 Case Report
Case Report
Mild Ocular Findings and Skeletal Anomalies Producing a Marfanoid Appearance, Along with Autoimmune/Inflammatory Findings Due to SNV of Unknown Significance in Genes ABCC6 and OPA1 Inherited from the Mother
Nicolas Chanes1, Jessica Tumolo2, James L Weber2, Srirangan Sampath2, Ricardo Gómez3, Alfonso Vargas3, Abraham Gedalia4, Alejandro León5, María D. Bernal6, Steffan Sernich7, Todd M Sanderson8, Daniel R Bronfin8, Regina M Zambrano9, Chandana B Keshavamurthy10, Katelyn A. Woolridge11, Sandra Urrea12 and Yves Lacassie9*
1Louisiana State University Health School of Medicine in New Orleans, Louisiana
2Prevention Genetics, Marshfield, Wisconsin
3Department of Pediatrics, Division of Endocrinology, LSU Health New Orleans and Children’s Hospital, New Orleans, Louisiana
4Department of Pediatrics, Division of Rheumatology, LSU Health New Orleans and Children’s Hospital, New Orleans, Louisiana
5Department of Ophthalmology, Children’s Hospital, New Orleans, Louisiana
6Department of Ophthalmology, LSU Health New Orleans
7Department of Cardiology, LSU Health New Orleans, and Children’s Hospital, New Orleans, Louisiana
8Department of Pediatrics, Ochsner Health Center for Children, New Orleans, Louisiana
9Department of Pediatrics, Division of Genetics, LSU Health New Orleans
10Rheumatology, Ochsner Health Center, New Orleans, Louisiana
11Dermatology, Ochsner Health Center, New Orleans, Louisiana
12New Orleans, Louisiana
Yves Lacassie MD, Professor Emeritus Department of Pediatrics, Louisiana State University Health, School of Medicine, 200 Henry Clay Ave., New Orleans, LA 70118, USA
Received Date: March 04, 2022; Published Date: March 22, 2022
Abstract
When faced with patients presenting with dolichostenomelia, ocular features, and Marfanoid appearance, most physicians often suspect the possibility of Marfan syndrome or one of its differential diagnoses. We report a 14-year-old Hispanic female with nanophthalmos with high hyperopia, dolichostenomelic appearance, mild musculoskeletal findings, xerosis, and positivity for some inflammatory/autoimmune markers. Although a detailed evaluation at age eight showed a Marfan systemic score of 0 and growth only between the 10th and 25th centile, the diagnosis of Marfan syndrome has been frequently raised until now. Without a specific diagnosis that could explain her syndromic findings, whole genome sequencing (WGS) was requested. This study did not detect a known reported variant but rather heterozygote single nucleotide variants in genes ABCC6 and OPA1 were found. These variants were inherited from her mother who also presents with some medical issues and positive antinuclear antibodies. Although these variants might explain these clinical findings, further confirmation will be necessary. We hope this report may encourage subsequent publications.
Keywords: Marfanoid appearance; Nanophthalmos; High hyperopia; Mild iritis; Minor musculoskeletal; Dermatological; and Rheumatological findings; Inherited ABCC6 and OPA1; Single Nucleotide Variants (SNV)
Introduction
Marfan syndrome is a common and well-known disorder involving skeletal findings such as dolichostenomelia, pectus excavatum or carinatum, arachnodactyly, kyphoscoliosis; ocular anomalies including myopia, astigmatism, and ectopia lentis, and fusiform aneurysms with the risk of aortic dissection due to a fibrillin defect [1]. The early diagnosis and referral to cardiology are of utmost importance due to the high risk of cardiovascular complications that may be life-threatening. We report a 14-year-old girl with an apparent Marfanoid phenotype due to a dolichostenomelic appearance, visual problems requiring thick eyeglasses, and other minor findings that motivated referral to orthopedics to rule out Marfan syndrome. Although the Marfan systemic score at age eight was negative [2], the possibility of Marfan syndrome was still a concern for the family. Suspecting an unreported syndrome, a WGS was requested which demonstrated heterozygote single nucleotide variants in the ABCC6 and OPA1 genes that were inherited from the mother who presents with very mild and unusual features. The clinical significance of these genetic variants should be further studied.
