- Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, et al. (2006) Incorporating Tropical Diseases with Programs for HIV/AIDs, Tuberculosis, and Malaria. PLoS Medicine 3(5): e102.
- USAID (2014) Hookworm.
- Nacher M (2011) Interactions between worms and malaria: Good worms or bad worms? Malaria J 10: 259.
- Hotez PJ, Diemert D, Bacon KM, Beaumier C, Bethony JM, et al. (2013) The Human Hookworm Vaccine. Vaccine 31(Suppl 2): B227-232.
- Tzur T, Weintraub AY, Sergienko R, Sheiner E (2012) Can anemia in the first trimester predict obstetrical complications later in pregnancy? J Matern Fetal Neonatal Med 25(11): 2454-2457.
- Envision (2014) Soil Transmitted Helminthiasis.
- Theresa W. Gyorkos, Nicolas L Gilbert (2014) Blood drain: Soil transmited helminths and anemia in pregnant women. PLoS Negl Trop Dis 8(7): e2912.
- World Health Organization (2014) Soil-Trnsmitted Helminth infections.
- Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, et al. (2012) Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic review and Meta-Analysis. PLoS Medicine 9(1): e1001162.
- World Health Organization (2006) Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelmintic Drugs in Control Intervention- A manual for health professionals and program managers. Geneva: WHO Press.
- World Health Organization (2012a) Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for IMplementation. Geneva: WHO Presss.
- World Health organization Global Programme to Eliminate Lymphatic Filariasis (2013) Lymphatic Filariasis: managing morbidity and preventing disability. World health organization.
- WASH and Neglected Tropical Diseases (2013) A manual for WASH implementers Kenya.
- Uniting to Combat Neglected Tropical Diseases (2014) Delivering on Promises and Driving Progress. London Declaration Stakeholders Working Group.
- Save the Children USA (2010) School Health and Nutrition Manual: A Guide for program planning and implementation in Bangladesh. Save the Children Country Office: 14.
- Save the Children (2009) School health and nutrition: An overview: Successes and lessons learned from Nasirnagar, Bangladesh, March 2009.
- Suchdev PS, Ruth LJ, Woodruff BA, Mbakaya C, Mandava U, et al. (2012) Selling Sprinkles micronutrient powder reduces anemia, iron deficiecny, and vitamin A deficiency in young children in Western Kenya: a clusterrandomized controlled trial. The American Journal of Clinical Nutrition 95(5): 1223-1230.
- World Health Organization (2012b) Action- CDC IMMPaCT: Effectiveness of selling micronutrient powders (Sprinkles) in Western Kenya- NICHA Project- Multiple micronutrient powder (point of use 15 fortification)- Stunted Child. Retrieved from Global database no the Implementation of Nutrition Action (GINA): https://extranet.who.int/nutrition/gina/fr/ node/6062
- The World Bank (2014b) Pregnant women receiving prenatal care.
- USAID (2013) Ventures: Franchising Human Waste in Kenya’s Slums.
- World Health Organization (2013) Weekly epidemiological record 88(24): 241-256.
- Evidence Action (2013a) Deworm the World Initiative Kenya. Evidence Action.
- Evidence Action (2013b) Dispensers for safe water Kenya.
- Evidence action (2014, 09).
- The End Fund (2013) 2013 Annual REport.
- The End Fund (2014) Co-Investment in Kenya – working together to target STH and Schistosomiasis in school-aged children.
- The World Bank (2014a) Indicators.
- United Nations Economic and Social Council (2009) African Review Report on Waste Management. United Nations.
- Water Aid (2014) Kenya.
-
Bin Zhao, Xia Jiang, Jinming Cao, Kuiyun Huang. Biostatistical Analysis on Hookworm Infection in Humans. AnnalBiostat & Biomed Appli. 2(2): 2019. ABBA.MS.ID.000534.
Basket trials; Master protocols; Phase II trials; Precision medicine
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






