 Research Article
Research Article
Molecular Identification of Aerococcus Associated with Bovine Mastitis and Determination of Antibiotic Susceptibilities
Kirkan Sukru*, Parin Ugur, Yuksel Hafize Tugba and Oklay Mehmet Ali
Department of Microbiology, Aydin Adnan Menderes University, Turkey
Kirkan Sukru, Department of Microbiology, Faculty of Veterinary Medicine, Aydin Adnan Menderes University, Turkey.
Received Date: September 23, 2018; Published Date: October 09, 2018
Abstract
Aerococcus species are saprophytic in the environment as well as many human and animal infections. It has also been reported to be detected in clinical and subclinical mastitis cases in cattle. The similarity with Streptococcus and Staphylococcus species is known and may lead to misdiagnosis of Aerococcus species. In this research, 100 milk samples were collected from the farms in Aydin where clinical and subclinical mastitis was detected. The samples were brought to Aydin Adnan Menderes University Veterinary Faculty Microbiology Department by cold chain. A total of 15 (15%) Aerococcus isolates were identified from clinical and subclinical mastitis cows with MALDI-TOF MS, which is an automated identification system, and genotypic identification were performed by targeting the 16S rRNA gene. In the antimicrobial susceptibility tests based on Minimal Inhibitor Concentration (MIC), the isolates were 100% sensitive to Ciprofloxacin, Tigecycline and Trimethoprim-sulfomethoxazole, 87% sensitive to Vancomycin, 80% sensitive to Erythromycin, 67% sensitive to Tetracycline, 53.3% sensitive to Penicillin and Linezolide; 93% resistant to Nitrofurantoin and 53.3% resistant to Teicoplanin. It is predicted that the obtained regional data will shed light on the role of A. viridans in pathogenesis of mastitis in cattle for future studies.
Keywords: Cattle; Aerococcus; 16S rRNA; Mastitis; Identification
Introduction
Aerococcus is the first species identified among Aerococcusspecies and has been reported as a human pathogen causing urinary tract infections, arthritis and endocarditis [1,2]. In clinical veterinary medicine, A. viridans has been described as the causative agent of arthritis, pneumonia and meningitis in cows [3,4] and also in pigs [5]. It has been reported that veterinarians cause infections in the field, lobsters [5], tilapia fish [6], pigs and cattle [7]. A. viridans has been reported to be associated with bovine mastitis in East Asia [4,8]. There is no concrete data on the prevalence and impact of the pathogen on both human and animal health. Other Aerococcusspecies have been further described using MALDI-TOF MS as a rapid and accurate method for microbial identification [9,10]. Several studies addressing the molecular characterization of A. viridans have observed that high genetic heterogeneity is independent of the applied technique [4,5]. Differences between antimicrobial susceptibility profiles among clinical isolates have also been reported [4,5,11,9].
A. viridans is generally saprophytic and found in air, dust, vegetation, soil and seafood. Bacteria can also be found in the upper respiratory tract as part of the micro flora in the skin of healthy individuals. Aerococci are phenotypically similar to bacterial strains of Staphylococci and Streptococci, which can lead to misidentification of the pathogen and thus to the prevention of Aerococcus infections [12].
Cattle mastitis is still a widespread and a costly disease for the dairy industry in many parts of the world. Mastitis also affects animal welfare [13] and is known to be one of the most important reasons why cows are removed from the herd. A. viridans belongs to the family Streptococcaceae and can be found in the environment as a saprophytic bacterium. Clinically, this bacterium is associated with some human and animal diseases [2,14]. Although pathogenic significance of this organism in bovine mastitis is indefinite, it has occasionally been isolated as a single species from subclinical mastitis infections in dairy cows [2,15].
The scope of this study was to determine the presence of Aerococcusspecies in milks obtained from cows with mastitis and to make appropriate antibiotic selection for treatment. Thus; it is aimed to make it possible to quickly identify A. viridans strain, one of the factors causing mastitis in dairy cattle farming enterprises, and to recommend effective treatment protocols.