Case Presentation
The proposita is a 14-year-old girl, born to a 36-year-old father and a 30-year-old mother with apparently negative family history. The pregnancy was facilitated by clomiphene therapy after several years of infertility. The proposita was born at 38 WGA via normal spontaneous vaginal delivery with a birth weight of 2,820 g (31st centile) and a length of 50.6 cm (71st centile). At the age of three, she was diagnosed with high hyperopia and strabismus (accommodative esotropia). She has had normal dilated fundus examination on yearly follow-up evaluations without signs of optic nerve pallor or retinal anomalies. Since infancy, she has had erythematous changes in the skin with peculiar appearance. At age 11, she was noted to have mild asymptomatic bilateral iritis (trace pigmented anterior chamber cells in both eyes). Due to the possible diagnosis iritis, the proposita was referred to rheumatology who reported an elevated with speckled pattern ANA (1:320, NV<1:40), slightly high IgM (287 mg/dl, NV 36-251), IgG (1587 mg/dl, NV 510-1275), and ESR (24 mm/h, NV 0-20). Cardiac evaluations including echocardiograms at age 5 and 14 were normal. On physical examination Figure 1 at age 13 4/12, her appearance was dolichostenomelic mainly because she was underweight (height was 151.6 cm (15th centile), and weight was 35 kg (4th centile). Head circumference measured 51.5 cm (4th centile) and her ears 6.8 mm (97th centile for head circumference) (father’s ears are > 97th centile). The palpebral fissures measured 24.5 mm (10th centile), and there was minor hand asymmetry with the right hand being slightly smaller than the left. The left clavicle and left upper sternum were more prominent. The patient had a minor sacrococcygeal dimple, minor cubitus valgus, and subluxation of the left thumb. Walker-Murdoch and Steinberg signs were negative (Figure 1).
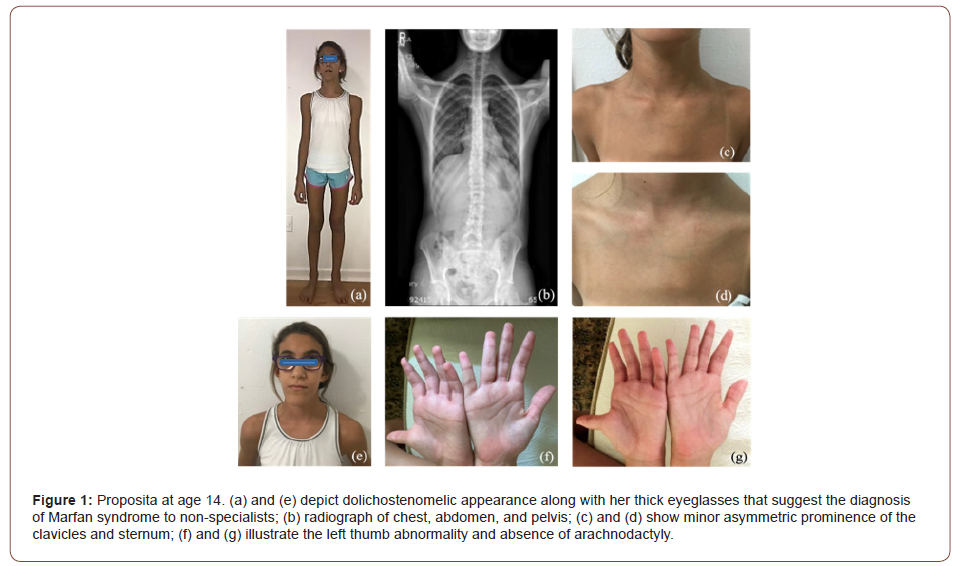
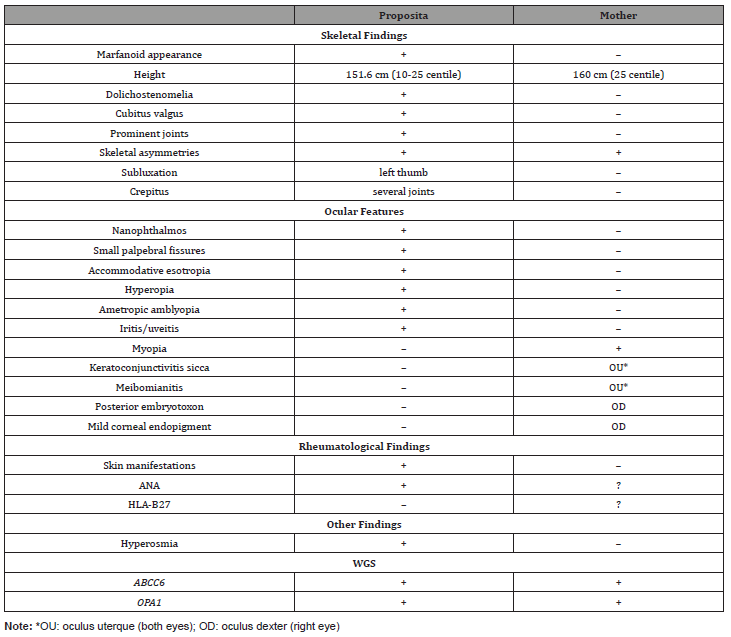
Evaluation of the father was negative. The evaluation of the mother revealed the presence of congenital asymmetry of the lower extremities with secondary scoliosis, history of spinal pain, decreased cartilage in several major joints, episodes of alopecia, skin rashes, the presence of positive ANA, and mild ophthalmological findings, including mild myopia OU, keratoconjunctivitis sicca OU, posterior embryotoxon, and mild corneal endopigment OD. The ophthalmological reevaluation of the proposita revealed bilateral nanophthalmos (axial length 18.5 mm) with persistent bilateral high hyperopia (10 diopters) with mild astigmatism (1.5 diopters), mild dry eye syndrome and meibomianitis, mild allergic conjunctivitis and the presence of rare, pigmented cells in the anterior chamber OU intermittently since age 11 without any symptoms of iritis. Table 1 summarizes the main findings in the proposita and her mother (Table 1).
