 Case Report
Case Report
Haemoglobin D Iran With Beta Thalassemia in A Primigravida With Anaemia
Arif Maqsood Ali*, Gule Raana Waseem and Shazia Arif
Rawalpindi Institute of Cardiology, Pakistan
Arif Maqsood Ali, Assistant Professor of Microbiology, Department of Pathology & Blood Bank, Rawalpindi Institute ofCardiology, Rawalpindi, Punjab, Pakistan.
Received Date: August 16, 2019 Published Date: August 23, 2019
Abstract
Anaemia is quite common in female population of developing countries including Pakistan. Beside haemoglobinopathies are often seen in India, Pakistan and Iran due to traditional practices of consanguineous marriages. Haemoglobin D-Punjab is one of the most common subvariants (55%) of Haemoglobin D, which can be inherited as a homozygous or a heterozygous trait with other haemoglobinopathies. Although, Haemoglobin D-Punjab is commonly seen but another variant of HB D like Hb D Iran with heterozygous trait of β thalassemia is rarely reported. We present a rare case of co-inheritance of Haemoglobin D- Iran and β thalassemia in a young primigravida of Kashmiri origin in her twenties. She was six months pregnant and was diagnosed to have anaemia. She had history of diarrhea. On examination, she was pale looking and mildly icteric. Systemic examination was insignificant except for mild splenomegaly. The fundal height was normal for age to date. On investigation, she had mild anaemia with microcytic hypochromic blood picture. Hemoglobin electrophoresis showed a band of Hb A2+Hb F+S/D. Molecular studies of combined Haemoglobin D –Iran and beta thalassemia. She was counseled about his disease and advised follow-up up at 6 months after delivery after delivery.
Keywords: Anaemia; Haemoglobinopathy; Primigravida; Thalassemia
Introduction
Hemoglobin D (Hb D), is a variant of hemoglobin that occurs mainly in north-west India, Pakistan and Iran [1]. The replacement of glutamic acid with glutamine in normal Hemoglobin A at 121 position on its beta chain leads to a new structurally different variant of haemoglobin called Hb D [2]. Hb D is clinically silent in its heterozygous state, but coinheritance of Hb D with either Hb S or thalassemia produces clinically significant conditions like sickle cell anemia and chronic hemolytic anemia of moderate severity [3].
The Hb D Iran trait and homozygous cases have been already reported in the literature [4,5]. However, few studies have reported compound heterozygotes of Hb D Iran with other Hb variants [6]. We report a rare case of compound heterozygous condition of Hb-D β thalassemia trait in a Kashmiri family living in Azad Kashmir, Pakistan.
Case Report
A young six-month prime gravida of Kashmiri origin from Muzaffarabad, Azad Kashmir Pakistan in her twenties presented with chronic diarrhea with history of chronic anemia. She was nonsmoker, nonalcoholic. There was no past history of parasitic infestation, blood loss, weight loss, tuberculosis, any other disease. She was treated by various GPs with oral hematinics without much improvement of her symptoms & correction of anaemia. She was believed to have diarrhea due to iron therapy and was lately administered parenteral iron therapy. However, her Hb remained 9gm/dl and did not improve. On examination, she was conscious and oriented in time place and person. Her BP was100/70mm Hg and pulse rate of 80 beats per minute. She was fair colored with mild jaundice. There was no cyanosis, clubbing, koilonychia, lymphadenopathy or edema. Systemic examination was normal except for mild splenomegaly. Ultrasound abdomen showed 24 weeks fetus, normal sized liver and an enlarged spleen of 5cms in size. Her stool examination revealed cysts of Entamoeba histolytica. She was treated with tablet metronidazole 800mg tid for 10 days. Her diarrheal symptoms disappeared and follow up stool examination was normal. Retrospectively, her family history revealed consanguine marriage of her parents. Complete blood picture showed Hb of 8.8g/dl, WBC count of 13.5 X 103/ul with 68% polymorphs. RBC count was 5.16x 106/ul. MCV was 60.8 fL, MCH 17.1pg and HCT 31.4%. Platelets were 232x103/ul. Peripheral blood film showed microcytic hypochromic blood picture with moderate anisopiokilocytosis and a few target cells. Reticulocyte count was 5.5%. Hemoglobin electrophoresis was advised to rule out haemoglobinopathy. It showed a band of Hb A2 plus Hb F plus Hb S/ D. Hb F was 0.8% and H A2 3.1%. Sickling test was negative. PCR for thalassemia showed a mutation in Fr 8-9(+G) and another Hb D (Iran). A diagnosis of combined haemoglobin D –Iran and β thalassemia were made. Urine examination was normal. The diagnosis was conveyed to the patient. No intervention was needed, however, she was counselled regarding the condition and advised to follow up at 6 months after delivery for blood screening for haemoglobinopathy.