Material and Methods
Sample collection
The study was carried out between March and August 2018 on dairy cattle breeding farms of private enterprises in the Aydin province in Turkey. One hundred milk samples from Holstein dairy cows, which showed signs of clinical and subclinical mastitis, on six farms with 25-250 head animal capacity were used. The animals chosen for samplings were in the lactation period, at 2-8 years of age, did not receive antibiotic treatment in one month of period. Clinical mastitis diagnosis was confirmed by California Mastitis Test (Bovivet®, Kruuse™, Denmark).
Aerococcus sp. isolation
Milk samples brought to the laboratory were directly inoculated onto blood agar containing 7% sheep blood. After 24 hours incubation at 37 °C, the biochemical characteristics of the strains evaluated as Gram positive cocci by Gram staining were evaluated by standard laboratory procedures [16]. Round shaped bacteria with Gram (+) character were identified by microscopy. The preliminary identification of Aerococcussp. isolates was carried out by catalase reaction.
Identification of ,Aerococcus sp. with VITEK (MALDI-TOF) MS
In this study VITEK MS/IVD/V.3.0 (bio Mérieux, France) database was used and all the operations performed according to manufacturer directions. Bacterium agar subculture was obtained from our Aerococcal isolates determined as Gram positive cocci and catalase negative by preliminary tests. One colony from fresh colonies after 18-24 hours was taken with a 1μL aliquot and spread as a thin layer on VITEK MS slides. On the same slide, 15 isolates were prepared. The calibration curve, located in the middle of each 16 wells on the prepared slide, was spread in a thin layer of the Escherichia coli ATCC 8739 (used as standard control strain in MALDI-TOF analyses) with mass spectrum profile for quality control and calibration purposes. A 1μl matrix MALDI-TOF solution of CHCA (α-cyano-4-hydroxycinnamic acid) was added to each of the prepared wells and allowed to dry in the chamber. After waiting for 2 minutes, the slide was inserted into the device and the operation started. After the vacuum and calibration process lasted about 10 minutes, the spectra of each of the isolates began to be taken when the device was first turned on. The total reporting time of our slide with 15 samples and 3 calibration points was measured as 26 minutes (about 1.7 Minutes per sample).
Genotypic identification
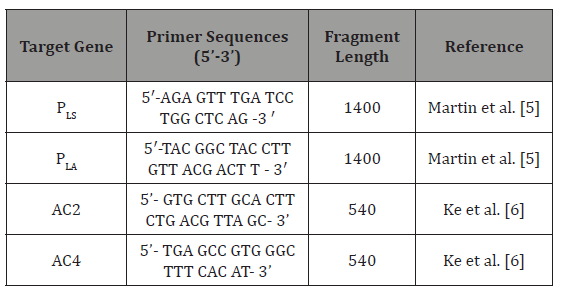
Primers: The primers (Macrogen™, South Korea) used in the PCR method are shown in (Table 1).
Table 1: The primers used in the PCR method.
Aerococcussp PCR: In our study, DNA extraction for PCR in Aerococcus strains isolated in our study was performed with Thermo Scientific® DNA extraction kit. Genus-specific PCR procedures of isolating Aerococcussp. isolates carrying the 16S rRNA gene were performed according to the protocol reported by [17]. PCR amplification for a sample in PCR reactions for the detection of 16S rRNA primer-specific products was performed in a final volume of 25μl with a final concentration of 2 × MASTER Mix (Genet Bio® ExPrime Taq Premix, Cat no.G-5000N), 10pmol primer (0.5μl for each, AC2 and AC4 (Table 1)), and 3μl 100ng template DNA. Thermal cycle and time diagram of PCR process used in 16S rRNA analyses was as follows initial denaturation at 94 °C for 5 min, 31 cycles consisting denaturation at 94 °C for 1 min, annealing at 59 °C for 1 min, extension at 72 °C for 1 min, and a cycle of final extension at 72 °C for 5 min [18].
