 Research Article
Research Article
AI/Deep Learning in Angle Closure Glaucoma
Joseph W Eichenbaum*
Dept Ophthalmology, Icahn School of Medicine at Mount Sinai, USA
Joseph W Eichenbaum, Dept Ophthalmology, Icahn School of Medicine at Mount Sinai, USA.
Received Date:June 29, 2025; Published Date:July 18, 2025
Abstract
Aim: To illustrate the potential new role of AI in the detection of Angle Closure Glaucoma, ACG.
Methods: Google scholar and PubMed were searched for: Deep Learning, DL, in ACG, convoluted neural networks (CNN) in ACG, decision tree
in ACG, random forest in ACG, linear regression in ACG, and AI studies in ACG. Promontory papers from these categories, especially in CNN were
selected and summarized for their variations in CNN classification, tuners and or subtypes of ACG studied. Area Under the Curve value achieved from
test and validate datasets was presented at the conclusion of the article review when appropriate; the DL in ACG references were searched as well
for review articles and meta- analysis which were used to gain perspective in gonioscopy vs AS-OCT or SS-OCT, (Anterior Segment or Swept Source
Optical Coherence Tomography), sensitivity in predicting ACG. After covering a brief history of ACG, salient papers in DL and ACG, including the role of gonioscopy vs CNN image categorization of: AS-OCT, SS-OCT or goniolens images are described.
Significance: This study finds a possible new role niche for DL in ACG, especially in modest ophthalmic care settings which have no expert
gonioscopy capability but can communicate with larger centers that can deal with representative patient AS-OCT or gonioscopic images.
Keywords: AI, deep Learning, Convolutional Neural Network, Angle closure glaucoma; Gonioscopy, Angle closure glaucoma suspect; Angle closure glaucoma disease; AS-OCT, SS-OCT; Peripheral Anterior Synechiae (PAS); Occludable angle closure structure; Appositional angle closure structure; Train, Test and validate data sets
Introduction
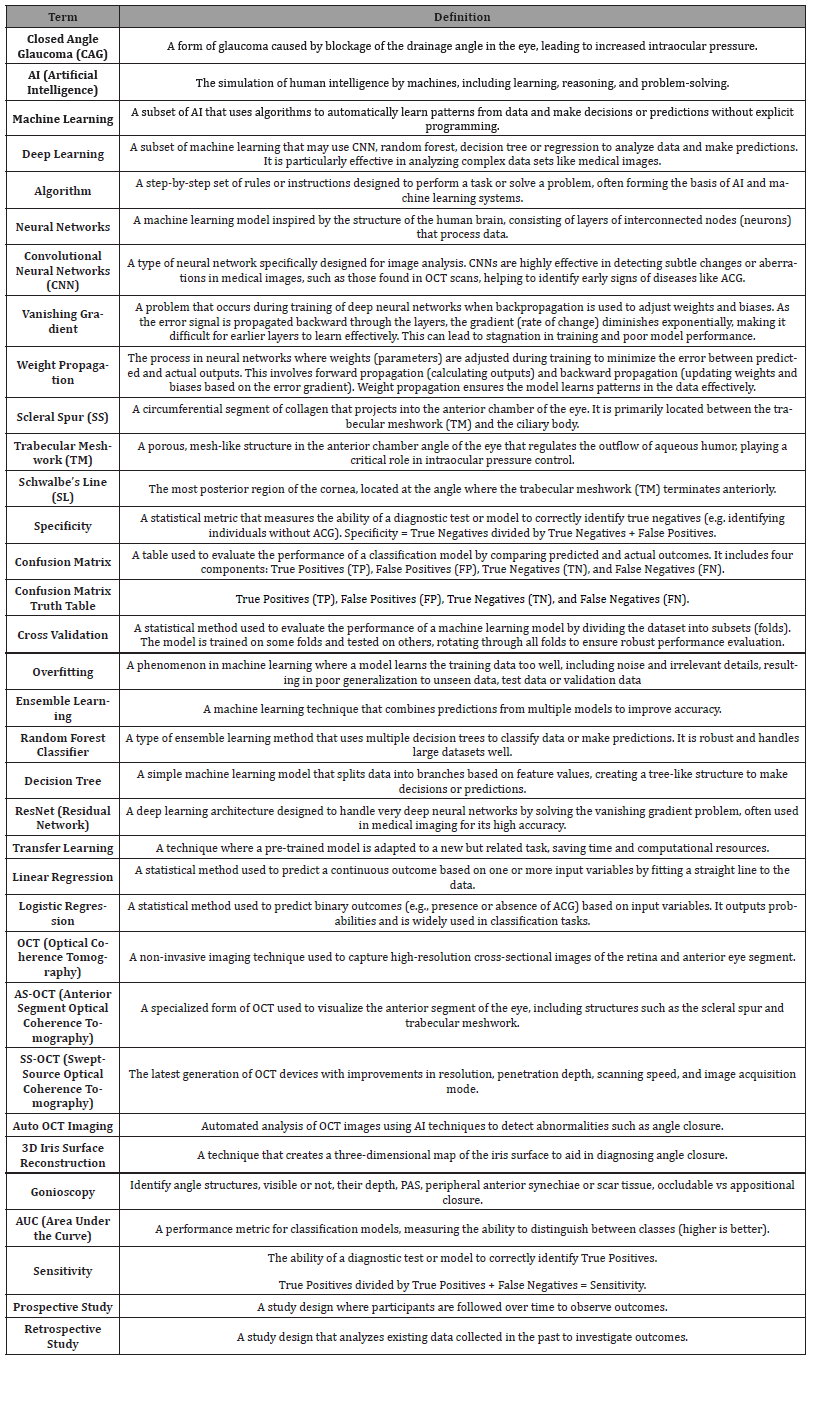
Terms and Definitions Table: Lexicon AI, Data Science and Glaucoma Terms
Table 1:
Closed-angle glaucoma brief history
Circa 1857 a German ophthalmologist, Albrecht von Graefe, first described surgical iridectomy for closed angle glaucoma, heretofore a uniformly blinding disease [1, 2]. Alexios Trantas used a direct ophthalmoscope along with digital pressure on the limbus to examine the anterior chamber angle, observe aqueous humor flow and its relationship to glaucoma diagnosis [3]. In 1907 Trantas presented detailed angle drawings and in 1918 he coined the term gonioscopy.