Discussion
Hemoglobinopathies are one of the common blood disorders seen in Pakistani population. Mutations in the globin genes can cause either a quantitative reduction in output from that gene or alter the amino acid sequence of the protein produced. Quantitative defects cause thalassemia, whereas qualitative changes are referred to as Hb variants. These hereditary disorders of Hb pose a massive health problem in many third world countries including India, Pakistan, and Iran [7].
In a retrospective study conducted at National Institute of Blood Disease & Bone Marrow Transplantation, Karachi, Pakistan, a total of 2739 patients blood samples were analyzed from 2010-2014. The overall frequency of hemoglobinopathies was 34.2%, among which beta Thalassemia minor was 51.8% and Hb D trait was 6.7% and Beta Thalassemia major was 24.1%.7 Nearly similar findings were observed in other studies conducted by in Pakistan [8,9].
Thalassemias are inheritable common genetic disorder worldwide due to alteration in haemoglobin (Hb) production. About 4.83% of world’s populations carries of globin chain variants including 1.67% of the population which is heterozygous for α-thalassemia and β-thalassemia [7].
Beta thalassemia occurs as a result of mutation in Hb-β gene on chromosome-11 and follows autosomal recessive pattern of inheritance. It spreads from asymptomatic to symptomatic depending on genetic conditions [4]. β thalassemia represents a heterogeneous group of hemoglobin disorders and is caused by reduced or absent beta globin gene expression. The disease has high frequency in Mediterranean regions, Africa, Southeast Asia and Indian subcontinent. It is estimated that there are 270 million carriers of thalassemia world over and 80 million of them have beta thalassemia traits. It is a significant health problem in Pakistan and 5-7% of our population (approx 9-13 million) has thalassemia minor [10].
Hb-D is a haemoglobin variant is seen mainly in North West India, Pakistan and Iran [4]. The co-inheritance of Hb-D and β thalassemia minor is rarely reported [11].
Hb D was first reported as a new Hb by Itano in 1951 in a North American family [12].
Hb D Iran reported by Rahbar in a family in central part of Iran is the replacement of glutamic acid by glutamine at 22 (helical residue B4) [13]. In a study carried out at a largest medical center in Iran from November 2002 to December 2010, hemoglobin analysis of 220 patients with Hb D variants showed 92 cases of Hb D Punjab and 88 patients with Hb D Iran. There are few studies in literature regarding prevalence of Hb D in different countries [13]. There is scarcity of data regarding compound heterozygous Hb D Iran with β Thalassemia trait. Hb D occurs in four forms: heterozygous Hb D trait, Hb D- thalassemia, Hb S-D disease and homozygous Hb D disease, amongst them Hb D-Punjab is by far the commonest [3].
During 22 years of thalassemia screening in a Thalassemia Prevention Unit in northern Greece that covers the regions of central and western Macedonia, northern Greece, with a population of around 2.5 million, only 30 cases were found heterozygous of Hb D, 1 case of compound heterozygous for Hb S / Hb D-Punjab and 1 case of compound heterozygous for β-thalassemia / Hb D-Punjab among 80,401 subjects screened.
β thalassemia trait is very common and is found in more than half of pregnant anaemic patients in our region. Various studies have reported that the quantity of Hb D Iran eluting in the Hb A2 window in high performance liquid chromatography varies from 36.0 to 47.7% in a heterozygous condition, while in compound heterozygous states, the quantity varies between 47.3 and 94.4%.
Table 1: Hematological profile.
