 Research Article
Research Article
Correlation Between Pregravid Nutritional Status, Obstetric Nutritional Risk and Maternal Morbidity in High Risk Pregnancy: A Case Control Study
Roberto Anaya-Prado1,2,4*, Michelle Marie Anaya-Fernández2, Roberto Anaya-Fernández2,3, Consuelo Cecilia Azcona-Ramírez2, Jonathan Ulises Martínez-Escobar3, Diana Paulina Ochoa-Yudiche2,4, Ana Paula Cárdenas-Fregoso2,4, Daniela Reyes-González2,4 and Diego Alberto Marín-Esparza2,4
1Direction of Research and Education at Hospital of Obstetrics and Gynaecology; at Western National Medical Centre; the Mexican Institute for Social Security, México
2Division of Research at Centro Médico Puerta de Hierro, México
3School of Medicine at Centro Universitario de Ciencias de la Salud, University of Guadalajara, México
4School of Medicine and Health Sciences, Tecnológico de Monterrey; Guadalajara, Jalisco. México
Roberto Anaya-Prado MD, MSc, PhD, FACS. Blvd, Puerta de Hierro 5150. Edificio B, segundo piso. Despacho 201-B, Fraccionamiento, Corporativo Zapopan, CP. 45110. Zapopan, Jalisco, México.
Received Date:September 12, 2022; Published Date: September 27, 2022
Abstract
Background: Maternal nutrition has been reported to play a critical role in both the development and outcome of pregnancy. But, a causal correlation between obstetric nutrition risk (ONR), pregravid and gravid nutritional status (NS) and pregnancy outcome has not been established. The purpose of this work was to analyze the possible association between ONR, pregravid and gravid NS and maternal morbidity (MM).
Methods: This case control study included 180 pregnant patients admitted for high risk pregnancy (HRP). Patients were allocated in two groups (n = 90 p/group) using the ONR criteria on hospital admission: no nutritional risk group (Group A, ONR score < 3) and nutritional risk group (Group B, ONR score > 3). Study variables included: ONR scores, pregravid and gravid NS and MM.
Results: Average ONR scores were 1.24 ± 0.04 and 3.58 ± 0.07, on admission and 2.63 ± 0.18 and 4.88 ± 0.08 at discharge, on Groups A and B, respectively (p <0.001). Patients were “overweight” and “underweight” in Groups A and B, respectively (p <0.05). Hospital morbidity was identified in 10 (11.11%) and 44 (48.88%) patients in the no nutritional risk and nutritional risk groups, respectively (p <0.05. RR = 2.23; 95% CI 0.36 - 0.81).
Conclusion: There was a positive association between pregravid NS, Obstetric Nutritional Risk and MM in HRP. Nutritionally at-risk patients were underweight, showed a significantly higher morbidity and had longer hospital stays.
Keywords: Malnutrition; Nutritional risk screening; Nutritional status; Undernutrition; High risk pregnancy; Maternal morbidity
Introduction
A high-risk pregnancy (HRP) is one with either an abnormal or pathologic condition, concomitant to gestation or delivery, that threaten the life or health of the mother or fetus [1]. Prevalence of HRP has been reported between 12% and 60%, depending on the country. Some of the risk factors leading to an HRP are woman´s age, nutritional status (diet), diabetes, autoimmune disorders, hypertension, infectious diseases, history of chronic disease and complications in previous pregnancy, multifetal pregnancies and less than 1-year gestational interval [1-3]. A compromised maternal nutrition can result in an adverse outcome of pregnancy and childbirth [4-7]. A clear relationship between maternal malnutrition and adverse pregnancy outcome has been accepted. The consequences are limitless. But, stunting, preeclampsia and increased number of cesarean deliveries are some of them [8-12]. The most important contributing factors to birth weight, in HRP, are stunting and poor weight gain during gestation. Increased nutritional requirements during pregnancy, to maintain fetal growth and maternal metabolism, may further contribute to low weight gain during pregnancy [4,5]. Furthermore, obesity and overweight are additional contributing risk factors to pregnancy outcome. And, prevalence of these two conditions has rocketed in recent years [9,13,14]. The risk of preeclampsia and gestational hypertension increase in the presence of obesity and high body mass index (BMI); because plasma lipids raise significantly during gestation. Women who develop preeclampsia show more considerable lipid changes [13]. It seems likely that women in developing countries are at greater risk of underweight. While, overweight and obesity are more prevalent in women of developed countries and those residing in urban areas with better economic condition [7,15]. Pregnancy complications, cesarean delivery, diabetes, hypertension and prolonged labor have all been associated to overweight and obesity. While stunting and wasting are higher in underweight women [9]. However, there is not a general agreement between Body Mass Index (BMI) measurements and pregnancy outcome. Some reports indicate that a higher BMI is associated to pregnancy complications; while others claim that a lower BMI is closely related to birth outcome. Therefore, maternal underweight, overweight or obesity (malnutrition as a whole) are a threat to maternal and infant health [9,16].
Clinical practice has been affected by the burden of “malnutrition”. It has been demonstrated that undernutrition has a direct impact on surgical complications, wound healing and immune function; which lead to prolonged hospital length of stay (LOS) [17-20]. Nutritional condition can further deteriore during hospital stay, worsen the patient´s outcome and increase health care costs [21-24]. Reports indicate that up to 50% of hospitalized patients are malnourished [25-27]. Therefore, the main concern for many years, has been to diagnose malnutrition, on hospital admission [18,22,23]. Accordingly, a nutritional risk screening tool (NRS- 2002) was developed by J Kondrup. This methodology is able to identify undernourished patients, the risk of developing undernutrition and whether nutritional support can improve the patient´s outcome in the hospital setting [28,29]. NRS-2002 includes MUST (malnutrition universal screening tool) components and a scoring of severity of disease as an evidence of an increased metabolic rate [7-19,22,28,29] proper nutritional screening has already been documented in patients with different pathologic conditions; such as, cancer, hip fractures, heart and lung diseases [21,22]. Therefore, screening for nutritional risk (NR), at hospital admission, is highly advisable [30-35].