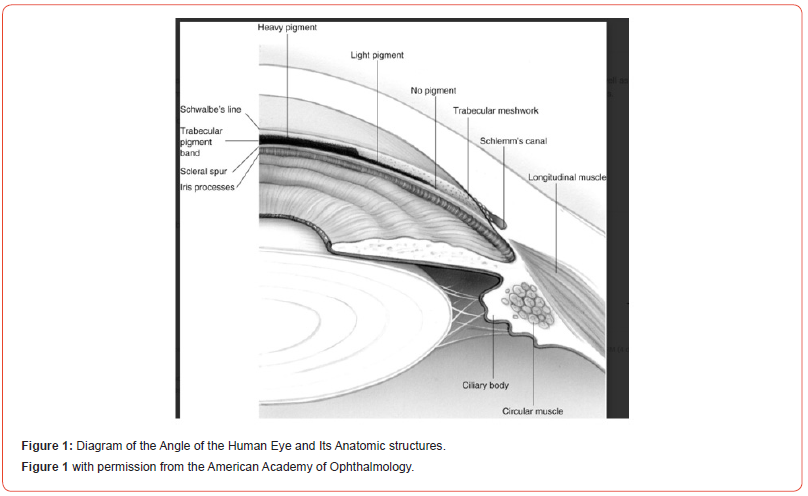
The introduction of the slit lamp by Alvar Gullstrand, 1911, manufactured by Zeiss, using an electric bulb focused on a slit that produced a rectangular beam run through the first Koeppe corneal contact lens, secured by a knotted bandage around the patient,1919, significantly advanced gonioscopy but only of the of the nasal and temporal sectors of the angle [3]. Manuel Uribe Troncoso in 1925 developed a self-illuminating gonioscope that enabled full visualization of the angle [3].
Thorburn in 1927 was the first to photograph a patient with angle closure brought on by a mydriatics and reversed by a miotic. He noted, however, that most of his patients had open angles, not closed angles [3]. Otto Barkan, 1936, developing better slit lamp illumination and magnification, using a Koeppe corneal contact lens, created the basis for modern clinical gonioscopy, made the broad distinction of “deep” and “shallow “anterior chamber and re-enforced the role of surgical iridectomy for shallow chamber glaucoma. In 1937, he first described goniotomy as treatment for glaucoma, under direct visualization [1, 3].
Goldmann in 1938 introduced indirect gonioscopic visualization through his mirrored contact lens. This lens type, with built- in angles and variable sized reflecting mirrors favoring different views with tilting and pressure, thereby throws light to different depths and structure of the angle. The Goldmann lens remains today as a major ophthalmology tool to assess anterior chamber angles [1,3].
Sugar and Gradle, 1940, and later Scheie, 1957, published grading systems that were based on the angle structures that were visible. Shaffer in 1960 and Spaeth in 1971 added to the grading system of the angle by: fuller descriptions of the angle, of the iris, and the level of the iris insertion [3]. The additional grading system functions, offer open and closed angle commentary of views at different clock hours, iris insertional level variability and give the ophthalmologist insights into static as well as dynamic features of the angle, such as: with mild goniolens compression on the corneal surface, is the occluded angle able to open? If it opens, are there PAS between iris and trabecular meshwork? Does the iris move forward through an iridotomy or iridectomy, e.g. if the ciliary body is expanding as in plateau iris, or if the vitreous is expanding as in a malignant glaucoma [3, 4].
Prior to 1920 the concept of relative pupillary block and its role in the pathogenesis of angle closure glaucoma was not understood. Feibel pointed out in an historical article on angle closure glaucoma that Edward J Curran’s statement of pupillary block as a major component of closed angle glaucoma as well as its cure by peripheral iridotomy were significant contributions to the management of closed angle glaucoma [5].
It would take some 40-50 years later for the trabecular meshwork to be logistically identified not only as the site of reduction of aqueous outflow with aging, but also as a significant factor in elevated intraocular pressure causing primary open angle glaucoma [6].
In fact, only recently have the cellular mechanics of progressively elevated intraocular pressure come under an organized framework of understanding. According the Quigley group, progressive elevation of intraocular pressure over stimulates axonal support functions at the optic nerve head. This results in genomic and proteomic attempts at extracellular matrix remodelling in retinal ganglion cell and glial cells. When genomic and proteomic support systems fail over time from continued elevated intraocular pressure, then retinal ganglion cells and supporting glial cells die and visual field loss from their absence follows [7].
Discussion
Returning to closed angle glaucoma technological historical elements, for angle closure documentation, Pavlin, Sherar, and Foster [8] 1990, developed ultrasound biomicroscopy (UBM) utilizing a high frequency transducer to deliver high resolution images of the anterior segment. UBM units operate at 50MHz, produce a tissue resolution of about 50 micrometers, and can penetrate 4-5mm of tissue. Typical B-mode ultrasonography performs at 8-15MHz with a resolution of 0.11mm, and penetrates between 30-40 mm [9, 10].
Epidemiology and pathophysiology of ACG, UBM visualizations
Primary acute angle closure glaucoma occurs when the iris mechanically blocks the trabecular meshwork and ciliary body face and raises the intraocular pressure, IOP. Before this or in conjunction with the iris mechanically blocking the trabecular meshwork, pupillary block of aqueous flow occurs, e.g. in patients with shallow anterior chambers, such as in Asians. The Framingham study found the prevalence of narrow angles in whites was 3.8% vs 8.5% in a Vietnamese population. Hyperopia and older age are also risk factors for pupillary block [10, 11].
Although the human lens grows in all people, the consequences of this growth in patients with shallow anterior chambers, as seen in some Asians, especially if the pupil dilates and the iris lies closer to the lens, the physiologic flow of aqueous through the pupil is blocked. Continued aqueous secretion fosters an aqueous pressure differential between the anterior and posterior chambers, pushing the iris forward, called iris bombe, and iris blockage of the trabecular meshwork, the major aqueous drainage site. UBM images of angle closure glaucoma typically show apposition of the peripheral iris against the trabecular meshwork, which mechanically elevates the ocular pressure [10].