Species-specific PCR for Aerococcus: Species specific PCR procedures for A. viridans were performed according to the protocol by [19]. PCR amplification for a sample in PCR reactions for the detection of 16S rRNA primer-specific products was performed in a final volume of 25μl with a final concentration of 2 × MASTER Mix (Genet Bio® Ex Prime Taq Premix, Cat no.G-5000N), 10 pmol primer (0.5μl for each, PLS and PLA (Table 1) and 3μl 100ng template DNA. Thermal cycle and time diagram of PCR process used in A. viridans specific analyses was as follows initial denaturation at 94 °C for 5 min, 31 cycles consisting denaturation at 94 °C for 1 min, annealing at 62 °C for 1 min, extension at 72 °C for 1 min, and a cycle of final extension at 72 °C for 5 min [12]. The 10μl amplified PCR products were detected by staining with 0.5μg/ml ethidium bromide after electrophoresis at 80 Volt for 40min in 2% agarose gel. The expected amplicon size was 1400 for Aerococcussp. (16S rRNA PCR), 540 for A. viridans (Species-specific PCR).
Antibiotic susceptibility test
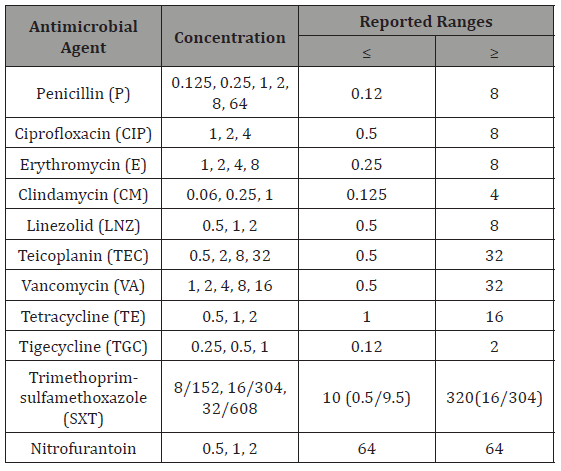
VITEK AST-P641 cards with reference number 418591 were used for antibiogram. The antibiotics, concentrations and reported ranges of VITEK AST-P641 are given in (Table 2). A total of 280μL bacterial suspension at 0.50-0.63 McFarland concentration prepared in the VITEK GP cards was transferred to another sterile 3mL saline solution and mixed homogenously by automatic pipetting. As it is in the process of identification with VITEK Cards, it was transferred to the incubator module after it was filled by the device first. Results were received by the device within 8 hours.
Table 2: The antibiotics, concentrations and reported ranges of VITEK AST-P641.
Result
Of the 100 milk samples examined in our study, 15 (15.0%) of the samples were isolated as Aerococcussp. As a result of identification studies with MALDI-TOF MS device, 15 (15.0%) of the samples were isolated as A. viridans. As a result of genus specific PCR in our study, all isolates (n=15) were identified as Aerococcussp. (Figure1). Species-specific PCR for Aerococcussp. isolates showed that all of the isolates were confirmed as A. viridans (Figure 2).
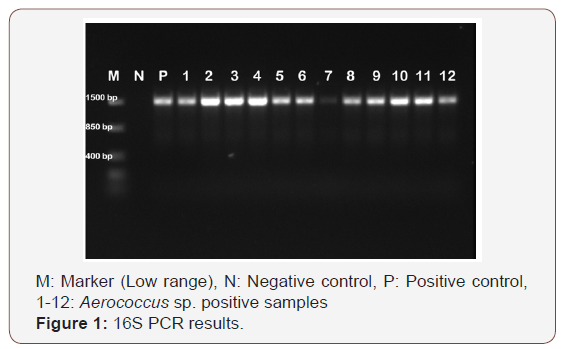
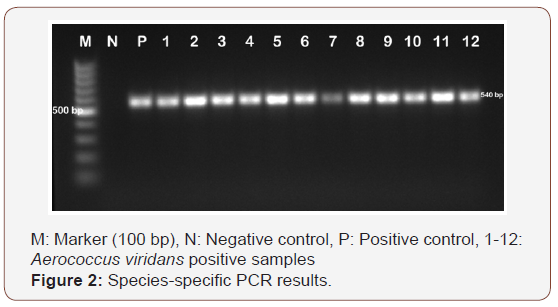
Table 3: CLSI standard minimal inhibitory concentration (MIC) values (CLSI, 2017).