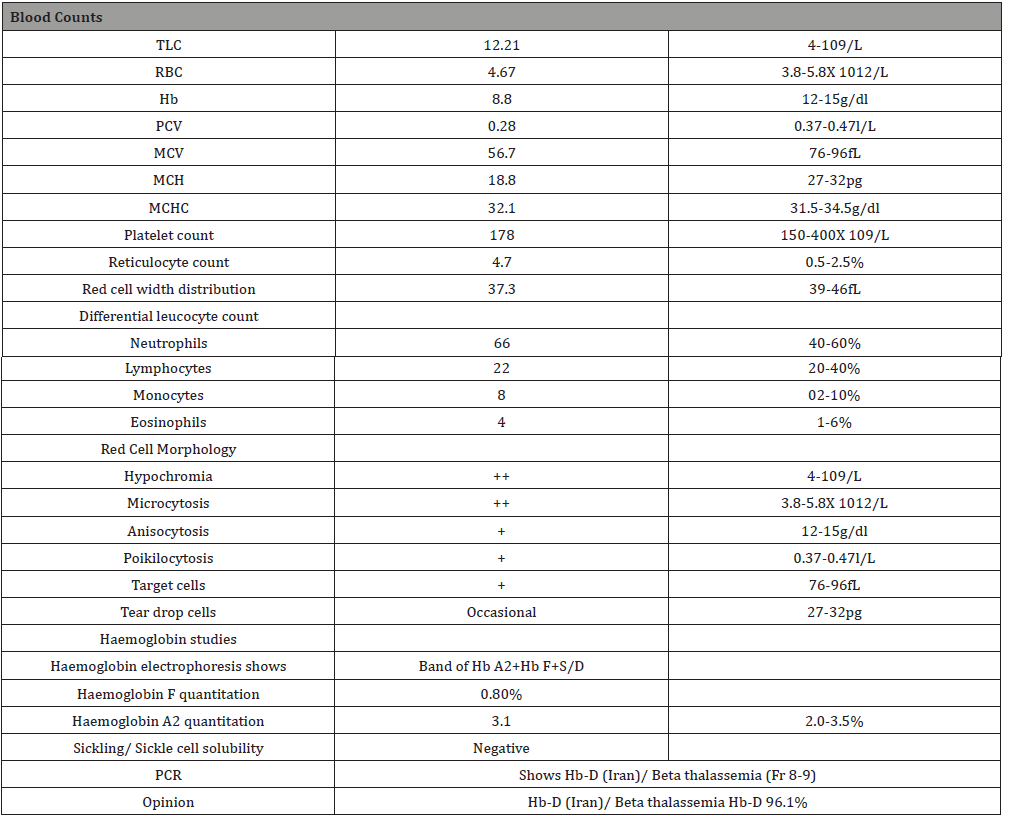
Hb D-Iran is commonly found to be co-existent with β-Thalassemia and generally doesn’t get diagnosed until patient comes for the diagnosis or treatment of the Thalassemia. In homozygous cases, such as one reported by Thornburg CD et al., Hb D-Iran can present with anaemia, poikilocytosis and mild hemolysis [5]. When co-existing with β-Thalassemia, the patient’s clinical symptoms arise due to the thalassemia component and not due to the Hb D-Iran counterpart. One such case has been reported by Agrawal MG et al., where Hb D-Iran was co-existing with β-Thalassemia [14]. In heterozygous form with Hb A (Hb D-Iran trait), the patient is usually clinically silent and goes undetected as is evident from this index case (Table 1). Another case was reported by Gupta et al., where Hb D-Iran was present along with Hb D-Punjab [15]. It should be noted that in all these cases, Hb D-Iran was diagnosed by gene sequencing as was in our patient (Table 2).
Table 2: Molecular genetics - PCR for Thalassemia.

To our knowledge, we reported the first case of co-inheritance of compound heterozygous Hb D Iran with β thalassemia trait in Pakistan. The coinheritance of Hb D Iran with β thalassemia can be found in Pakistani population. Therefore, it is important to investigate patients suspected of haemoglobinopathy in an endemic area who either fail to respond to correction therapy for anaemia or present with clinical features of the disease. In order to prevent and ensure successful control programs of thalassemia and hemoglobinopathy, it is important to screen young couples for genetic before marriage [16].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Firkin F, Chesterman C, Penington D, Rush B (1996) Disorders of hemoglobin structure and synthesis. De Gruchi's Clinical Haematology in Medical Practice, (5th edn) Oxford: Blackwell Science, USA, p. 137-171.