Alternatively, plateau iris, a rare, closed angle glaucoma variant where the ciliary processes are anterior and rotated forward so that the far peripheral iris is thrust forward to the drainage angle. Plateau iris has been also documented by UBM. However, because the angle obstruction is bunching of the peripheral iris, especially with dilation, iris bombe is not present [10].
Swelling of the ciliary body for example due to an idiosyncratic reaction to Topiramate, a sulfamate moiety, an anti-seizure medication, (or perhaps even in cases of chronic uveitis), in the setting of shallow angle may result in forward rotation of the iris-lens diaphragm resulting in acute myopia and angle closure glaucoma. This (Topiramate) ciliary body swelling inducing angle closure glaucoma has been documented as well with UBM [10].
OCT in ACG
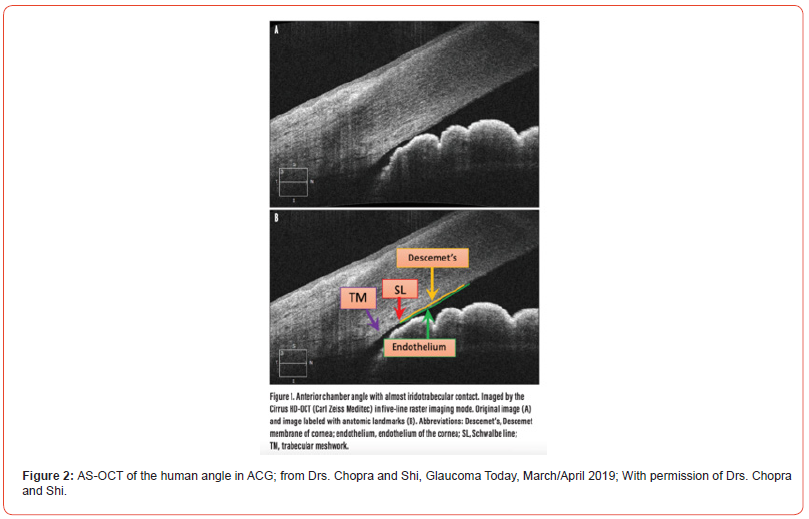
Anterior segment OCT (AS-OCT) was first reported in 2001 [12]. With a wavelength of 1310nm, it was optimized for examination of the iridocorneal angle, but because of its relatively higher cost and early lack of approval for insurance reimbursement, it was not initially well adopted in the US. However, several research studies and more recent data has demonstrated the clinical utility of AS-OCT, it’s reproducibility and role in delineating closed angle glaucoma See Figure 2 from Y Shi and V Chopra, Glaucoma Today, Mar/Apr 2019 [13].
Compared to gonioscopy which may take a while to master and may inadvertently open the angle by unintentional indentation, ASOCT does not require an eye contact lens; it can be done in the light or the dark; it can image pupil size; it can visualize Schwalbe’s line, which is adjacent to the trabecular meshwork in addition to scleral spur, which is further removed from the trabecular meshwork, and offers a more precise way of documenting the degrees of angle opening in considering laser iridotomy. Most important the AS-OCT can be shown to the patient and family to explain the individual’ s disease and treatment. Thus, if one wanted to capture 180 degrees of angle closure or greater for laser iridotomy consideration, or the extent of peripheral anterior synechiae, the AS-OCT is quite helpful [13].
Additional technological/deep learning methods of documenting ACG 1. 3 D quantification of iris surface in ACG
Because existing methods of angle closure detection, e.g. in ASOCT, only use 2D slices of structural angle properties, J. Hao and H. Fu et al. [14]. proposed a reconstruction and quantification of 3d iris surface from AS-OCT volume. Using a 15 degree radiant AS-OCT sector, the iris is segmented; the segmentation is then converted into a 3D point cloud and mesh; Poisson disk sampling (a computer graphics technique for optimized texture generation and particulate simulation) then employs iterative sampling of representative iris seed image samples and then the elements are subject to independent integrative trials to see if the sampled element fits and becomes part of the new iris sample; this ideally can provide a more accurate iris surface and different surface components are measured and computed to either achieve or not achieve the diagnosis of angle closure glaucoma [14].
However, Hao, et al. [14]. noted initial 3D point cloud deficiencies with iris surface misrepresentations with irregular, “more dramatic” geometrical changes and perhaps a lower quality mesh. Thus, they adapted a more “constrained Poisson-disk sampling” and found that maximum curvature of a given point in the cloud was larger than the global average, e.g. radii set “to 6 or 10”, then optimal point cloud and mesh were able to show more effective iris geometric details. The article goes on to explain that quantitative iris parameters, such as curvature were independently associated with the degree of angle closure progression and that their method was better able to identify the true iris root than the other iris segmentation models [14].
While their Table 1, in their paper: Comparative angle closure glaucoma classification of results obtained by different methods (2D vs 3D) does show that their 3D Point Net with optimization had high: percentage accuracy, percentage sensitivity, and percentage area under the curve, most of the 2D studies also had higher than 95% sensitivity and they did not require special graphic calibrations and optimized point curvature settings to work with 2D images [14].
2. Using deep learning of OCT images in ACG, CNN module
(Multi-Sequence Deep Network)
2a. AS-OCT image pattern analysis under dark and bright
illumination conditions separates appositional-angle images
from occludable angle images; CNN module (Multi-Sequence
Deep Network), ConvlSTM TC module
Using 66 eyes to produce 1584 AS-OCT sequences and 16,896 images related to ACG, H Hao, et al. [14]. in an endeavor to compare AS-OCT outcome data to gonioscopy, (which is often done with low and regular lighting conditions), compared AS-OCT images under low and regular lighting conditions, using a CNN module (Multi- Sequence Deep Network). In addition, a time weighted Convl STM TC. Module was used to optimize DL classification features. The Casia-2 AS-OCT machine, Tomey Inc, Japan, scans counter clockwise from 180-0 degrees which captures a series of sequence AS- OCT images. Also, they modified these images to achieve a 3D image of the anterior chamber by background removal and realignment. Thus, they developed a spatial state of features from AS-OCT images dataset obtained under both bright, 1041 lux, and dark, 0.41 lux, illumination conditions. These; lighting changes resulted in a change in pupil size (which was supposed to simulate the pressure of the gonio lens), which can push the angle open and help determine the true configuration of the angle [14].
