 Research Article
Research Article
LH Priming Before Ovarian Stimulation in Poor Responders: Effects on Oocyte Recovery and Post ICSI/IVF Pregnancy Rates
Mario Mignini Renzini1, Claudio Brigante1, Silvana Gippone1, Letizia Brienza1, Diana Turchi1, Elena De Ponti2, Mariabeatrice Dal Canto1 and Jose Buratini1,3*
1Biogenesi, Reproductive Medicine Centre, Istituti Clinici Zucchi, Monza, Italy
2Department of Medical Physics, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy
3Department of Structural and Functional Biology, Institute of Biosciences, Sao Paulo State University, Botucatu, Brazil
Jose Buratini, 1Biogenesi, Reproductive Medicine Centre, Istituti Clinici Zucchi, Monza, Italy and Department of Structural and Functional Biology, Institute of Biosciences, Sao Paulo State University, Botucatu, Brazil.
Received Date: May 17, 2024; Published Date: June 13, 2024
Abstract
Background: Androgen-mediated LH activity stimulates early follicle growth. LH pre- treatment appears to improve ovarian responses in ICSI/ IVF practice but has not yet been sufficiently assessed in poor responders.
Methods: POSEIDON-4 patients (maternal age≥35; AFC<5 and/or AMH <1.2ng/mL) were stimulated with FSH+LH in a flexible antagonist protocol (Control; n=129) or subjected to the same stimulatory treatment preceded by a seven-day LH priming (150 IU/day) under GnRHa downregulation (LH priming; n=106). Follicular responses and ICSI/IVF outcomes were compared between treatment-groups in overall patients and in a subgroup of 136 patients with severely decreased follicular availability (AMH<0.75ng/mL).
Results: Overall POSEIDON-4 patients in the “LH priming” group achieved a twice higher clinical pregnancy rate (p=0.02), accompanied by a 40% reduction in cycle cancellation (p=0.03) and increased production of viable embryos (p=0.02). A multivariate analysis indicated a robust association between “LH priming” and pregnancy achievement, independent of confounding variables (OR=2.58; p=0.02). A two-fold increase in clinical pregnancy rate was also observed in severely restricted POSEIDON-4 patients in the “LH priming” group (p=0.05), this time, accompanied by a higher oocyte yield (p≤0.04).
Conclusion: A pre-ovarian stimulation LH priming can markedly improve ICSI/IVF outcomes of POSEIDON-4 patients, benefiting in different ways subgroups of this challenging patient group.
Keywords: Luteinizing hormone; Ovarian stimulation; Oocyte; ICSI; IVF; Poor responders; Advanced maternal age
Introduction
Poor ovarian response (POR) has been considered an important obstacle for IVF success since its early days [1]. Because the availability of early antral follicles for gonadotropin-induced follicular recruitment diminishes with maternal age [2], the increasing prevalence of advanced maternal age (AMA) in couples seeking for fertility treatment has substantially increased the incidence of POR in IVF practice [3]. In addition to ovarian reserve depletion, the most common cause of POR in AMA patients, agerelated loss of oocyte quality further increases the challenge for these patients to achieve their goal [4-6]. Hence, the combination AMA/POR has become one of the most important and complex topics in the field of reproductive medicine [3,5].
Importantly, POR patients are heterogeneous regarding the underlying causes and severity of their condition, thus requiring specific treatment strategies [6,7-9]. While POR patients presenting gonadotropin hypo-responsiveness due to mutations in the FSH or FSHR genes usually respond well to the inclusion of LH activity in OS [10,11], those responding poorly due to a diminished ovarian reserve (DOR) would particularly benefit from treatments capable to increase the number of recruitable small follicles before ovarian stimulation (OS). Therefore, the proposition of the so-called POSEIDON classification, distinguishing patients according to POR aetiology and age [6,12-14], was an important step towards the improvement of POR management. Among four groups proposed, POSEIDON-4 patients are those presenting DOR and AMA (age ≥35; AFC <5 and/or AMH <1.2 ng/mL), estimated to account for around 75% of the entire POR population according to the Bologna criteria [15,16]. Several management strategies including adjuvants and different OS protocols have been considered for these patients, but none of them has been capable to markedly improve their outcomes [16,17].