Nutritional status has been associated with the occurrence of numerous diseases [17-20]. Actually, maternal nutrition has a critical role in both the development and outcome of pregnancy [4]. In recent years, the main issue has been to screen for nutritional status (NS) of the hospitalized patient. The idea has been to identify undernourished patients and those at increased risk of malnutrition. But, the lack of evidence about the possible link between obstetric nutritional risk (ONR) and pregravid nutritional status, in the obstetric patient, justified the inclusion of a protocol in patients admitted for HRP. The benefits of NR screening in the obstetric patient with HRP have recently been documented [36]. A higher hospital morbidity was demonstrated in HRP patients who were nutritionally at-risk. Furthermore, their nutritional status worsened during hospital stay. Therefore, individual awareness about the adverse effects of malnutrition might reduce the number of pregnancy complications. Unfortunately, BMI is not stable over time. Yet, current recommendation is to record BMI either before pregnancy or in the first trimester [9]. Still, the main concern is whether preconception nutritional status remains the same throughout pregnancy. Accordingly, a causal correlation between pregravid BMI, obstetric nutritional Risk (ONR) and pregnancy outcome has not been established [37]. The purpose of this study was to analyze the possible association between ONR, pregravid and gravid nutritional status (NS) and maternal morbidity (MM).
Material and Methods
In this case control study, we included 180 pregnant patients admitted for High Risk Pregnancy (HRP) to Hospital of Obstetrics and Gynaecology at Western National Medical Centre, Mexican Institute of Social Security (IMSS). Patients were distributed in two groups (n = 90 p/group) using the Obstetric Nutritional Risk (ONR) Screening criteria reported by Anaya-Prado R et al.: no obstetric nutritional risk group (Group A, ONR score < 3) and obstetric nutritional risk group (Group B, ONR score > 3).(36) Groups were then compared to identify possible association between ONR status (on hospital admission), pregravid and gravid NS and maternal morbidity. Study variables included: ONR screening score, pregravid and on admission BMI (nutritional status) and weight, maternal age, gestation weeks, height, hospital length of stay (LOS) and maternal morbidity. This study included all adult HRP pregnant patients (> 18 y/o) who stayed for a minimum of 3 days in the department of HRP (Figure 1).
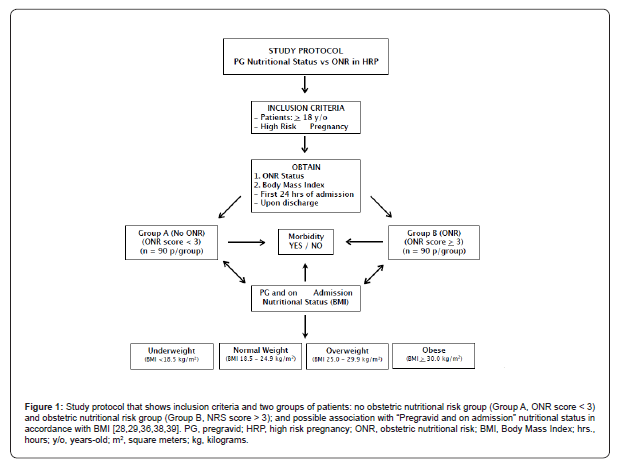
Study design
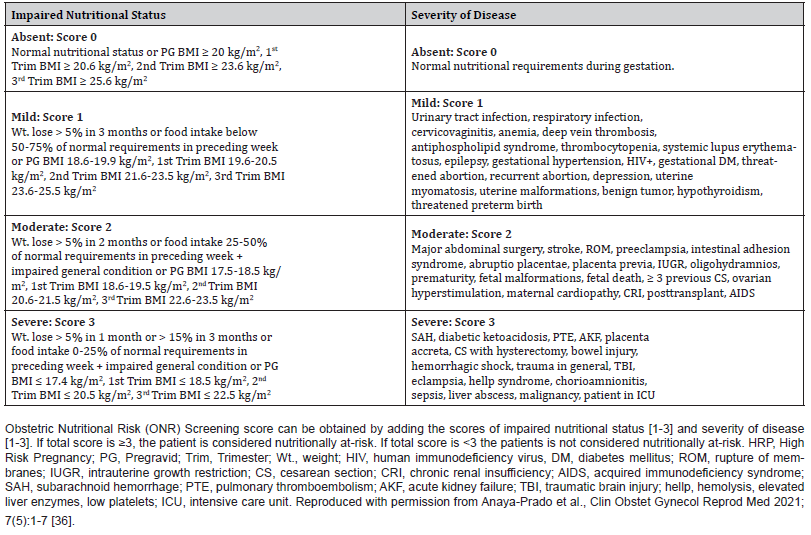
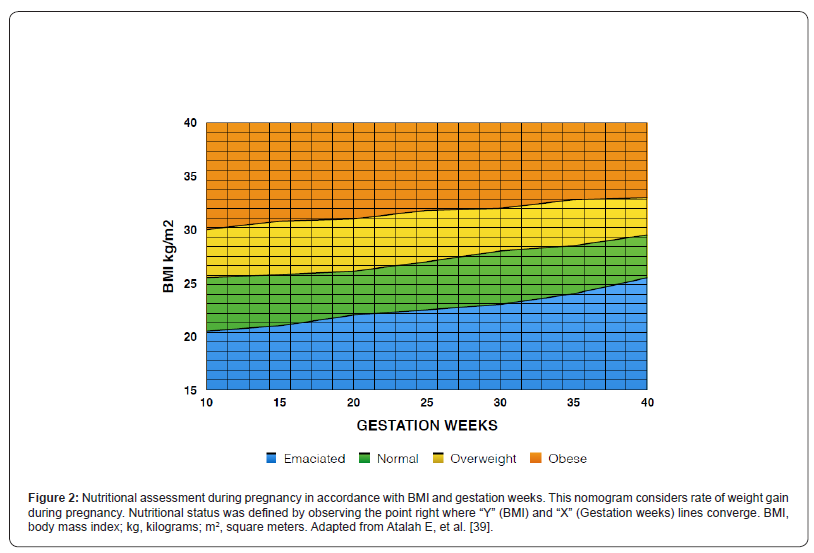
HRP patients were assessed by the group of investigators at the first 24 hours of admission about their Obstetric nutritional risk status in accordance with ONR screening criteria (Table 1). Thereafter, ONR screening was performed upon discharge. Accordingly, every patient received an ONR screening score based on two main categories: nutritional status and disease severity. Nutritional Risk Screening methodology, described by Kondrup et al., and adapted for the obstetric patient with HRP by Anaya-Prado R, et al., has previously been described [28,29,36]. Briefly, the ONR score (0 - 6) was obtained by adding nutritional status score (0 - 3) and disease severity score (0 -3). A total score > 3 was considered nutritionally at-risk. Nutritional status was scored as absent, mild, moderate and severe (0 - 3) based on three different variables: a) changes in estimated food intake, measured in quartiles; b) changes