The dataset was constructed from Zhongshan Ophthalmic Center, China from 66 eyes of 60 subjects with primary open angle glaucoma, POAG, primary closed angle glaucoma, PCAG, suspects, PACGS, or primary angle closure glaucoma disease PACD, annotated by dynamic gonioscopic exams. 21 eyes, with POAG; 13 eyes are angle closure suspects with appositional angle closure; 32 eyes with appositional and closed angles. Net, with 11 AS-OCT slices in each 15 -degree region, annotated from gonioscopic exam by a senior professor, a total of 1584 image sequences in each of the two eyed dark and bright datasets was generated or 504 for open angle, 742 for appositional, and 338 fully closed. Five- fold cross validation to train and test data was applied [14].
In summary, using the MSDN CNN classifier to compare AS-OCT
and gonioscopy in bright and dark data set resulted in the best set
of correlates of the two diagnostic modalities:
Kappa (the agreement between two classifiers) 0.80, F1
(performance of a data set when the classes are imbalanced) .85,
Accuracy .92, sensitivity .84 and specificity .92 resulted from the 3D
group [14]. By way of comparison of other Chinese ACG populations,
manual gonioscopy is still the gold standard for assessing angle
closure glaucoma. Static gonioscopy examines whether the angle
is occludable or not without light exposure or pressure on the eye
[15, 16]. Dynamic gonioscopy uses a deliberate, controlled degree
of pressure and light exposure to distinguish between appositional
vs synechial status of iris over trabecular meshwork, closed vs
occluded and can push the angle open. Since there are several
subjective elements such as patient discomfort with gonioscopy,
amount of globe pressure and variable anterior chamber angle
deformations, the possibility of false positives and negatives is not
to be ignored [15, 16].
2b. Automated* AS-OCT images (*referenced without SS and trabecular meshwork re-identification per image, but rather by expert identification and new reference dataset comparison, with expert labels); VGG-16 Learning Neural Network
Using AS-OCT with automated* classification subtypes of open and closed angle glaucoma, Fu, et al. 2018 and Fu, et al 2019 using large numbers of train and test validation images produced higher area under the curve or greater sensitivity of picking up the closed angle glaucoma, 0.98, than gonioscopy. [17,18]. These two studies by Fu, et al., Angle closure glaucoma evaluation challenge (AGE), used state of the art deep learning-based classification networks with excellent, 1600 test and 1600 train images [17, 18] *Hu, et al. in [19] explain the development of their automation for AS-OCT. The main element of ACG diagnosis is identifying iridotrabecular, ITC, contact on AS-OCT. But most AS-OCT images because of limited resolution cannot reliably identify ITC. Consequently, clinicians use scleral spur, SS, a landmark for trabecular meshwork, TM, which lies just anterior to the SS. Clinicians can look for iris contact at the SS. But even SS may be difficult to identify in AS-OCT images 20- 30% of the time. Thus, if one could adapt an automated system that could, independent of SS, identify TM, that would help. So, using a deep learning quantitative image measurement of AS-OCT features, against the clinicians AS-OCT grading of known image features, could automate identifying ITC [19].
Hu et al. recruited Chinese, Indian, and Malay descent patients, 2113, from glaucoma clinics in Singapore. Using AS-OCT, Zeiss Meditec, with examiner masked to gonioscopic findings, under darkroom conditions, low scan Visante mode images were obtained, 16mm x 6mm, 256 A-scans per line, optical resolution axial 18um: transverse to center: 60um. Patients with corneal conditions, pseudophakia, laser procedures other than iridotomy, motion artifacts or incomplete images were excluded [19].
Quantitative physical measurements, including segmentation method, unified frame, clinical quantitative parameter measurement generated a reference dataset of AS-OCT images containing markers labeled by ophthalmologists identifying landmarks such as SS and end of cornea. Then with a different set of AS-OCT images, the K-nearest similar reference images were retrieved from the reference dataset. The manual labels from the reference images were transferred to the different set of images as initial markers. These markers were then used to estimated major clinical boundaries such as SS/end of cornea. Then 20 quantitative clinical parameters, e.g., pupil diameter, anterior chamber depth and volume, SS to SS distance between iris endpoints, trabecular iris angle area, central points of iris pigment epithelium, etc. were calculated. A linear support vector machine classifier (a soft- ware package that helps with training algorithms to solve multi-class parameter problems and provide probability estimates, (dl.acm. org/doi/10.1145/19611189.1961199) was then used to predict the probability of angle closure in reference labeled and unlabeled data sets [19].
The VGG-16 deep learning neural network, NN, system, which takes the AS-OCT reference and the quantitatively fine-tuned, labeled AS-OCT image data sets through the multilayer perception NN to predict open vs closed angle [19].
For comparison to the ACG AS-OCT reference and quantitatively fine-tuned image data set as well as the normals, Gonioscopy, static and dynamic was performed with a 2mirror lens and PAS were ascertained. Spaeth and Scheie gonioscopy classification systems were used. If the posterior TM was seen for < or = 180 degrees during static gonioscopy, angle closure was diagnosed. AS-OCT image angle closure was defined as one third of the visible TM is in contact with the iris. These criteria were corroborated by a senior glaucoma specialist [19].
Gonioscopy patient data was compared to AS-OCT angle images patient data. Gonioscopy diagnosed 6771 open angle images and 1299 closed angles. AS-OCT 1323/8270 was found to have poorly discernible TM or SS and needed a second examiner. However, kappa for inter-observer reproducibility for angle open or closed was 0.8 [19].
AS-OCT images with clinician quantitative features had an AUC, 0.90, sensitivity 0.79, specificity 0.87.
AS-OCT images with deep learning had AUC 0.96, sensitivity 0.90, and specificity 0.92 [19]. Poorly delineated AS-OCT SS or TM were responsible for some false negative failure cases. Also, shadow interference in low contrast regions misled the computer vision system [19].