Patients presenting DOR and AMA appear to be refractory to increased gonadotropin dose and do not seem to lack FSH receptors in recruited antral follicles [18,19]. Therefore, as commented above, these patients are expected to benefit from strategies specifically capable of increasing the population of FSH- responsive small follicles before the onset of OS. Since androgens have been long known to enhance early follicular development through increased FSH receptor expression/activity in granulosa cells, they have been utilized in pre-OS therapies with this aim [20-25]. Indeed, androgen pre-treatment increased oocyte yield in POSEIDON-4 patients [26] and has been suggested to positively impact pregnancy and live birth rates of poor responders in general [27-30].
However, in the face of safety concerns and the fact that excessive androgen signaling can otherwise disrupt follicular homeostasis [25,31-33], an interesting alternative would be enhancing androgen paracrine action through the stimulation of thecal androgenesis with LH receptor agonists [34], which, indeed, has already been proposed [35-37]. In the first study exploring this alternative, a seven-day long r-LH priming (300 IU/day) associated with deep GnRH agonist down-regulation and OS with FSH increased oocyte yield and embryo production in normal-responders aged 19-39 years [35]. Subsequently, a four-day long LH treatment (150 IU/ day) preceding the onset of FSH administration in a long agonist protocol improved oocyte yield and live birth rates in women younger than 38 years with repeated poor ovarian responses [37]. Recently, an eight-week long hCG pre-treatment (260 IU/day) did not alter follicular dynamics but did increase oocyte recovery in DOR women aged 18-40 years [38]. Interestingly and also recently, LH pre-treatment for 1-2 months increased the antral follicle count (AFC) and AMH serum levels in two patients with hypothalamic amenorrhea, both achieving a live birth following OS/ICSI [39]. Importantly, to the best of our knowledge, no previous study has assessed the impact of a pre-OS LH priming on IVF/ICSI outcomes of POSEIDON-4 patients.
Motivated by the rationale and preliminary data described above, we have designed for POSEIDON-4 patients a protocol combining a seven-day long pre-OS LH priming with GnRHa hypothalamic downregulation. Herein, aiming to assess the utility of such strategy for this prevalent and challenging patient group, we compared OS and ICSI/IVF outcomes obtained with the new protocol (Group “LH priming”) with those obtained with our previous treatment of choice for this patient group (OS with FSH+LH combined with flexible antagonist downregulation and synchronization of antral follicle development with oestradiol pre-treatment; “Control”), in accordance with POSEIDON recommendation [40]. We hypothesised that the new protocol including the LH priming would promote greater availability of large/total follicles for oocyte retrieval, greater oocyte recovery and higher post-IVF/ICSI pregnancy rates.
Methods
Patients and experimental designed
This retrospective study was conducted at the Biogenesi Reproductive Medicine Centre, Monza, Italy, from January 2022 to June 2023. The study includes retrospective data from 235 POSEIDON-4 patients (maternal age ≥ 35; AFC <5 and/or AMH <1.2 ng/mL) [12,16], each providing a single IVF/ICSI cycle, during which they were subjected to one of two alternative OS protocols. Patients in the group defined as “Control” (n=129) were stimulated with FSH+LH (2:1) in a flexible antagonist protocol with estradiol pre-treatment, whereas patients in the group designated as “LH priming” (n=106) were subjected to the same stimulatory treatment (FSH+LH; 2:1) preceded by a seven-day LH priming in a long GnRH agonist protocol (see details below). Of note, previous evidence indicate that antagonist and long agonist downregulation provide similar IVF/ICSI outcomes in POR patients [16,41,42]. Therefore, the use of different downregulation protocols in the experimental groups is not expected to significantly affect the results. In addition, aiming to promote equivalent pre-OS follicular synchronization in the treatment-groups, only antagonist cycles with estradiol pretreatment were included in the “Control” group [16].
In a second step, in order to assess in further depth the impact of the LH priming in poor responders, “Control” and “LH priming” groups were also compared in a subpopulation of POSEIDON-4 patients (n=136) with severely diminished ovarian reserve as indicated by AMH serum concentrations <0.75 ng/mL.
Clinical pregnancy rate was the primary outcome in the present study, while percentage of failed/cancelled cycles (those not achieving a fresh transfer or embryo cryopreservation), number of total and large follicles (>16 mm) at trigger, number of total oocytes recovered per cycle and per pick, and FORT (follicular output rate) [43] and FOI (follicle to oocyte index) [4] (Alviggi et al., 2018) indexes were secondary outcomes.