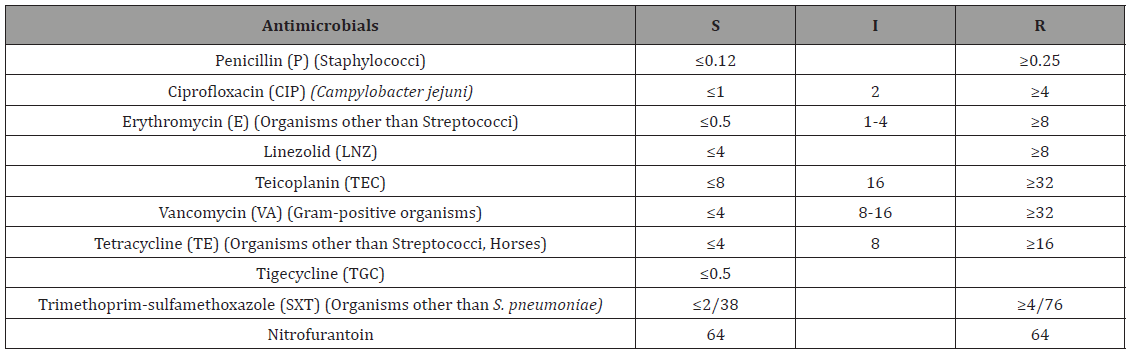
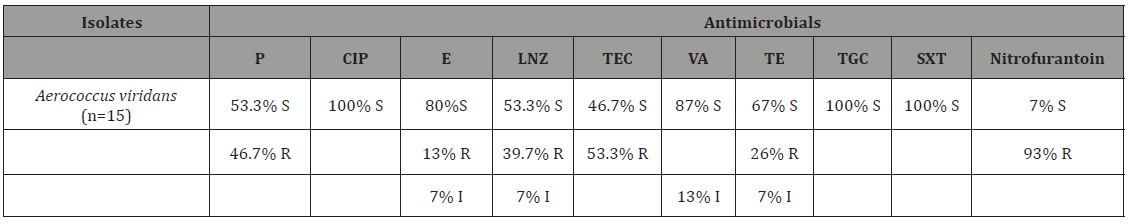
Table 4: The result of antibiogram in this study.
Discussion
As previous studies have reported, due to incorrectly identifying mastitis cases as Streptococcus or Staphylococcus species, A. viridans isolation rates are estimated to be at a minimum level [12]. However, the developments in identification techniques, particularly the application of molecular assays, have led to a more reliable identification of A. viridans.
The gold standard for species identification of Aerococcus species is based on the gene sequence encoding 16S rRNA. These sequences are trusted in the Aerococcus identification and distinguish them from other species at genus level [18]. However, this method is time-consuming and impractical for routine use. On the other hand, matrix-assisted laser desorption ionization mass spectrometry (MALDI-TOF MS) has been reported to give good results for the identification of Aerococcusspecies. When used for the identification to species level among thirty-five Aerococcus species, MALDI-TOF MS correctly identified all isolates to species level [19]. According to that study result, all 22 isolates were identified by MALDI BioTyperTM with log (score) values> 2.0 for A. viridans. Partial 16S rRNA gene sequencing was performed with isolates U45, U62 and U83; Sequences have been reported to show 99% identity to A. viridans ATCC 11563. As demonstrated for other strains of the Streptococcaceae family, MALDI-TOF MS has proven to be a reliable method for the diagnosis of A. viridans [20].