2c. Automated AS-OCT, multi-sequence deep neural network, UNet CNN, separating appositional (non-PAS); from occludable ACG (with PAS); variable illumination to generate AS-OCT images and other parameters to help distinguish PAS occludable from non-PAS appositional with geometric considerations
Since all of the automated AS-OCT angle closure methods thus far were based on binary classification of open vs closed angle glaucoma, H Huaying, et. al. [15]. introduced a multi-sequence deep neural network method that separates appositional vs occludable, closed or closed with synechiae [15]. This technique, using: (1) changes in pupil size @ standard light source: dark, 0.41 lux and bright, 104 lux illumination to simulate goniolens pressure, (2) global b scan alignment of the AS-OCT scans (involuntary eye movement and improper placement of the optical axis may misalign the 2 D scans), and (3) localize the anterior chamber angle by iris segmentation under different lighting conditions. The changing pupil size under different lighting, alters the iris and the anterior chamber angle. Thus, while synechiae retain the same angle appearance, appositional iris trabecular meshwork changes illustrate variable geometric images. The diamond pattern algorithm of Zhu and Ma 2000 helped computation to variable x, y ocular motion in datasets [20]. Also, the diamond search strategy of Cheng et al. 2016 helped with translation alignment of data [21].
U-Net based convolutional deep learning neural network, Ronnegerger [22] was used for automatic iris segmentation. U-Net was used to upgrade the iris image. This network comprised of a contracting-encoder and an expanding-decoder, such that the ASOCT reader obtains a label classification for each pixel.
Huaying et al randomly sampled 80% of the iris image for train data and 20% was left for test data. A five-fold validation method was used, a statistical method used to evaluate the performance of a machine learning model by dividing the dataset into subsets (folds).
The model is trained on some folds and tested on others, rotating through all folds to ensure robust performance evaluation, to train the model for best results and then a Mean Square Error loss is used; the resulting iris segmentation accuracy was 0.99 %. Thus, the anterior chamber specific region may be determined by the iris root with quite high accuracy [15].
The multi-sequence deep neural network generates dark and light captured images and the spatial state of the iris to achieve glaucoma classification of the angle. To reduce problems of “overfitting”, (i.e. the model fits too closely to the training data so it cannot make accurate predictions on test or validation data), a ResNet-34, [18] is used as a baseline neural network, which also reduces “vanishing gradients” (loss of model training data with smaller propagation weights in the layers of the neural network.) The input sequential data to the CNN encoder module are resized to 224x224 pixels for the same patient with two different lighting conditions.
In addition to the imaging standard iris characteristics, using ResNet-24, ConvLSTM module either for anomaly or motion detection [23] helped dealing in time weighted anomalies or appearance of motion changes respectively in CNN output. Thus, achieving “automatic” data set analysis, keyed to a glaucoma specialists angle image reference marks has many benefits. Either for standard image, say gonioscopic vs AS-OCT reference, or ophthalmologist labeled, or dark vs light, iris 3 D, all involved “tuning” of the CNN deep learning networks to more accurately identify angle structures e.g. AUC or sensitivity to determine open vs. closed angle, angle closure in dark or in bright light, or even glaucoma subtype determination, angle closure suspect vs angle closure disease, PAS (occludable) vs appositional.
Thus, even though gonioscopy remains the gold standard in diagnosing ACG, from the above studies, (J Hao, H Hao, H Fu) sensitive tuning of deep learning convolutional neural networks have been instrumental in taking AS-Oct images of angles to the level of respectable credibility in diagnosing ACG.
SS-OCT, CNN modelling, and angle closure, parametric review
The Shan paper is reviewed in detail because it embodies the majority of the most current ACG measurement parameters, the application of SS-OCT imaging, and the use of CNN AI deep learning.
Using a convolutional neural network model, Shan, et al. [24] (drawing from two eye centers in China and one eye center in Singapore), examined subtypes of angle closure glaucoma: angle closure glaucoma suspects, 488, vs angle closure glaucoma disease, 183, and compared 100 control eyes with 100 angle closure suspects and 100 angle closure patients with glaucoma.
The rationale for this study technique [24] is that while angle closure glaucoma is characterized by appositional or synechiae by the iris over the trabecular meshwork, early angle closure is the only first stage leading to the most severe condition of complete angle closure disease, which may result in irreversible vision loss [24-27]. Therefore, early identification of patients with angle closure glaucoma disease would auger well for early treatment and saving vision. Worldwide 1% of the population have ACG and 3.5% have open angle glaucoma. The majority of both types is often silent, with sudden loss of vision prominent in ACG and gradual loss of vision in open angle glaucoma [26].
While gonioscopy is the current standard for the diagnosis of closed angle glaucoma, clinically it has limitations. Thus, Shan et al used swept source AS-OCT(SS-OCT), which is a non-contact imaging method that can provide 360 degrees of anterior segment, 128 circumferential scans in seconds and studies with SS-OCT show significant differences between patients who are closed angle suspects vs those that have closed angle disease [28,29]. Angle opening distance, trabecular iris space area, and angle recess area are different in closed angle glaucoma suspects vs closed angle glaucoma disease patients. SS-OCT, however, may require expert manual validation of landmarks such as scleral spur and there is a large volume of data to be processed and analyzed, which may have slowed the application of SS-OCT [24]. Since convolutional neural network, CNN, is one of the best deep learning techniques for image analysis, using a CNN automated image processing algorithm was chosen for this population-based screening of closed angle glaucoma suspect from disease [24].
In Shan et al study [24] gonioscopy was done with 4-mirror Zeiss gonioprism (Volk Optical) by three experienced glaucoma specialists who were masked to SS-OCT findings. If the trabecular meshwork was not visible, dynamic gonioscopy was done to assess peripheral anterior synechia; Based on the clinical exam, patients were divided into control, closed angle glaucoma suspect or closed angle glaucoma disease.
Eyes with non-visualization of the posterior trabecular meshwork for at least 180 degrees on gonioscopy were categorized as angle closure disease; suspects for angle closure were indicated by no increased intraocular pressure, IOP, no PAS, and no glaucomatous nerve damage. Optic nerve damage was defined as; vertical cup to disc ratio of less than or equal to 0.6, interocular vertical cup to disc ratio difference of less than 0.2, no optic disc hemorrhages, no optic nerve rim loss or notch, no retinal nerve fiber layer defects on OCT and no Humphrey visual filed defects. Angle closure glaucoma eyes were defined by IOP >21mm Hg, PAS, or glaucomatous optic nerve damage; control eyes had normal gonioscopy with or without other intraocular diseases apart from cataract [24].