Ovarian stimulation protocols
In the “Control” group, pituitary downregulation was achieved through a conventional flexible GnRH antagonist protocol starting when the largest follicle reached 13-14 mm in diameter. In all antagonist cycles, in order to promote follicular synchronization before OS, oestradiol pre-treatment (4 mg/day orally) starting at the previous mid-luteal phase (18th- 21st cycle day) was performed. In the “LH priming” group, pituitary downregulation was induced with administration of a GnRH agonist (triptorelin acetate; 0.1 mg/ day) starting in the previous mid-luteal phase (18th- 21st cycle day). From the first day of the following menstrual cycle, follicular status and oestradiol serum levels were monitored every other day, and in the absence of follicles ≥8 mm and oestradiol >50 pg/mL, a seven day-long r- LH treatment (150 IU/day) was started. Follicular activity and oestradiol levels were again monitored at the end of the LH priming period, and in the absence of follicles ≥8 mm and oestradiol >50 pg/mL, OS was started. Ovarian stimulation and trigger treatments were identical for all patients participating in the study. Follicular growth was stimulated with daily administration of FSH (300 IU/day) and LH (150 IU/day). Oocyte maturation was triggered with hCG (0.25 mg) 36 h prior to oocyte collection, when the leading follicles reached 17-18 mm in diameter.
ICSI, embryo culture and embryo transfer
ICSI/IVF and embryo culture were performed according to local routine as previously described [40]. Fresh single (SET) or double (DET) embryo transfer was performed on Day 2 or 3 according to the American Society for Reproductive Medicine guidelines [44]. The number of transferred embryos (1 or 2) was determined by embryo availability and quality following IVF/ICSI and embryo culture. In the presence of more than two viable embryos, embryo selection was based on morphology, as instructed by the Istanbul Consensus [45]. Embryos from patients with insufficient endometrial quality or progesterone levels higher than 1.5 ng/mL on the day of trigger were frozen. Implantation was assessed 12 days after ET with a βhCG test, and clinical pregnancy was diagnosed 7 weeks after ET by ultrasound monitoring.
Statistical analysis
Categorical variables are described by percentages and continuous variables are presented by mean values and standard deviation. End points in the form of percentages were compared with the Fisher’s exact test, whereas differences in continuous variables were assessed with the Wilcoxon sum rank test. The statistical analysis was conducted utilizing the Stata Software 9.0 (Stata Corporation, College Station, Texas, USA), and differences with p≤0.05 were considered significant. Differences with p values between 0.05 and 0.1 were considered as statistical trends.
Univariate and multivariate analyses were performed in order to control for the potential confounding interference of unequal variations in maternal age, AMH serum concentration, insemination method (IVF vs. ICSI) and incidence of male infertility factor in the association of OS treatment (Control vs. LH priming) with clinical pregnancy. Therefore, in such analyses, achievement of clinical pregnancy was the dependent variable, while treatment (Control vs. LH priming) and the aforementioned potential confounding factors were independent variables.
Results
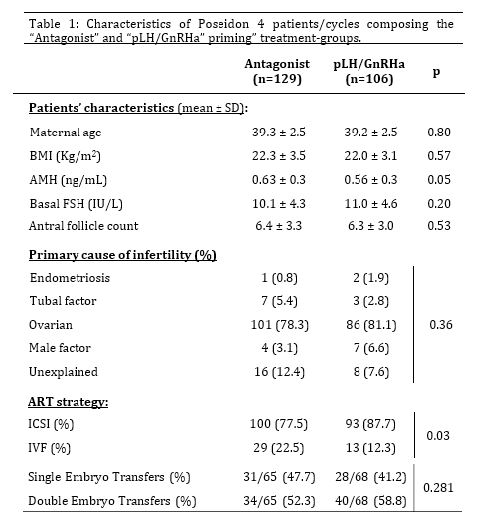
Of 235 overall POSEIDON-4 patients included in this study, 136 (57.87%) presented AMH serum levels lower than 0.75 ng/mL and were classified as POSEIDON-4 patients with severely diminished follicular availability. Overall POSEIDON-4 patients composing the “Control” and “LH priming” groups did not differ regarding maternal age, BMI, basal FSH, AFC, distribution of infertility causes and percentages of single and double ET, but those subjected the “LH priming” treatment presented slightly lower AMH serum concentrations (p=0.05) and more frequent utilization of ICSI in relation to IVF as compared to controls (p=0.03; Table 1).