in body weight within the last 1 - 3 months, measured in percentage of body weight loss, and; c) changes in BMI, measured in kg/m2. Gestational weight gain (GWG) by trimester and pregravid BMI status according to the World Health Organization (WHO) categories: Underweight < 18.5 kg/m2; Normal weight 18.5 - 24.9 kg/m2; Overweight 25.0 - 29.9 kg/m2; Obese > 30 kg/m2, were considered for BMI range in different scores. Thus, BMI was categorized in accordance with gestational age, GWG by trimester and pregravid BMI (Figure 2) [38,39]. The disease severity score was categorized as absent, mild, moderate and severe (0-3) based on admission diagnosis. Table 1 summarizes how ONR scores were calculated to categorize patients in either group: not at-risk or nutritionally at-risk.
Table 1: Obstetric Nutritional Risk (ONR) Screening Scores in HRP Patients.
Ethics
The protocol was submitted and approved by the Local Hospital Ethics and Research Committee and all information and patient data were handled and processed by the investigators, ensuring confidentiality at all times. Even though this investigation adhered to principles of good clinical practice and that the risk was less than the minimum, informed consent was obtained, and all patients agreed to participate in this study.
Statistical analysis
Outcome variables are presented in raw numbers or percentages. For qualitative variables, the Pearson´s correlation and Chi2 test, with Yates correction, or Fisher exact test were utilized; and results are presented in percentages and proportions. Quantitative variables are expressed as mean ± standard deviation of the mean (SDM), compared by Student’s t test for independent samples and results are reported on averages. The Mann-Whitney rank sum test was applied when normality test failed. Data were analyzed by one-way analysis of variance (ANOVA) procedure, followed by the Student-Newman-Keuls’ test to determine differences between individual means. Turkey test and Dunn method were also utilized for paired multiple comparisons. The analysis was performed using SigmaStat® (release 4.0), SPSS (release 8.0) and SAS (release 6.12). A P value equal to or less than 0.05 was considered statistically significant.
Results
Average gestation age, maternal age, height and length of stay was 31.8 ± 7.5 and 31.3 ± 8.3 weeks (p =0.181); 29.6 ± 0.6 and 27.7 ± 0.6 years (p =0.036); 1.58 ± 0.06 and 1.64 ± 0.03 meters (p <0.001); and 4.78 ± 0.30 and 7.44 ± 0.46 days (p <0.001) for the not at-risk and nutritionally at-risk groups, respectively (Groups A and B). Difference between the two groups was statistically significant, except for gestation age (Table 2).
Anthropometric measurements
Average prepregnancy, on admission and at discharge body weight was 66.8 ± 13.05, 76.6 ± 12.32, and 73.6 ± 12.39 kg (p <0.001); and 49.5 ± 4.13, 58.7 ± 5.0 and 54.1 ± 4.4 kg (p <0.001) for the not at-risk and nutritionally at-risk groups, respectively (Groups A and B). Average prepregnancy, on admission and at discharge BMI was 26.5 ± 4.64, 30.3 ± 4.21 and 29.2 ± 4.34 kg/m2 (p <0.001); and 18.0 ± 1.07, 21.4 ± 1.43 and 19.8 ± 1.35 kg/m2 (p <0.001) for Groups A and B, respectively. All pair-wise multiple comparisons (Dunn Method) demonstrated a statistical significant difference (p <0.05), except when comparing weight and BMI on admission vs discharge in the no nutritional risk group; and when comparing BMI on pregestation vs discharge in the not at-risk and nutritionally at-risk groups, respectively (Table 2, Figures 3A,3B).
Table 2: ONR Screening in HRP and Interval Anthropometric Measurements.