SS-OCT was done by masked experienced examiners in a dark room, ~20 lux, using 3 D image viewer across the anterior chamber and then software extracted raw data and fed it into the deep learning software pipeline. 128 SS-OCT images, each from 841 eyes totalling 107,648 images were split into 80% training set and 20% test, validation set. The training set was used to train the deep learning model, and the validation data set was used to evaluate the model’s performance. The neural net used was Inception V3: the images from the SS-OCT were re-sized to 299x299 matrix and their pixel values were normalized between 0 and 1. Random horizontal shifts of 0 to 3 pixels were set to optimize neural net pixel learning.
The area under the curve, AUC, performances of the models was as follows: for controls, 0.99, for angle closure suspects 0.95, for angle closure glaucoma: 0.94. The confusion matrix showed 1 control mis-identification out of 170, 4 angle closure glaucoma suspect mis- identifications out of 488, and 5 angle glaucoma with the disease out of 183 mis-identifications. Precision, or positive predictive value for control was 0.93, ACG suspect was 0.70, and ACG disease was 0.64, resulting in an average precision of 0.75- 0.76. Recall or number of true positives divided by the number of true positives + false negatives was control 0.87, ACG suspect 0.66, and ACG disease 0.72 [24]. Despite five-fold cross validation, (for definition, see Lexicon), the moderately disappointing spread of the control to ACG suspect and to ACG disease in the Precision and Recall of AI evaluative metrics leaves the AI model for ACG prediction, at least in this population, to require additional information to diagnose ACG: e.g. retinal nerve fiber layer, percentage PAS or percentage trabecular meshwork covered by iris.
Table 2:ACG Reference, Technology/Deep Learning Technique, Author and Year.
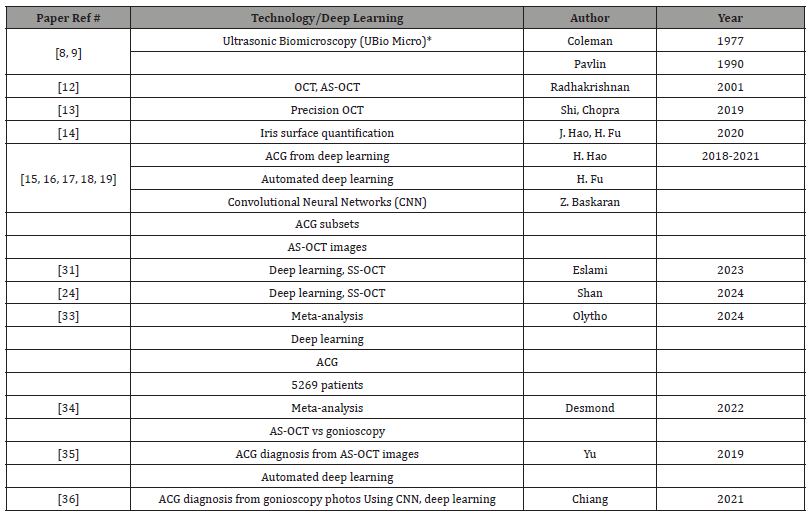
Expanded Abbreviations and Notes:
1. UBio Micro (Ultrasonic Biomicroscopy): A high-resolution imaging technique that uses ultrasound to visualize anterior segment structures of the eye.
2. AS-OCT (Anterior Segment Optical Coherence Tomography): A non-invasive imaging modality used to capture cross-sectional images
of the anterior segment.
3. Convolutional Neural Networks (CNN): A type of deep learning neural network particularly effective in image analysis tasks, such as
detecting subtle changes in medical images.
4. SS-OCT (Swept-Source Optical Coherence Tomography): The latest generation of OCT devices with improvements in resolution, penetration
depth, scanning speed, and image acquisition. Added under references 31 and 24.
In a similar vein, Eslami et al used AS-Oct images for analysis with five different neural networks, Mnasnet, MobileNet, resNet18, ResNet50 and EfficientNet, test and training set models. MobileNet performed the best identification for detecting normal (out of 87 eyes), CAG suspects (66 eyes), and CAG disease (66 eyes) at 0.99, 0.77, and0.77, category respectively [30]. In a meta-analysis of 6 studies and 5269 patients, deep learning algorithms applied to ASOCT demonstrated a pooled sensitivity of 94% in diagnosing ACG [31].
Gonioscopy, in a recent study vs. Van Herick method of detecting angle closure, which uses a slit lamp at the limbus to assess anterior chamber depth, was superior to Van Herick in identification of ACG. Also, in the same study, looking at 131 patients, with observers masked, compared resident and attending gonioscopy agreement in diagnosing angle closure glaucoma at 94%, but technician comparison to attending physician ophthalmologist ACG was about 58% [32]. Of note, the paper pointed out that the Van Herick method, for detecting ACG even when performed by experienced ophthalmologists incorrectly identified about 1 in 8 open angle eyes as closed [32].
ACG using SS-OCT accuracy in PAS vs appositional determination, using CNN, Incepiton Resnet V2CNN
Yang, et al. [33] used masked gonioscopy (performed by six glaucoma experts) and SS-OCT (Casia SS-1000, SS-OCT viewer software, version 4.7; Tomey Corp, Japan) in 278 patients from Zhongshan Ophthalmologic center in China from 2018 to 2020 to obtain 128 cross sectional images in JPEG format. Each image was split in two with vertical midline to match right and left sides; image motion artifacts or incomplete images were excluded from analysis. Each image of an anterior chamber angle was classified by two trained observers as open or closed. The static angle closure anterior chamber angle images in the first SS-OCT dataset were combined with gonioscopy results to reclassify those anterior chamber angle images as appositional or synechial angle closure. Appositional angle closure in the static images meant there were no PAS seen on gonioscopy and synechial images were defined as PAS recorded on gonioscopy. This division resulted in a second SS-OCT image dataset [33].