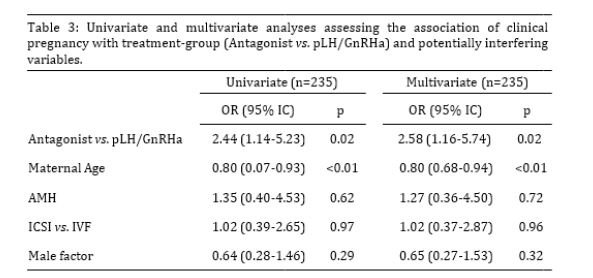
With regard to cycle outcomes (Table 2), overall POSEIDON-4 patients in the “LH priming” group achieved a fresh ET more often (p=0.02) and presented a 40% lower incidence of failed/cancelled cycles as compared to the “Control” group (p=0.03). Consistently, patients in the “LH priming” group produced more viable embryos (p=0.02) and embryos selected for fresh ET per cycle (p=0.02), as well as more viable embryos per oocyte inseminated (p=0.01). In addition, patients in the “LH priming” group tended to produce more viable embryos per oocyte recovered (p=0.09). More importantly and in agreement with the central hypothesis of the study, the “LH priming” group achieved an approximately twice higher clinical pregnancy rate per cycle (p=0.02), while presenting tendentially higher rates of implantation (p=0.1) and clinical pregnancy per ET (p=0.07) as compared to the “Control” group. Our multivariate analysis reinforced the strong positive association between the “LH priming” treatment and clinical pregnancy, while indicating that this association is independent of the potential confounding influence of heterogeneous variations between treatment-groups for maternal age, AMH serum levels, insemination method and presence of male infertility factor (OR=2.52; p=0.02; Table 3).
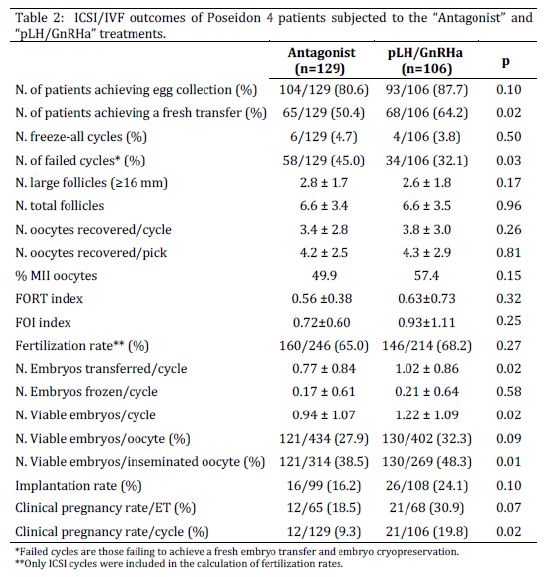
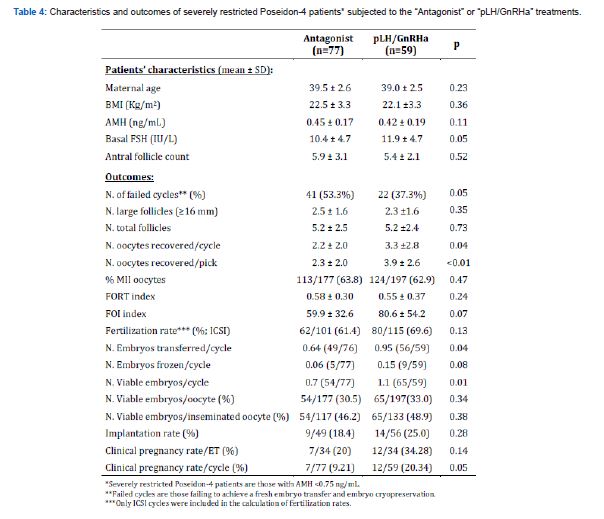
However, in disagreement with the hypotheses of the study, in the overall POSEIDON-4 patient group, the difference in pregnancy rates was not associated with corresponding differences in numbers of large or total follicles present at trigger, nor oocyte recovery or FORT/FOI indexes. Differently, in the subgroup of POSEIDON 4 patients with severely restricted follicular availability, although again no differences in follicular outputs were observed between treatment-groups, patients in the “LH priming” group recovered more oocytes per cycle (p=0.04) and per pick (p<0.01), produced more viable embryos (p=0.01) and achieved a more than two-fold higher clinical pregnancy rate as compared to the “Control” group (p=0.05; Table 4).