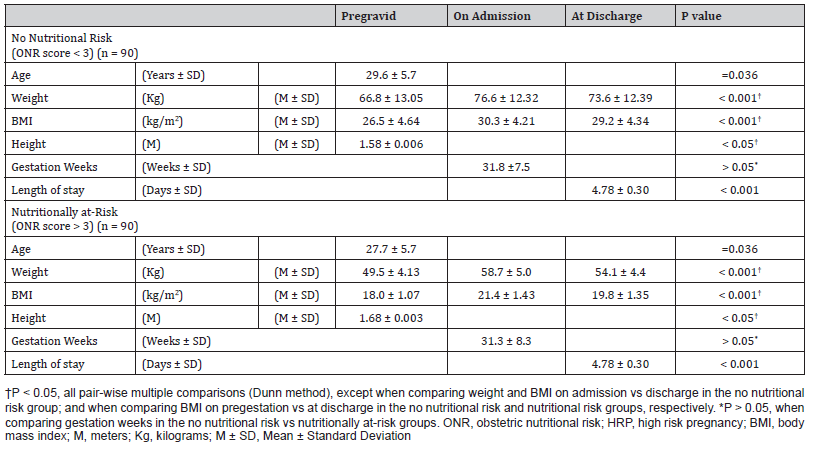
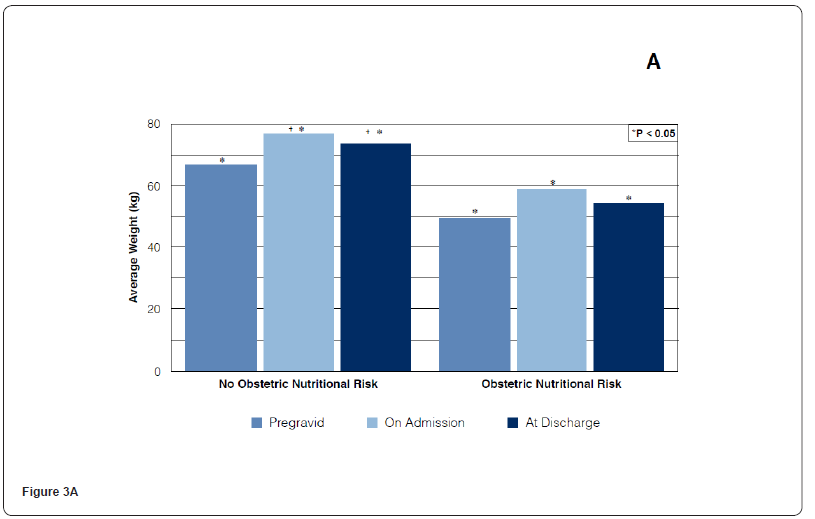
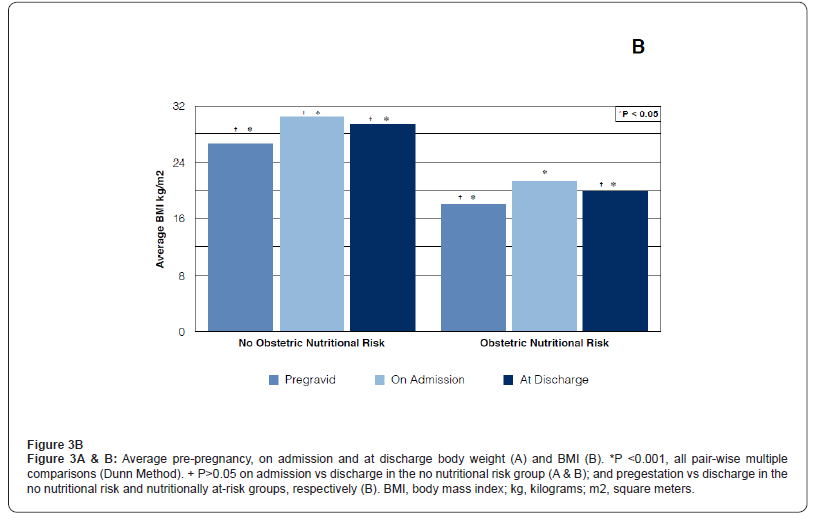
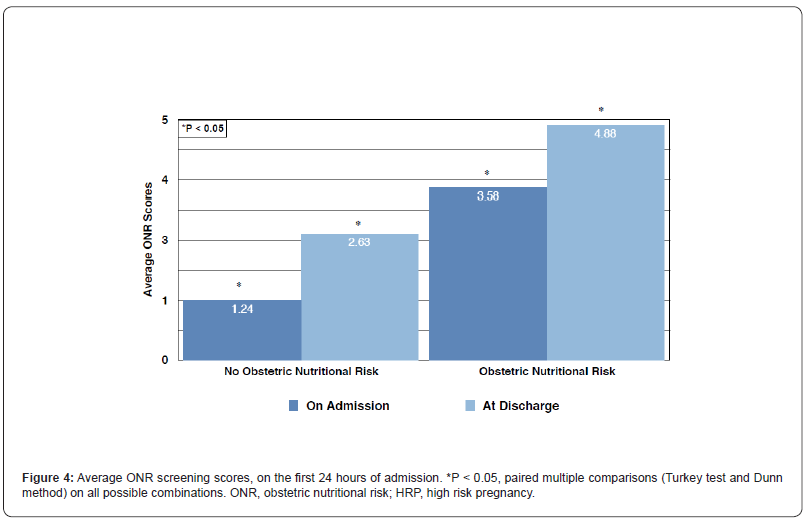
In accordance with “Pregravid” and “Gravid (on admission)” BMI measurements, patients were “overweight” and “underweight” in the no ONR and the ONR groups, respectively (Groups A and B). Difference between both groups was statistically significant (p <0.05). That is, Pregravid nutritional status remained the same throughout pregnancy, up to hospital admission (Table 2). Average ONR screening scores were 1.24 ± 0.04 and 3.58 ± 0.07, on admission and 2.63 ± 0.18 and 4.88 ± 0.08 at discharge, for the “not at-risk and nutritionally at-risk” groups, respectively (Figure 4). Comparison of on admission and at discharge median scores were significantly different (p <0.001) in both groups (Kruskal-Wallis ANOVA on ranks). All pair-wise multiple comparisons: on admission vs discharge and no nutritional risk vs nutritionally at-risk demonstrated a statistically significant difference (Turkey test and Dunn method). Additionally, thirty-three percent (n =30) of the patients in the “no nutritional risk” group became “nutritionally at-risk when screened at discharge (p <0.03). Similarly, 73.33% (n = 66) of the patients identified as “nutritionally at-risk” on admission, developed higher scores when screened at discharge (p <0.05). Hospital morbidity was observed in 10 (11.11%) and 44 (48.88%) patients in the no nutritional risk and nutritional risk groups, respectively (Figure 5). The difference between the two groups was statistically significant (p <0.05. RR = 2.23; 95% CI 0.36 - 0.81). There was a positive association between pregravid and on admission nutritional status (underweight), obstetric nutritional risk and hospital morbidity (p <0.05, X2 = 30.58).