Two deep learning classifiers, both based on the IncepitonResnetV2CNN, a CNN, or a convolutional neural network, consisting of 244 convolution layers, 204 batch normalization layers, and four pooling layers. The activation factor function and another binary cross entropy loss function are applied to the final output neural layer as well as other adaptive optimizers and a new CNN architecture was achieved. Net output of the neural net with 80% of the images as training set and 10% as test set, with two sets of each (11-15 degree angles vs >25-degree angle) and the neural net associated adjustment parameters, (layers, activation functions, normalization, alluded to above), the Area Under the Curve was .9 for their first classifier: static angle closure. The second deep learning classifier, containing over 21,000 images of appositional images and 11,784 synechial angle glaucoma images, validation set with 261 total images, 1399 appositional ACG and 1213 synechial ACG achieved an AUC of 0.88, with 83% sensitivity and 80% specificity [33].
Meta analysis Studies and Analysis
Despite the deep learning boost in utility that AS-OCT and SSOCT have rendered in diagnosing ACG in the last 5-7 years, even against the perennial backdrop of gonioscopy variability from discomfort to the patient, to subjectivity of observations, even of the most experienced glaucoma expert ophthalmologists’ hands, gonioscopy remains the gold standard in diagnosing ACG [34].
Desmond et al in Diagnostic accuracy of AS-OCT vs gonioscopy for detecting angle closure concluded, out of 727 studies, 23 of which were included in the final analysis, there was large variation in the parameters being studied and methodologies. The sensitivity of AS-OCT varied from 46-100%, median 87%. Although AS_OCT demonstrates good sensitivity in detecting angle closure and may prove useful in Asian countries with significant levels of undiagnosed ACG, “AS-OCT is not yet able to replace gonioscopy. More studies are needed to determine the utility of AS-OCT when AS-OCT classifies eyes to have closed angles but are open on gonioscopy” [34].
Yang et al stated that despite the most sophisticated SS-OCT/ deep learning dataset model training, they could “still could not identify the precise features between the appositional angle closure and synechial angle closure from heat maps. So, further study is needed to learn what enables deep learning classifiers to distinguish the different statuses of ACG [33]. Regarding the different statuses of ACG (appositional vs PAS closure), Shan et al concluded “CNN classifiers can effectively distinguish ACG from controls, on AS-OCT with good generalizability across different patient cohorts. However, their performance is moderate when trying to distinguish ACG suspects vs actual angle closure vs angle closure glaucoma disease” [24].
Olyntho, et al. in Artificial Intelligence in Anterior Chamber Evaluation: A Systematic Review and Meta-Analysis from 6 studies and 5269 patients concluded that while “deep learning algorithms applied to AS-OCT has excellent sensitivity and specificity in the identification of angle closure, this may be a valuable resource in the screening of populations without access to experienced ophthalmologists who perform gonioscopy [31].
On the other hand, Yu, et al. [35] working with three competing CNN classifiers with large cross validation datasets found in the best classifier transfer to the ResNet-18 architecture, the detection of gonioscopic angle closure was AUC .933 on cross validation data set and 0.928 on test dataset; for detecting gonioscopic ACG disease on two and three quadrant definitions, the ResNet classifier achieved AUCs of 0.964 and 0.952, respectively on test data. Thus, Yu et al concluded, “Deep learning classifiers effectively detect gonioscopic angle closure and angle closure disease on automated analysis of AS-OCT images. These methods could be used to automate clinical evaluations of the anterior chamber angle and improve eye care in high-risk populations” [35].
Eyecam goniophotography, CNN classifiers (Resnet-50), glaucoma specialists on the pigmented trabecular meshwork, reference labels; remote detection
In a slightly different vein from AS-OCT or SS-OCT in conjunction with CNN classifiers, Chiang et al used: EyeCam gonio photography in 4 angle quadrants, a CNN classifier based on ResNet-50 architecture trained to detect angle closure, (defined as inability to visualize the pigmented trabecular meshwork), using reference labels by a single experienced glaucoma specialist. The CNN classifier performance was assessed using independent test data set with reference labels by the single glaucoma specialist or a panel of 3 glaucoma specialists. This performance was compared to that of 9 graders with a variable range of clinical experience.
The CNN classifier gave an AUC of 0.996 for the single grader and 0. 952 for the 3 glaucoma specialists, but less for the human graders, which improved with clinical experience. Chiang et al concluded, “A CNN classifier can effectively detect ACG in gonio photographs. This provides an automated method to support remote detection of patients for primary angle closure glaucoma” [36].
Thus, it would be interesting to try the CNN classifier(s) with AS-OCT or gonio photographs or even technician gonio images in lesser advantaged world areas to learn if the predictive ability is better than the oblique light from the side of the anterior chamber to predict ACG [37].
Conclusion
Although gonioscopy is the gold standard for determining ACG and its subtypes, the advances in gonio biomicroscopy, AS-OCT, SSOCT, goniophotography and the application of convolutional neural network (with iterative fine tuning) offer sophisticated and efficient classifiers of ACG and its subtypes. These recent technologies have provided an exciting new horizon for more pedestrian imaging of glaucoma and perhaps a larger platform for glaucoma treatment worldwide.
Acknowledgements
I would like to acknowledge my appreciation to the Andrew Ng, who first provided me with exposure to Machine Learning, Python and AI, and to the professors at MIT who gave me the opportunity to go further into Deep Learning and its techniques in data science analysis.
Conflict of Interest
None.
References
- History of Ophthalmology (1982) George Gorin:137.
- The History of Ophthalmology.
- WM Alward, RA Longmuir (2017) American Academy of Ophthalmology Brief History Gonioscopy. color atlas of Gonioscopy AAO.org.
- Steven M Podos. Mt Sinai Medical School, Chair Dept Ophthalmology, personal communication 1980.
- RM Feibel (1981) Edward J. Curran and the concept of relative pupillary block. Surv Ophthalmol 25(4): 270-278.
- Rohen JW, Witmer R (1972) Electron microscopic studies on the trabecular meshwork in glaucoma simplex. Graefes Arch Clin Exp Ophthalmol 183: 251-266.
- Ian Pitha, Liya Du, Thao D Nguyen, Harry Quigley (2023) IOP and glaucoma damage: The essential role of optic nerve head and retinal mechanosensors. Prog Retin Eye Res 99: 101232.
- Pavlin CJ, Sherar MD, Foster FS (1990) Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology 97: 244-250.