Discussion
In the view of the strong association between AMA and POR and the increasing prevalence of AMA in couples seeking for fertility treatment, the duet AMA/POR has become one of the most important challenges in current IVF practice, requiring approaches capable to overcome limited oocyte quantity and quality at the same time [3]. LH pre-treatment has been previously proposed as a promising strategy to enhance OS responses through androgen paracrine action [27,46,47]. However, in the lack of specific data for AMA poor responders, the utility of a pre-OS LH priming for this important patient group has remained elusive. Herein, we provide novel evidence that an LH priming preceding OS can indeed markedly improve ICSI/IVF outcomes of POSEIDON-4 patients. Interestingly, in parallel, our findings suggest that the positive impact of the LH priming on IVF/ICSI outcomes is mainly a consequence of decreased cycle cancellation and increased oocyte quality in overall POSEIDON-4 patients, while greater oocyte yield appears to account for improved ICSI/IVF results specifically in patients with severely diminished follicle availability.
As hypothesized, overall POSEIDON-4 patients subjected to OS preceded by an LH priming achieved a markedly higher clinical pregnancy rate. Importantly and in agreement with a previous smaller study assessing the effects of a four-day long LH pretreatment in predominantly pre-AMA poor responders [37], the positive impact on pregnancy rate was associated with a lower incidence of failed/cancelled cycles. Indeed, cycle cancellation has been long recognized as a major impediment associated with POR [48]. On the other hand, in disagreement with our hypothesis, these findings were not accompanied by differences in numbers of total or large follicles at trigger, FORT/FOI indexes or greater oocyte yield. Our results are nevertheless in line with a previous study assessing a similar OS strategy (seven-day 300 IU/day LH priming with agonist downregulation and OS with FSH) in normal responders aged 19-39 years, in which LH pre-treatment did not significantly affect follicular responses to OS, nor oocyte yield [35]. Also, of note, our findings partly agree with a recent study, in which an eight-week long hCG pre-treatment did not affect the follicular output rate but did increase oocyte yield in POR patients aged 18- 40 years stimulated with FSH in an antagonist protocol [38]. The differences above most likely reflect heterogeneous treatments and populations across studies; the present study is the first to investigate the impact of a pre-OS LH priming on IVF/ICSI outcomes of POSEIDON-4 patients.
Interestingly, in the overall POSEIDON-4 population, the robust difference between pregnancy rates achieved by the treatmentgroups, together with the absence of a treatment effect on oocyte yield and with the observation of an increased percentage of inseminated oocytes producing viable embryos, suggest that the LH pre-treatment has favored pregnancy achievement by improving oocyte quality. This interpretation is also in line with statistical trends towards higher rates of implantation and pregnancy per ET in the “LH priming” group (p<0.1). The identification of the mechanisms by which the LH priming may have improved oocyte quality requires further investigation. We speculate that androgenmediated LH activity may have increased the number/proportion of small follicles capable to efficiently respond to FSH at the beginning of OS [20-25], thus promoting more gradual/homogeneous oocyte maturation during antral follicle growth, even if morphological differences were not observed. This speculation is in line with the positive effect of a similar LH pre-treatment on the number of small antral follicles at the beginning of OS but not on the number of large follicles at the end of OS [35], as well as with recent evidence that oocyte developmental competence benefits from gradual maturation and prolonged cumulus-oocyte communication [49].
In order to better assess the effects of the LH priming on the outcomes of poor responders at AMA, a sub-analysis including only POSEIDON-4 patients with severely restricted follicular availability was performed. Despite the lower number of patients in relation to the overall analysis, a significantly and substantially increased clinical pregnancy rate, accompanied by a 30% decreased incidence of cycle cancelation, was again observed in the “LH priming” group. However, while again no differences in follicular responses were observed, severely restricted POSEIDON-4 patients subjected to LH pre-treatment recovered more oocytes than those in the control group. The increase in oocyte yield alongside with no observable impacts on follicular parameters is intriguing. As speculated above, the LH priming may have promoted more homogeneous maturation within the follicle cohort, increasing the proportion of healthy/ adequately matured follicles at OPU and, consequently, the retrieval of cumulus-oocyte complexes. We cannot discard, however, that our ultrasound monitoring may not have been sufficiently accurate to detect subtle but still relevant differences in follicular responses.