Discussion
In this study, we were able to demonstrate a positive association between pregravid nutritional status, obstetric nutritional risk (on hospital admission) and maternal morbidity in HRP patients. In accordance with pregravid and gravid BMI, nutritionally at-risk patients were underweighted and showed a significantly higher morbidity. While, not at-risk patients were, on average, obese. This shows that maternal nutritional status remained the same from preconception up to delivery, in both groups. Our main concern was to identify whether obstetric nutritional risk (ONR) was linked to either pregravid or gravid nutritional condition (BMI). The importance of screening NR on hospital admission has been shown in different specialties, such as cancer patients and those being treated for heart and lung disease [28-30,34,35,40,41-45]. And, recent evidence demonstrates that ONR has a negative effect in morbidity on HRP patients [36]. The development and outcome of pregnancy can be affected by maternal nutrition [4]. Actually, NS has been associated to the occurrence of numerous diseases [17- 20]. The global prevalence of maternal undernutrition and its short and long-term consequences, in low and middle-income countries, has already been documented [8,11,12]. For some years now, the issue has been to screen for malnutrition of the hospitalized patient. Identifying malnourished patients, on hospital admission, may help structure adequate nutritional plans; so, in-hospital morbidity and mortality can be lowered.
Although it is believed that there is a physiologic adaptation (hypothesis of nutritional fetal origins) to nutritional status during pregnancy; nutritional requirements increase in order to cope with the needs of fetal growth [4-7,46,47]. Some studies suggest that giving nutritional supplements to pregnant women might have a positive impact on adult life of their offspring’s. However, improving nutritional status during pregnancy has not demonstrated a direct impact on offspring outcome [46]. Furthermore, a link between reduced birthweight and poor fetal growth or stunting has been accepted [8,11,12]. It has been argued that a baby of low birthweight (which may be the consequence of poorer nutrition in utero), who is stunted and underweight in early childhood and gains weight rapidly, can suffer from cardiovascular and metabolic diseases in adult life [46]. Actual consequences of poor nutritional status during pregnancy reported by these observational studies. So, maternal undernutrition has a real impact on outcome for both the mother herself and her offspring [4,8,11,12]. Thus, the risk of malnutrition with its consequences, in the hospital setting, is latent and pregnant women are not the exception [27].
Following BMI measurements, we observed that nutritionally at-risk patients were underweight; while not at-risk patients were obese. We also noticed that HRP patients, who were nutritionally at-risk, had significantly longer hospital stays, as compared to patients who were in the no nutritional risk group. Thus, screening for ONR at hospital admission should be performed on HRP patients, as suggested for other conditions. Some studies have reported Mini Nutritional Assessment (MNA) questionnaire to be superior to NRS-2002 in predicting hospital length of stay (LOS). Nonetheless, other reports indicate that NRS-2002 is a better predictor of hospital full length than MNA, Subjective Global Assessment (SGA) and Malnutrition Universal Screening Tool (MUST) [17,47,49]. Irregardless of the screening tool, a significant association between nutritionally at-risk and increased LOS has been accepted by different studies [21,44]. Actually, previous investigations have demonstrated that protein-energy malnutrition is a strong and independent risk factor associated with morbidity, mortality, prolonged LOS and higher complication rates, including infections [50-52]. Fasting, loss of appetite, depression, along with a poor hospital diet may further compromised NS. This might as well explain why, in our study, ONR screening scores worsened during hospital stay, in both groups, when screened at discharge. Most patients are fasted on hospital admission; which is a common practice in many hospitals. These results demonstrate that ONR is a useful tool to identify HRP patients with either poor or good nutritional condition. Whether nutritionally at-risk HRP patients might benefit from some type of nutritional support, remains to be investigated. Thus, our results are consistent with those previously reported by others; but, in conditions different than pregnancy [17,21,53].