- Coleman DJ, Lizzi FL, Jack RL. (1977) Ultrasonography of the Eye and Orbit. Philadelphia PA: Lea & Febiger 26.
- DJ Rhee, G Spaeth, J Katz, JS Myers (2005) Ultrasound biomicroscopy and Angle Closure. Glaucoma.
- Nguyen N, Mora JS, Gaffney MM (1996) A high prevalence of occludable angles in a Vietnamese population. Ophthalmology 103: 1426-1431.
- Radhakrishnan S, Rollins AM, Roth JE, et al. (2001) Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 119(8): 1179-1185.
- Y Shi, V Chopra (2019) Anterior segment OCT: Precision Angle Imaging, an underutilized but advantageous window into angle closure glaucoma. Glaucoma Today 41-43.
- J Hao, H Fu, Yanwu Xu, Yan Hu, Fei Li, et. al. (2020) Reconstruction and Quantification of 3D Iris Surface for Angle-Closure Glaucoma Detection in Anterior Segment OCT. Springer Cham.
- Huaying Hao, Yitian Zhao, Qifeng Yan, Risa Higashita, Jiong Zhang, (2021) Angle-closure assessment in anterior segment OCT images via deep learning. Med Image Anal 69.
- M He, PJ Foster, J Ge, W Huang, D Wang, et al. (2006) Gonioscopy in adult chinese: the liwan eye study. Invest ophthalmol & vis sci 47(11): 4772-4779.
- H Fu, F Li, X Sun, X Cao, J Liao, et al. (2018) Mult-context deep network for angle-closure glaucoma screening in anterior segment oct. MICCAI 356-363.
- H Fu, Y Xu, S Lin, DWK Wong, M Baskaran, et al. (2019) Angle-closure detection in anterior segment oct based on multilevel deep network. IEEE Trans Cybern.
- Huazhu Fu, M Baskaran, Y Xu, S Lin, D Wong, (2019) A Deep Learning System for Automated Angle-Closure Detection in Anterior Segment Optical Coherence Tomography Images. Am J Ophthalmic 203: 37-45.
- S Zhu, KK Ma (2000) A new diamond search algorithm for fast block-matching motion estimation. IEEE Trans. Med Imaging 9 (2): 287-290.
- J Cheng, D Tao, Y Quan, DWK Wong, et al. (2016) Speckle reduction in 3d optical coherence tomography of retina by a-scan reconstruction. EEE Trans Med Imaging 35(10): 2270-2279.
- O Ronneberger, P Fischer, T Brox (2015) U-net: Convolutional networks for biomedical image segmentation. MICCAI 234-241.
- S Xingjian, Z Chen, H Wang, DY Yeung, WK Wong, et al. (2015) Convolutional lstm network: A machine learning approach for precipitation nowcasting. Proceedings of NIPS 802-810.
- J Shan, Z Li, P Ma, T Tun, S Yonamine, et al. (2024) Deep Learning Classification of Angle Closure based on Anterior Segment OCT. Ophthalmol Glaucoma 7(1): 8-15.
- M He, PJ Foster, J Ge, Whuang, Y Zheng (2006) Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District Guangzhou. Invest Ophthalmol Vis Sci 47: 2782-2788.
- HA Quigley, AT Broman (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90: 262-267.
- YCT Hons, X Li, T Y Wong, HA Quigley, T Aung, (2014) Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 121(11): 2081-2090.
- P Ma, Y Wu, J Oatts, J Patlidanon, Yu, et al. (2022) Evaluation of the Diagnostic Performance of Swept-Source Anterior Segment Optical Coherence Tomography in Primary Angle Closure Disease. Am J Ophthalmol 233: 68-77.
- S Moghimi, A Torkashvand, M Mohammadi, M Yaseri, LJ Saunders, et al. (2018) Classification of primary angle closure spectrum with hierarchical cluster analysis. Plos.
- Y Eslami, ZM Kouzahkanan, Z Farzinyash, M Safizadeh, R Zarei (2023) Deep Learning-Based Classification of Subtypes of Primary Angle-Closure Disease with Anterior Segment Optical Coherence Tomography. J Glaucoma 32(6): 540-547.
- M Olyntho, J Carlos, EB Castanha, A Gonçalves, B Silva (2024) Artificial Intelligence in Anterior Chamber Evaluation: A Systematic Review and Meta-Analysis. J Glaucoma 33(9): 658-664.
- TV Johnson, PY Ramulu, HA Quigley, EL Singman, (2018) Low Sensitivity of the Van Herick Method for Detecting Gonioscopic Angle Closure Independent of Observer Expertise. Am J Ophthalmology 195: 63-71.
- Y Yang, Y Wu, C Guo, Y Han, M Deng, (2022) Diagnostic Performance of Deep Learning Classifiers in Measuring Peripheral Anterior Synechiae Based on Swept Source Optical Coherence Tomography Images. Front Med Sec Ophthalmology: 8.
- T Desmond, V Tran, M Maharaj, N Carnt, A White, (2022) Diagnostic accuracy of AS-OCT vs gonioscopy for detecting angle closure: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol 260(1):1-23.
- B Yu, M Chiang, S Chadhury, S Kulkarni, A Pardeshi, et al. (2019) Deep Learning Classifiers for Automated Detection of Gonioscopic Angle Closure Based on Anterior Segment OCT Images. Am J Ophthalmol 208: 273-280.
- M Chiang, D Guth, A Pardeshi, J Randhawa, A Shen, et al. (2021) Glaucoma Expert-Level Detection of Angle Closure in Goniophotographs with Convolutional Neural Networks: The Chinese American Eye Study. Am J Ophthalmol 226:100-110.
- K Shikino, Y Hirose, M Ikusaka (2016) Oblique Flashlight Test: Lighting Up Acute Angle-Closure Glaucoma. J Gen Intern Med 31(12): 1538.
-
Joseph W Eichenbaum*. AI/Deep Learning in Angle Closure Glaucoma. W J Opthalmol & Vision Res. 5(2): 2025. WJOVR.MS.ID.000610.
-
Glaucoma, Blinding disease, Gonioscopy, Koeppe corneal, Visualization, Ultrasound biomicroscopy, Angle closure glaucoma, Hyperopia, Ophthalmic, Angle images
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