Importantly, considering the difficulty faced by POSEIDON-4 patients with a severely reduced follicular population to achieve a live birth, the present data suggest that this patient subgroup may particularly benefit from the use of a pre-OS LH priming. Interestingly, similar clinical pregnancy rates were observed in overall and severely restricted POSEIDON-4 patients treated with LH priming in the present study. In a context where oocyte donation has been increasingly preconized to overcome AMA-related subfertility [3,50], the present study strongly indicates that through a tailored OS strategy including LH pre-treatment, the chances for these particularly challenging couples to achieve their primordial goal, i.e., the live birth of a baby conceived from their own gametes, may be substantially increased.
The present study is limited by its retrospective nature and, although the interference of major confounding variables was controlled for by a multivariate analysis, we cannot rule out the influence of other possibly non-identified confounding factors. We also recognize the utilization of different downregulation protocols in the treatment-groups under comparison as a limitation of our study. Nevertheless, in the view of the magnitude of the differences in pregnancy rates reported herein, together with reassuring evidence from the literature indicating that these downregulation schemes perform equivalently in poor responders [13,41,42], one can conclude that the robust differences reported herein are mostly, if not entirely, determined by the utilization of LH pretreatment. Finally, although the number of patients included in the study was sufficient to demonstrate a significant and substantial treatment effect on our primary outcome for both, overall and severely restricted POSEIDON-4 patients, we recognize that greater replication would favor the assessment of important variables reflecting oocyte/embryo quality such as implantation and pregnancy rates per ET, for which statistical trends were observed. Of note, given the potential impact/benefit of our findings for a challenging and growing group of patients, to whom treatment delay may further compromise fertility prognosis, we chose to divulgate our results before reaching higher replication and collecting live birth data. Further studies, ideally with a randomized/controlled design, are needed to confirm whether the robust impact of the LH priming on clinical pregnancy reported herein is accompanied by a corresponding increase in live birth rates of POSEIDON-4 patients.
Conclusion
The present findings provide novel evidence that the incorporation of a LH priming to the OS strategy can substantially improve post-ICSI/IVF pregnancy rates of AMA poor responders with DOR. Interestingly, this appears to be predominantly a consequence of decreased cycle cancellation and improved oocyte quality, while increased oocyte yield appears to specifically contribute for the positive impact of the LH priming in POSEIDON- 4 patients with severely limited follicle availability.
Acknowledgement
The authors thank Thaisy T Dellaqua for assistance during manuscript preparation.
Authors’ contributions
M.M.R. and C.B: clinical intervention, data interpretation, critical reading, study design; S.G.: clinical intervention, data interpretation, critical reading, study design; L.B.: clinical intervention, data preparation, critical reading; D.T.: laboratorial work, data preparation critical reading; E.D.P.: statistical analysis, critical reading; M.D.C.: laboratory support, data analysis and interpretation and critical reading. J.B.: study design, data analysis and interpretation, manuscript writing.
Conflict of Interest
None of the authors have any conflict of interest to declare.
Funding
This study was entirely funded by the leading fertility clinic.
References
- Garcia JE, Jones GS, Acosta AA, Wright G Jr (1983) Human menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase II, 1981. Fertil Steril 39(2): 174-179.
- Wallace WH, Kelsey TW (2010) Human ovarian reserve from conception to the menopause. PLoS One 5(1): e8772.
- Ubaldi FM, Cimadomo D, Vaiarelli A, Fabozzi G, Venturella R, et al. (2019) Advanced maternal age in IVF: still a challenge? The present and the future of its treatment. Front Endocrinol (Lausanne) 10(94): 1-18.
- Broekmans FJ, Soules MR, Fauser BC (2009) Ovarian aging: mechanisms and clinical consequences. Endocr Rev 30(5): 465-493.
- Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, et al. (2018) Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne) 9(327): 1-8.
- Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, et al. (2018) Defining Low Prognosis Patients Undergoing Assisted Reproductive Technology: POSEIDON Criteria-The Why. Front Endocrinol (Lausanne) 9: 461.
- Polyzos NP, Devroey P (2011) A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil Steril 96(5): 1058-1061.
- Oudendijk JF, Yarde F, Eijkemans MJ, Broekmans FJ, Broer SL (2012) The poor responder in IVF: is the prognosis always poor?: a systematic review. Hum Reprod Update 18(1): 1-11.