- Baglioni C (1962) Abnormal human hemoglobin. VII. Chemical studies on hemoglobin D. Biochem Biophys Acta 59: 437-449.
- D Desai, H Dhanani, M Shah, N Dayal, A Kapoor, et al. (2003) Homozygous Hemoglobin D Disease: A Case Report. The Internet Journal of Pathology 3(1): 1-4.
- De Marco EV, Crescibene L, Bagalh A, Brancati C, Quatierl A, et al. (1994) Hb D-Iran [B22(B4) Glu->Gln] In Southern Italy. Hemoglobin 18(1): 65-69.
- Thornburg CD, Zimmerman SA, Schultz WH, Ware RE (2001) An infant with homozygous hemoglobin D-Iran. J Pediatr Hematol Oncol 23(1):67-68.
- Mohanty PK (2017) Compound heterozygote of Hb DIran [HBB: c.67G>C, b 22(B4) Glu>Gln] with b0 -thalassemia [cds 41/42 (-CTTT)] from Eastern India. Rev Bras Hematol Hemoter.
- Shabbir S, Nadeem M, Sattar A, Ara I, Ansari S, et al. (2016) Type and frequency of hemoglobinopathies, diagnosed in the area of Karachi, in Pakistan. Cogent Medicine 3(1): 1188875.
- Saleem M, Ahmad PA, Mubarik A, Ahmed SA (1985) Distribution pattern of haemoglobinopathies in northern areas of Pakistan. J Pak Med Assoc 35(4): 106-109.
- Waheed U, Satti HS, Farooq N, Zaheer HA (2012) Frequency of haemoglobinopathies: a single-centre, cross-sectional study from Islamabad, Pakistan. East Mediterr Health J 18(12): 1257-1259.
- Rajashekar K, Harieesha KB, Hemanth M, Karthik JK, Sharmada BK (2017) Evaluation and comparison of bracket slot dimensions of commercially available pre adjusted edgewise appliance – an in-vitro study, International Journal of Current Research 9(04): 48804-48807.
- Qadir M, Amir S (2017) Frequency of beta thalassemia trait in pregnant anemic patients attending Khyber Teaching Hospital, Peshawar-Pakistan. Khyber Med Univ J 9(4): 185-187.
- Itano HA (1951) A Third Abnormal Hemo-globin Associated with Hereditary Hemolytic Anemia. Proc Natl Acad Sci U S A 37(12): 775-784.
- Zakerinia M, Ayatollahi M, Rastegar M, Amanat SH, Askarinejad AR, et al. (2011) Hemoglobin D (Hb D Punjab/Los Angeles and Hb D Iran) and co-inheritance with alpha-and beta-thalassemia in southern Iran. Iranian Red Crescent Medical Journal 13(7): 493-498.
- Agrawal MG, Bhanushali AA, Dedhia P, Jeswani KD, Dayanand S, et al. (2007) Compound heterozygosity of Hb DIran (β22 Glu →Gln) and β0-thalassemia (619 bp-deletion) in India. Eur J Haematol 79(3): 248-250.
- Gupta A, Saraf A, Dass J, Mehta M, Radhakrishnan N, et al. (2014) Compound heterozygous hemoglobin D-Punjab/hemoglobin D-Iran: a novel hemoglobinopathy. Indian J Hematol Blood Transfus 30 Suppl 1: 409-412.
- Panyasai S, Rahad S, Pornprasert S (2017) Coinheritance of hemoglobin D-Punjab and β0-thalassemia 3.4 kb deletion in a Thai girl. Asian journal of transfusion science 11(2): 199-202.
-
Arif Maqsood Ali, Gule Raana Waseem, Shazia Arif. Haemoglobin D Iran With Beta Thalassemia in A Primigravida With Anaemia. A Case. W J Gynecol Women’s Health. 2(4): 2019 WJGWH.MS.ID.000545.
Anaemia, Haemoglobinopathy, Primigravida, Thalassemia, Chronic hemolytic anemia, Diarrhea, Tuberculosis, Parenteral, Splenomegaly, Mutation
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