[8] also reported the presence of 8% of A. viridans in clinical mastitis cases in Japan. For this reason, in this study, it was determined that the incidence of mastitis caused by A. viridans was higher than previously reported. Before working, an improvement in hygienic milking practices was observed in dairy animals, which was reported to result in a significant reduction in the incidence of mastitis caused by infectious pathogens such as S. aureus. However, this practice has been reported to cause a reduction in the incidence of other pathogens such as environmental Streptococci, A. viridans, and coagulase negative staphylococci, which are opportunistic bacteria, in well-controlled herds. In addition, various management practices can significantly affect the incidence of mastitis. This data emphasizes the possibility that A. viridans can play an important role in bovine mastitis and that it is present as a secondary to subclinical mastitis cases. As a result of identification made from samples collected from dairy cows with clinical and subclinical mastitis in this study, 15% A. viridans were isolated and identified parallel to previous studies using new generation identification systems and genotypic identification methods. Here, the presence of A. viridans strains was revealed in bovine mastitis cases [21].
The results of this study are consistent with the findings of a study showing that animal-borne A. viridans isolates are highly susceptible to β-lactams and Vancomycin. Resistance to Trimethoprim/sulfamethoxazole, Tetracycline and Streptomycin [4,5]. Sensitivity to Tigecycline, vancomycin and macrolides have also been reported in our study and in previous studies. A. viridans isolates showed resistance to Nitrofurantoin and Teicoplanin. According to the findings and developing multidrug resistance, antibiogram examination is necessary to assure efficacy of antibiotic treatment in cases of A. viridans-induced mastitis.
Conclusion
For years, Aerococcussp. could only be identified by specialised laboratories for Aerococcussp. Today, increasing awareness of Aerococcalsp. as human and animal pathogens, coupled with tools developed for the identification of Aerococcussp has made it possible to identify this genus more effectively. In order to accurately identify Aerococcussp., MALDI-TOF MS is used instead of biochemical methods. Aerococcal strains can be isolated from clinical or subclinical mastitis cases even though mastitis cases may be missed due to mistakes made in identification. A total of 15 (%) A. viridans were identified in clinical and subclinical mastitis cows with MALDI-TOF MS, an automatic genotypic identification system targeting the 16S rRNA gene. In addition, antibiotic susceptibility tests based on Minimal Inhibitor Concentration (MIC) detection method have established that the Aerococcus isolates have developed multiple antibiotic resistances. Due to the risk of misidentification of species involved in mastitis, a disease that causes serious economic losses in dairy cattle farming enterprises, diagnosis must be done with care. The addition of PCR assays and MALDI-TOF MS analyzes amongst the routine inspection procedures is recommended in order to minimize the errors in isolation and identification of A. viridans. It is also important to identify the antibiotics for maximum treatment efficacy. It is hoped that the obtained data will shed light on the role of the causative agent in future studies on cattle for clinic and subclinical mastitis.
References
- Razeq JH, Thomas GM, Alexander (1999) The first reported case of Aerococcus bacteremia in a patient with HIV infection. Emerging Infectious Diseases Journal 5(6): 838-839.
- Zadoks RN, González RN, Boor KJ, Schukken YH (2004) Mastitis-causing streptococci are important contributors to bacterial counts in raw bulk tank milk. Journal of Food Protection. 67(12): 2644-2650.
- Devriese LA, Hommez J, Laevens H, Pot B, Vandamme P, et al. (1999) Identification of aesculin-hydrolyzing streptococci, lactococci, aerococci, and enterococci from subclinical intramammary infections in dairy cows. Veterinary Microbiology 70(1-2): 87-94.
- Liu G, Liu Y, Ali T, Ferreri M, Gao J, et al. (2015) Molecular and phenotypic characterization of Aerococcus associated with subclinical bovine mastitis PLoS One 10(4): 0125001.
- Martin V, Vela AI, Gilbert M, Cebolla J, Goyache J et al. (2007) Characterization of Aerococcus isolates from swine clinical specimens. Journal of Clinical Microbiology 45(9): 3053-3057.
- Ke X, Lu M, Ye X, Gao F, Zhu H, et al. (2012) Recovery and pathogenicity analysis of Aerococcus isolated from tilapia (Orecohromis niloticus) cultured in southwest of China. Aquaculture 43(1):18-23.