As stated by Kondrup, the goal of assessing NR, at hospital admission, has been to anticipate the possibility of a worse or better outcome due to nutritional conditions and whether nutritional support can benefit patients´ outcome [28,29]. Therefore, in an effort to demonstrate that nutritional support lowers morbidity and mortality in nutritionally at-risk patients, a great deal of research has been done in different scenarios (pathologies). In a large cross-sectional study performed by Skeie, et al., in mixed surgical patients, a positive association between nutritional risk and incidence of surgical site infection (SSI) was demonstrated [54]. Among the questions utilized to define NR, both reduced dietary intake and weight loss were the strongest associated with infection in this study. Furthermore, nutritionally at-risk patients were older, had urgent surgeries, had lower BMI and tended to have higher ASA scores [54]. In our study, lower BMI (underweight) was associated with ONR and higher morbidity. Our results are consistent with other reports that indicate higher odds of stunting and wasting among underweight women [9]. In our study, only one patient in the nutritionally at-risk group developed sepsis. However, chorioamnionitis, preeclampsia, and Hellp syndrome were the most frequent complications reported in the nutritionally at-risk cohort. The role of maternal diet in the aetiology of preeclampsia has recently received significant attention. Current knowledge, in non-pregnant patients, indicates that specific nutrients may be involved in some steps in the pathogenesis of preeclampsia. It has also been suggested that nutrients such as trace elements, fatty acids and folic acid can contribute to insulin resistance which is a key risk factor for preeclampsia [10]. Authors argue that maternal nutritional imbalance can lead to altered gene expression and methylation and homocysteine metabolism and to an increased inflammatory response with oxidative stress.
In order to lower morbidity and mortality, as well as the consequences of a prolonged hospital stay, proper nutritional interventions should be instituted on nutritionally at-risk patient [21,22]. Since food is commonly utilized to treat nutritional deficiencies within the hospital setting, Munk et al. compared an energy-enriched food menu versus a standard hospital food menu [55]. These authors found a significant positive impact on overall protein intake and on weight-adjusted energy intake in nutritionally at-risk patients. Thus, increasing protein and energy intake in hospitalized patients is feasible through hospital food menus. As suggested, NR screening can help tailor some type of nutritional support which will certainly improve the outcome of the hospitalized patient. In our hospital, ONR is not performed nor does nutritional support is indicated on a regular basis. That is, nutritional support is indicated only whenever there is a complication. Possible explanations, as recognized by others, are lack of nutritional education, undefined responsibilities and time of the healthcare personnel [21,44,56]. Nevertheless, we acknowledge the need for an adequate nutritional screening and therapy in order to lower maternal morbidity and mortality as well as to improve pregnancy outcome.
Since we found a direct association between pregravid, gravid and “obstetric nutritional risk status”; maternal weight management is highly advisable to prevent adverse mother and birth outcomes. The importance of this association relies on how well we provide appropriate prenatal care. It has been demonstrated that good prenatal care is associated with decreased maternal mortality, preterm birth, neonatal death and stillbirth [57]. Nonetheless, clinicians should be aware that even with the best care approach, HRP patients will still be at risk of poor neonatal outcome. So, the goal should be aimed at reaching a term pregnancy with a not at-risk nutritional status, on hospital admission; thereby, an improved maternal and childbirth outcome. Our results indicate that prenatal care is somehow missing maternal weight control; since nutritionally at-risk patients were underweight all along pregnancy. Controlling weight may significantly reduce the number of pregnancy complications. An acceptable maternal nutritional status might as well improve pregnancy outcome; thereby reducing the incidence of HRP. Identifying patients who are nutritionally at-risk, on hospital admission, will surely help us tailor some type of nutritional plan. However, every effort should be aimed at counselling women prior to or early in pregnancy, in order to avoid underweight, overweight or obesity. Informed women may as well try to optimize their BMI before conception. Whether HRP patients, who are nutritionally atrisk, will benefit from some type of nutritional support remains to be elucidated. Though, it has been demonstrated that outcome improves when nutritionally at-risk non-obstetric patients are properly treated [58]. Therefore, ONR screening should be implemented on HRP patients, not only to lower morbidity and mortality; but to identify whether perinatal outcome can also be improved. It can be presumed that, there would be a positive effect on hospital LOS and costs, should proper nutritional therapy be instituted. Further investigation will be necessary to demonstrate this hypothesis. Finally, HRP has been associated to perinatal morbidity and mortality; however, the impact of ONR on neonatal outcome remains to be investigated [59].
Acknowledgement
None.
Conflict of Interest
Authors declare no conflict of interest.
References
- Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, et al. (2018) Williams Obstetrics. 25th (edn), New York. McGraw-Hill, USA.
- Farajnezhad F, Shaahmadi F, Fashi Z, Daaylar L (2017) Prevalence of high risk pregnancy and some relevant factors in referred women to health centers. Journal of Scientific Achievements 2(12): 4-7.
- Pasdar Y, Heidari N, Safari Y, Safari Faramani R, et al. (2012) Prevalence of Some Risk Factors in Pregnant Women. Iranian Journal of Obstetrics, Gynecology & Infertility 15(21): 14-23.
- Bianchi CM, Mariotti F, Verger EO, Huneau JF (2016) Pregnancy requires major changes in the quality of the diet for nutritional adequacy: Simulations in the French and the United States populations. PLoS One 11(3): 1-17.
- Christian P, Stewart CP (2010) Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr 140(3): 437-445.
- Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, et al. (2012) Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr 96(5): 1032-1041.
- Bath SC, Steer CD, Golding J, Emmett P, Rayman MP (2013) Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382(9889): 331-337.