- Humaidan P, Chin W, Rogoff D, D'Hooghe T, Longobardi S, et al. (2017) Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. Hum Reprod 32(3): 544-555.
- Ferraretti AP, Gianaroli L, Magli MC, D'Angelo A, Farfalli V, et al. (2004) Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril 82(6): 1521-1526.
- Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, et al. (2018) Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril 109(4): 644-664.
- Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, et al. (2016) A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril 105(6): 1452-1453.
- Esteves SC, Alviggi C, Humaidan P, Fischer R, Andersen CY, et al. (2019) The POSEIDON Criteria and Its Measure of Success Through the Eyes of Clinicians and Embryologists. Front Endocrinol (Lausanne) 10: 814.
- Esteves SC, Conforti A, Sunkara SK, Carbone L, Picarelli S, et al. (2021) Improving Reporting of Clinical Studies Using the POSEIDON Criteria: POSORT Guidelines. Front Endocrinol (Lausanne) 12: 587051.
- Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, et al. (2011) ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26(7): 1616-1624.
- Haahr T, Dosouto C, Alviggi C, Esteves SC, Humaidan P (2019) Management Strategies for POSEIDON Groups 3 and 4. Front Endocrinol (Lausanne) 10: 614.
- Pandian Z, McTavish AR, Aucott L, Hamilton MP, Bhattacharya S (2010) Interventions for 'poor responders' to controlled ovarian hyper stimulation (COH) in in-vitro fertilization (IVF). Cochrane Database Syst Rev (1): CD004379.
- Regan SL, Knight PG, Yovich JL, Stanger JD, Leung Y, et al. (2017) Infertility and ovarian follicle reserve depletion are associated with dysregulation of the FSH and LH receptor density in human antral follicles. Mol Cell Endocrinol 446: 40-51.
- van Tilborg TC, Torrance HL, Oudshoorn SC, Eijkemans MJC, Koks CAM, et al. (2017) Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 1: The predicted poor responder. Hum Reprod 32(12): 2496-2505.
- Harlow CR, Hillier SG, Hodges JK (1986) Androgen modulation of follicle-stimulating hormone-induced granulosa cell steroidogenesis in the primate ovary. Endocrinology 119(3): 1403-1405.
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA (1998) Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 101(12): 2622-2629.
- Weil S, Vendola K, Zhou J, Bondy CA (1999) Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 84(8): 2951-2956.
- Luo W, Wiltbank MC (2006) Distinct regulation by steroids of messenger RNAs for FSHR and CYP19A1 in bovine granulosa cells. Biol Reprod 75(2): 217-225.
- Sen A, Hammes SR (2010) Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol 24(7): 1393-1403.
- Astapova O, Minor BMN, Hammes SR (2019) Physiological and Pathological Androgen Actions in the Ovary. Endocrinology 160(5): 1166-1174.
- Chen SN, Tsui KH, Wang PH, Chern CU, Wen ZH, et al. (2019) Dehydroepiandrosterone Supplementation Improves the Outcomes of in vitro Fertilization Cycles in Older Patients with Diminished Ovarian Reserve. Front Endocrinol (Lausanne) 10: 800.
- Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, et al. (2012) The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update 18(2): 127-145.
- Gleicher N, Kim A, Weghofer A, Shohat-Tal A, Lazzaroni E, et al. (2013) Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). J Assist Reprod Genet 30(1): 49-62.
- Nagels HE, Rishworth JR, Siristatidis CS, Kroon B (2015) Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev 11: CD009749.
- Walters KA, Rodriguez Paris V, Aflatounian A, Handelsman DJ (2019) Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol 242(2): R23-R50.
- Harlow CR, Shaw HJ, Hillier SG, Hodges JK (1988) Factors influencing follicle- stimulating hormone-responsive steroidogenesis in marmoset granulosa cells: effects of androgens and the stage of follicular Endocrinology 122(6): 2780-2787.
- Zeleznik AJ, Little-Ihrig L, Ramasawamy S (2004) Administration of dihydrotestosterone to rhesus monkeys inhibits gonadotropin-stimulated ovarian steroidogenesis. J Clin Endocrinol Metab 89(2): 860-866.
- Prizant H, Gleicher N, Sen A (2014) Androgen actions in the ovary: balance is key. J Endocrinol 222(3): R141-151.
- Hillier SG, Whitelaw PF, Smyth CD (1994) Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol 100(1-2): 51-54.