- Guccione J, Nizza S, Mallardo K, Cantiello A, Fiorito F (2013) Penicillinresistant Aerococcus bacteremia associated with bovine severe respiratory syndrome. Open Journal of Veterinary Medicine 3(2): 131- 135.
- Saishu N, Morimoto K, Yamasato H, Ozaki H, Murase T (2015) Characterization of Aerococcus isolated from milk samples from cows with mastitis and manure samples. Journal of Veterinary Medical Science 77(9): 1037-1042.
- Rasmussen M, (2013) Aerococci and aerococcal infections. Journal of Infection 66(6): 467-474.
- Senneby E, Nilson B, Petersson AC, Rasmussen M (2013) Matrix-assisted laser desorption ionization-time of flight mass spectrometry is a sensitive and specific method for identification of Aerococci. Journal of Clinical Microbiology 51(4): 1303-1304.
- Popescu GA, Benea E, Mitache E, Piper C, Horstkotte D (2005) An unusual bacterium, Aerococcus, and four cases of infective endocarditis. The Journal of Heart Valve Disease 14(3): 317-319.
- Bradley A (2002) Bovine mastitis: an evolving disease. The Veterinary Journal 164(2): 116-128.
- Chen LY, Yu WC, Huang SH, Lin ML, Chen TL, et al. (2012). Successful treatment of Aerococcus endocarditis in a patient allergic to penicillin. Journal of Microbiology, Immunology and Infection 45(2): 158-160.
- Stebbing PD, Pond MJ, Peeler E, Small HJ, Greenwood SJ, et al. (2012) Limited prevalence of gaffkaemia (Aerococcus var. homari) isolated from wild-caught European lobsters Homarus gammarus in England and Wales. Diseases o Aquatic Organisms 100(2): 159-167.
- Zhou W, Niu D, Zhang Z, Ning M, Shen H, et al. (2014) Vancomycin resistance due to VanA in an Aerococcus isolate. Indian Journal of Medical Microbiology, 32(4): 462-465.
- Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, et al. (2011) Veterinary Microbiology and Microbial Disease. Wiley-Blackwell, West Sussex, UK, P.137.
- Gopalachar A, Akins RL, Davis WD, Siddiqui AA (2004) Urinary tract infection caused by Aerococcus a case report. Medical Science Monitor 10(11): 73-75.
- Lawson PA, Falsen E, Ohlen M, Collins MD (2001) Aerococcus urinaehominis sp. nov., isolated from human urine, International Journal of Systematic and Evolutionary Microbiology 51(2): 683-686.
- Christensen JJ, Dargis R, Hammer M, Justesen US, Nielsen XC, et al. (2012) Matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis of Gram-positive, catalase- negative cocci not belonging to the Streptococcus or Enterococcus genus and benefits of database extension. Journal of Clinical Microbiology 50(5): 1787-1791.
- Hijazin M, Alber J, Lämmler C, Weitzel T, Hassan AA, et al. (2012) Identification of Trueperella (Arcanobacterium) bernardiae by matrixassisted laser desorption/ionization time-of-flight mass spectrometry analysis and by species-specific PCR. Journal of Medical Microbiology 61(3): 457-459.
- Wayne, (2017) CLSI National Committee for Clinical Laboratory Standarts (M11). Performance Standards for Antimicrobial Susceptibility Testing. 27th Informational Supplement.
-
Kirkan S, Parin U, Yuksel H T, Oklay M A. Molecular Identification of Aerococcus Associated with Bovine Mastitis and Determination of Antibiotic Susceptibilities. Arch Animal Husb & Dairy Sci. 1(1): 2018. AAHDS.MS.ID.000501.
-
Footwear Insole, Shock absorption, Comfort level, Joggers, Amateur runner, Patellofemoral, Plantar fasciitis spin splints, Injury, Micro trauma, Ankle, Knee, Deformation, Pedar-x, Capacitive sensors, Wear trial, Visual analogue scale, ANOVA, Silicone gel, Walking, T-Test
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