- Khanam R, Lee ASC, Ram M, Quaiyum M, Begum N, et al. (2018) Levels and correlates of nutritional status of women of childbearing age in rural Bangladesh. Public Health Nutr 21(16): 3037-3047.
- Khan MN, Rahman MM, Shariff AA, Rahman MM, Rahman MS, et al. (2017) Maternal undernutrition and excessive body weight and risk of birth and health outcomes. Arch Public Health 75: 12.
- Xu H, Shatenstein B, Luo ZC, Wei S, Fraser W (2009) Role of nutrition in the risk of preeclampsia. Nutr Clin Care 67(11): 639-657.
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, et al. (2008) Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371(9609): 340-357.
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, et al. (2013) Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382(9809): 427-451.
- Samur G, Akkus ÖÖ, Ede G, Ayaz A, Akyol A, et al. (2016) Nutritional status among women with preeclampsia and healthy pregnant women. Proper Nutr 18(4): 360-368.
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 348(9945): 766-781.
- Jayawardena R, Byrne NM, Soares MJ, Katulanda P, Hills AP (2013) Prevalence trends and associated socio-economic factors of obesity in South Asia. Obes Facts 6(5): 405-414.
- Rahman MM, Abe SK, Kanda M, Narita S, Rahman MS, et al. (2015) Maternal body mass index and risk of birth and maternal health outcomes in low-and middle-income countries: a systematic review and meta-analysis. Obes Rev 16(9): 758-770.
- Budzyński J, Tojek K, Czerniak B, Banaszkiewicz Z (2016) Scores of nutritional risk and parameters of nutritional status assessment as predictors of in-hospital mortality and readmissions in the general hospital population. Clin Nutr 35(6): 1464-1471.
- Koren-Hakim T, Weiss A, Hershkovitz A, Otzrateni I, Anbar R, et al. (2016) Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin Nutr 35(5): 1053-1058.
- van Bokhorst-de van der Schueren MA, Guaitoli PR, Jansma EP, de Vet HC (2014) Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr 33(1): 39-58.
- Ulltang M, Vivanti AP, Murray E (2013) Malnutrition prevalence in medical assessment and planning unit and its association with hospital readmission. Aust Health Rev 37(5): 636-641.
- Khalarbari-Soltani S, Marques-VIdal P (2016) Impact of nutritional risk screening in hospitalized patients on management, outcome and costs: A retrospective study. Clin Nutr 35(6): 1340-1346.
- Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Kristensen H, et al. (2004) Prevalence of patients at nutritional risk in Danish hospitals. Clin Nutr 23(5): 1009-1015.
- Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krahenbuhl L, et al. (2008) EuroOOPS: an international, multicenter study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr 28(3): 340-349.
- Jie B, Jiang ZM, Nolan MT, Efron DT, Zhu SN, et al. (2010) Impact of nutritional support on clinical outcome in patients at nutritional risk: a multicenter, prospective cohort study in Baltimore and Beijing teaching hospitals. Nutrition 26(11-12): 1088-1093.
- Waitzberg DL, Caiaffa WT, Correia MITD (2001) Hospital malnutrition: the Brazilian national survey (IBRANUTRI): a study of 4000 patients. Nutrition 17(7-8): 573-580.
- Calleja A, Vidal A, Cano I, Ballesteros M (2014) Malnutrition in hospitalized patients receiving nutritionally complete menus: prevalence and outcomes. Nutr Hosp 30(6): 1344-1349.
- Knudsen AW, Naver A, Bisgaard K, Nordgaard-Lassen I, Becker U, et al. (2015) Nutrition impact symptoms, handgrip strength and nutritional risk in hospitalized patients with gastrointestinal and liver diseases. Scand J Gastroenterol 50(10): 1191-1198.
- Kondrup J, Allison SP, Elia M, Vellas B, Plauth M (2003) ESPEN Guidelines for nutrition Screening 2002. Clin Nutr 22(4): 415-421.
- Kondrup J, Rasmussen HH, Hamberg O, Stanga Z (2003) Nutritional Risk Screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 22(3): 321-336.
- Zanetti M, Cappellari GG, Ratti C, Ceschia G, Murena L, et al. (2019) Poor nutritional status but not cognitive or functional impairment per se independently predict 1 year mortality in elderly patients with hip fracture. Clin Nutr 38(4): 1607-1612.
- Shan X, Liu J, Luo Y, Xu X, Han Z, et al. (2015) Relationship between nutritional risk and exercise capacity in severe chronic obstructive pulmonary disease in male patients. Int J Chron Obstruct Pulmon Dis 10(1): 1207-1212.
- Bloomer RJ (2007) The role of nutritional supplements in the prevention and treatment of resistance exercise-induced skeletal muscle injury. Sports Med 37(6): 519-532.
- Hoppeler H, Weibel ER (2000) Structural and functional limits for oxygen supply to muscle. Acta Physiol Scand 168(4): 445-456.
- Illa P, Tomiskova M, Skrickova J (2015) Nutritional risk screening predicts tumor response in lung cancer patients. J Am Coll Nutr 34(5): 425-429.
- Bodan M, Laviano A, Persic V, Rotim A, Jovanovic Z, et al. (2014) Characteristics of NRS-2002 nutritional risk screening in patients hospitalized for secondary cardiovascular prevention and rehabilitation. J Am Coll Nutr 33(6): 466-473.
- Anaya-Prado R, Torres-Mora LV, Anaya-Fernández MM, Anaya-Fernández R, Izaguirre-Pérez ME, et al. (2021) Obstetric Nutritional Risk Screening in High Risk Pregnancy and its Association with Maternal Morbidity: A Prospective Cohort Study. Clin Obstet Gynecol Reprod Med 7(5): 1-7.