Table 1: Main Findings of Proposita and her Mother.
With the suspicion of an undetermined genetic syndrome, WGS was requested. The WGS demonstrated that this patient is heterozygous in the ABCC6 gene for a maternally-inherited sequence variant designated c.3770C>A, which is predicted to result in the amino acid substitution p.Pro1257His. This patient is also heterozygous in the Optic Atrophy (OPA1) gene for a maternally-inherited sequence variant designated c.2506C>T, which is predicted to result in the amino acid substitution p.Arg836Trp. Of note, we did not detect any copy number, structural, or sequence variants that are likely to be a primary cause of disease in genes associated with Marfan syndrome and related aortopathies (ACTA2, AEBP1, BGN, CBS, COL3A1, COL5A1, COL5A2, EFEMP2, ELN, FBLN5, FBN1, FBN2, FLNA, FOXE3, LOX, LTBP3, MAT2A, MED12, MFAP5, MYH11, MYLK, NKAP, NOTCH1, PLOD1, PRKG1, SKI, SLC2A10, SMAD2, SMAD3, SMAD4, SMS, TGFB2, TGFB3, TGFBR1, TGFBR2).
Discussion
In genetics, when a syndrome is suspected, it is of paramount importance to determine the specific etiological diagnosis [3, 4]. This allows patients to receive proper genetic counseling, including not only the possibility of preventive medicine, but also upcoming gene therapies. Although the patient could be a mosaic, the possibility of an unreported genetic syndrome due to an autosomal dominant mutation involving the skeleton, eyes, and immune system led us to perform WGS (“PGnome” at PreventionGenetics) over WES due to the capture-free and PCR-free library prep, which offers several advantages over WES. WGS covers portions of the genome that are not included in WES including noncoding regions and deep intronic regions, often resulting in fewer dropped coding regions, and provides more accurate analysis of paralogous regions of the genome, resulting in greater sensitivity for SNVs compared to WES. Additionally, WGS yields improved sensitivity in the detection of structural variants (SVs), which includes copy number gains and losses that may also be detected by WES plus genome-wide detection of insertions, inversions, and translocations. In comparison, WES cannot detect SVs outside of coding regions of the genome that are not captured. It is important to note that WGS is more limited in its ability to detect mosaic variants compared to WES due to lower average depth of cover.
The WGS demonstrated that this patient is heterozygous in the ABCC6 gene for a maternally-inherited sequence variant. This variant has been reported in the heterozygous state in a patient with calcific coronary artery disease and hypertonia; however, further evidence of pathogenicity was not provided [5]. At this time, the clinical significance of this variant is uncertain due to the absence of conclusive functional and genetic evidence. Pathogenic variants in ABCC6 are also associated with autosomal recessive generalized arterial calcification of infancy and pseudoxanthoma elasticum (PXE) [6]. In PXE, the nonfunctional ABCC6 protein leads to ectopic mineralization that is most evident in the elastic tissues of the skin, eyes, and blood vessels [7]. Overlapping clinical features in this patient include nanophthalmos, pectus deformities, and scoliosis. We did not detect a second plausible causative variant in ABCC6.