- Durnerin CI, Erb K, Fleming R, Hillier H, Hillier SG, et al. (2008) Effects of recombinant LH treatment on folliculogenesis and responsiveness to FSH stimulation. Hum Reprod 23(2): 421-426.
- Lossl K, Andersen CY, Loft A, Freiesleben NL, Bangsboll S, et al. (2008) Short-term androgen priming by use of aromatase inhibitor and hCG before controlled ovarian stimulation for IVF. A randomized controlled trial. Hum Reprod 23(8): 1820-1829.
- Ferraretti AP, Gianaroli L, Motrenko T, Feliciani E, Tabanelli C, et al. (2014) LH pretreatment as a novel strategy for poor responders. Biomed Res Int 2014: 926172.
- Wang FN, Bogstad JW, Pors SE, Petersen MR, Pinborg A, et al. (2023) Eight weeks of androgen priming by daily low-dose hCG injections before ICSI treatment in women with low ovarian reserve. Hum Reprod 38(4): 716-725.
- La Marca A, Longo M (2022) Extended LH administration as a strategy to increase the pool of recruitable antral follicles in hypothalamic amenorrhea: evidence from a case series. Hum Reprod 37(11): 2655-2661.
- Humaidan P, La Marca A, Alviggi C, Esteves SC, Haahr T (2019) Future Perspectives of POSEIDON Stratification for Clinical Practice and Research. Front Endocrinol (Lausanne) 10: 439.
- Huang MC, Tzeng SL, Lee CI, Chen HH, Huang CC, et al. (2018) GnRH agonist long protocol versus GnRH antagonist protocol for various aged patients with diminished ovarian reserve: A retrospective study. PLoS One 13(11): e0207081.
- Drakopoulos P, Bardhi E, Boudry L, Vaiarelli A, Makrigiannakis A, et al. (2020) Update on the management of poor ovarian response in IVF: the shift from Bologna criteria to the Poseidon concept. Ther Adv Reprod Health: 14.
- Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, et al. (2011) Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod 26(3): 671-677.
- Practice Committee of American Society for Reproductive Medicine, & Practice Committee of Society for Assisted Reproductive Technology (2013) Criteria for number of embryos to transfer: a committee opinion. Fertil Steril 99(1): 44-46.
- ALPHA Scientists in Reproductive Medicine, & ESHRE Special Interest Group Embryology (2011) Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reproductive biomedicine online 22(6): 632-646.
- Lossl K, Freiesleben NC, Wissing ML, Birch Petersen K, Holt MD, et al. (2020) Biological and Clinical Rationale for Androgen Priming in Ovarian Stimulation. Front Endocrinol (Lausanne) 11: 627.
- Buratini J, Dellaqua TT, Dal Canto M, La Marca A, Carone D, et al. (2022) The putative roles of FSH and AMH in the regulation of oocyte developmental competence: from fertility prognosis to mechanisms underlying age-related subfertility. Hum Reprod Update 28(2): 232-254.
- McIlveen M, Skull JD, Ledger WL (2007) Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod 22(3): 778-785.
- Buratini J, Dellaqua TT, de Lima PF, Renzini MM, Canto MD, et al. (2023) Oocyte secreted factors control genes regulating FSH signaling and the maturation cascade in cumulus cells: the oocyte is not in a hurry. J Assist Reprod Genet 40: 1961- 1971.
- Kirkman-Brown J, Calhaz-Jorge C, Dancet EAF, Lundin K, Martins M, et al. (2022) Good practice recommendations for information provision for those involved in reproductive donation(dagger). Hum Reprod Open(1): hoac001.
- Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, et al. (2018) Understanding Ovarian Hypo-Response to Exogenous Gonadotropin in Ovarian Stimulation and Its New Proposed Marker-The Follicle-To-Oocyte (FOI) Index. Front Endocrinol (Lausanne) 9: 589.
-
Mario Mignini Renzini, Claudio Brigante, Silvana Gippone, Letizia Brienza, Diana Turchi, Elena De Ponti, Mariabeatrice Dal Canto and Jose Buratini*. LH Priming Before Ovarian Stimulation in Poor Responders: Effects on Oocyte Recovery and Post ICSI/IVF Pregnancy Rates. W J Gynecol Women’s Health. 6(2): 2024. WJGWH.MS.ID.000632.
-
Luteinizing hormone, Ovarian stimulation, Oocyte, ICSI, IVF, Poor responders, Advanced maternal age
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