- Chen Z, Du J, Shao L, Wu M, Ai M, et al. (2010) Prepregnancy body mass index, gestation weight gain, and pregnancy outcome in China. Int J Gynaecol Obstet 109(1): 41-44.
- Rasmussen KM, Yaktine AL (eds) ( 2009) Weight gain during pregnancy: Reexamining the Guidelines, Washington, DC: The National Academy Press.
- Atalah E, Castillo C, Castro R, Aldea A (1997) Proposal of a new standard for nutritional assessment of pregnant women. Rev Med Chil 125(12): 1429-1436.
- Naber TH, Schermer T, de Bree A, Nusteling K, Eggink L, et al. (1997) Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr 66(5): 1232-1233.
- Mc Whirther JP, Pennington CR (1994) Incidence and recognition of malnutrition in hospital. BMJ 308(6934): 945-948.
- Edington J, Boorman J, Durrant ER, Perkins A, Giffin CV, et al. (2000) Prevalence of malnutrition on admission to four hospitals in England. The malnutrition prevalence group. Clin Nutr 19(3): 191-195.
- Rasmussen HH, Kondrup J, Ladefoged K, Staun M (1999) Clinical nutrition in Danish hospitals: A questionnaire based investigation among doctors and nurses. Clin Nutr 18(3): 153-158.
- Kondrup J, Johansen H, Plum LM, Bak L, Hojlund L, et al. (2002) Incidence of nutritional risk and causes of inadequate nutritional care in hospitals. Clin Nutr 21(6): 461-468.
- Kelly IE, Tessier S, Cahill A, Morris SE, Crumley A, et al. (2000) Still hungry in hospital: identifying malnutrition acute hospital admissions. QJM 93(2): 93-98.
- Macleod J, Tang L, Hobbs FDR, Wharton B, Holder R, et al. (2013) Effects of nutritional supplementation during pregnancy on early adult disease risk: Follow up of offspring of participants in a randomized controlled trial investigating effects of supplementation on infant birth weight. PLoS One 8(12): e83371.
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, et al. (2004) Developmental plasticity and human health. Nature 430(6998): 419-421.
- Bauer JM, Vogl T, Wicklein S, Trögner J, Mühlberg W, et al. (2005) Comparison of the mini nutritional assessment, subjective global assessment, and nutritional risk screening (NRS 2002) for nutritional screening and assessment in geriatric hospital patients. Z Gerontol Geriatr 38(5): 322-327.
- Martins CPAL, Correia JR, Do Amaral TF (2005) Undernutrition risk screening and length of stay of hospitalized elderly. J Nutr Elder 25(2): 5-21.
- Merker M, Gomes F, Stanga Z, Schultz P (2019) Evidence-based nutrition for the malnourished, hospitalized patient: one bite at a time. Swiss Med Wkly 149: w20112.
- Felder S, Lechtenboehmer C, Bally M, Fehr R, Deiss M, et al. (2015) Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition 31(11-12): 1385-1393.
- Felder S, Braun N, Stanga Z, Kulkarni P, Faessler L, et al. (2016) Unraveling the link between malnutrition and adverse clinical outcomes: Association of acute and chronic malnutrition measures with blood biomarkers from different pathophysiological states. Ann Nutr Metab 68(3): 164-172.
- Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, et al. (2012) Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr 31(3): 345-350.
- Skeie E, Koch AM, Harthug S, Fosse U, Sygnestveit K, et al. (2018) A positive association between nutritional risk and the incidence of surgical site infections: A hospital-based register study. PLoS One 13(5): e0197344.
- Munk T, Beck AM, Holst M, Rosenbom E, Rasmussen HH, et al. (2014) Positive effect of protein-supplemented hospital food on protein intake in patients at nutritional risk: a randomized controlled trial. J Hum Nutr Diet 27(2): 122-132.
- Khalatbari-Soltani S, Marques-Vidal P (2018) Adherence to hospital nutritional status monitoring and reporting guidelines. PLoS One 13(9): e0204000.
- Dayal S, Fogel J, Griggs R (2022) Adequacy of prenatal care and stillbirth. Minerva Obstet Gynecol 74(1): 68-74.
- Rasmussen HH, Holst M, Kondrup J (2010) Measuring nutritional risk in hospitals. Clin Epidemiol 2: 209-216.
- Cao J, Peng L, Li R, Chen Y, Li X, et al. (2014) Nutritional risk screening and its clinical significance in hospitalized children. Clin Nutr 33(3): 432-436.
- Zhu X, Niu H, Wang H, Li X, Qi T, et al. (2019) High risk pregnancy associated perinatal morbidity and mortality: a second with population-based survey in Huai’an in 2015. BMC Pregnancy and Childbirth 19(1): 224.
-
Roberto Anaya-Prado*, Michelle Marie Anaya-Fernández, Roberto Anaya-Fernández, Consuelo Cecilia Azcona-Ramírez, Jonathan Ulises Martínez-Escobar, Diana Paulina Ochoa-Yudiche, Ana Paula Cárdenas-Fregoso, Daniela Reyes-González and Diego Alberto Marín-Esparza. Correlation Between Pregravid Nutritional Status, Obstetric Nutritional Risk and Maternal Morbidity in High Risk Pregnancy: A Case Control Study. W J Gynecol Women’s Health. 6(1): 2022. WJGWH.MS.ID.000626.
Malnutrition, Nutritional risk screening, Nutritional status, Undernutrition, High risk pregnancy, Maternal morbidity
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