This patient is also heterozygous in the Optic Atrophy (OPA1) gene for a maternally-inherited sequence variant. Optic atrophy 1 (OPA1) is the regulatory protein responsible for the fusion of mitochondrial intima, which not only helps ensure the stability of the mitochondrial intimal structure, but also participates in the remodeling of mitochondrial cristae [8]. This variant was previously reported in at least one individual with suspected optic neuropathy, although limited information was available regarding this patient [9]. At this time, the clinical significance of this variant is uncertain. Pathogenic variants in OPA1 have been associated with autosomal dominant Optic atrophy. At least some pathogenic variants in OPA1 are incompletely penetrant [9].
This study did not detect a known reported mutation but rather heterozygote single nucleotide variants in genes ABCC6 and OPA1 that were inherited from her mother who also presents with some medical issues and positive ANA. Although these variants might explain these clinical findings, further confirmation will be necessary. We hope this report will encourage subsequent publications.
Author Contributions
Nicolas Chanes performed chart and literature review as well as wrote and submitted the final version of the manuscript; Srirangan Sampath, Jessica Tumolo, and James Weber performed whole genome testing, literature review, and wrote sections regarding molecular aspects; Alfonso Vargas referred the patient, and with Ricardo Gómez participated in the diagnostic discussion, endocrinological evaluations and revised the manuscript; Abraham Gedalia performed rheumatological evaluation, the discussion of findings, and revision of the final manuscript; Alejandro León and María D. Bernal have performed all ophthalmological evaluations as well as assisted with literature review, discussion section, and final manuscript review; Steffan Sernich performed all cardiological evaluations and echocardiograms; Todd M. Sanderson and Daniel R. Bronfin have done the pediatric management, specialist referral, and review of final manuscript; Regina M. Zambrano performed the detailed genetic evaluation to rule out Marfan syndrome; Chandana B. Keshavamurthy and Katelyn A. Woolridge performed rheumatological and dermatological evaluations on the mother; Sandra Urrea performed data collection; Yves Lacassie performed current genetic and family evaluations, planned testing, and wrote the draft/revised the final manuscript.
Acknowledgement
None.
Conflict of Interest
Dr. James L. Weber is owner and director of PreventionGenetics. Dr. Jessica Tumolo is a Human Molecular Geneticist at PreventionGenetics. Dr. Srirangan Sampath is currently employed at Invitae. The rest of the authors declare no conflicts of interest.
References
- Dietz H (2017) Marfan Syndrome.
- Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, et al. (2010) The revised Ghent nosology for the Marfan syndrome. Journal of Medical Genetics 47(7): 476-485.
- Lacassie Y (1994) An International Multiaxial Diagnostic System in Clinical Genetics: Dysmorphology and Genetics of Cardiovascular Disorders. Athens, Greece: Zerbinis.
- Lacassie Y (2002) Use of a multiaxial diagnostic system in clinical genetics. Genetics in Medicine 4(2): 95-96.
- Hellman U, Mörner S, Henein M (2019) Genetic variants in cardiac calcification in Northern Sweden. Medicine 98(15): e15065.
- Chong CR, Hutchins GM (2008) Idiopathic infantile arterial calcification: the spectrum of clinical presentations. Pediatric and Developmental Pathology: The Official Journal of the Society for Pediatric Pathology and the Paediatric Pathology Society 11(5): 405-415.
- Germain DP (2017) Pseudoxanthoma elasticum. Orphanet Journal of Rare Diseases 12(1).
- Tan Y, Xia F, Li L, Peng X, Liu W, et al. (2021) Novel Insights into the Molecular Features and Regulatory Mechanisms of Mitochondrial Dynamic Disorder in the Pathogenesis of Cardiovascular Disease. Oxidative Medicine and Cellular Longevity, pp. 6669075.
- Le Roux B, Lenaers G, Zanlonghi X, Amati-Bonneau P, Chabrun F, et al. (2019) OPA1: 516 unique variants and 831 patients registered in an updated centralized Variome database. Orphanet Journal of Rare Diseases 14(1).
-
Nicolas Chanes, Jessica Tumolo, James L Weber, Srirangan Sampath, Yves Lacassie. Mild Ocular Findings and Skeletal Anomalies Producing a Marfanoid Appearance, Along with Autoimmune/Inflammatory Findings Due to SNV of Unknown Significance in Genes ABCC6 and OPA1 Inherited from the Mother. W J Opthalmol & Vision Res. 4(1): 2022. WJOVR.MS.ID.000578. DOI: 10.33552/WJOVR.2022.04.000578.
-
Marfanoid appearance, Nanophthalmos, High hyperopia, Mild iritis, Minor musculoskeletal, Dermatological, and Rheumatological findings, Inherited ABCC6 and OPA1, Single Nucleotide Variants (SNV)
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






