 Research Article
Research Article
A Nomogram for Prediction of Risk Factors for Preeclampsia during Antenatal Care at a Tertiary Maternity Hospital
Shaimaa A Khalaf1, Elham M Ahmed2, Rabaa H Hassanen1, Ahmed M Abbas3* and Ahmed A Youssef3
1Community Health Nursing Department, Faculty of Nursing, Assiut University, Egypt
2Nursing specialist, Faculty of Medicine and Health Sciences, Hodeida University, Yemen
3Obstetrics and Gynaecology Department, Faculty of Medicine, Assiut University, Egypt
Ahmed M Abbas, Department of Obstetrics and Gynaecology, Assiut University, Women Health Hospital, Egypt.
Received Date: April 09, 2020; Published Date: April 27, 2020
Abstract
Objective: The study aims to create a nomogram for prediction of risk factors for preeclampsia (PE) during antenatal care (ANC) in a tertiary maternity hospital.
Materials and Methods: A cross-sectional study was conducted between May 2016 and December 2017 in a tertiary maternity hospital. Two hundred thirty pregnant women were included, at first visit, personal data, family history of risk factors for PE, maternal medical, and obstetric history was collected. Physical examination, including blood pressure, weight, signs of edema, and urine analysis were done. Then follow up at 24 weeks and after 32 weeks gestation to know if she developed PE or not through the physician. Included nomogram, which was built based on the data of regression analysis, was used to predict the value of one or more responses from a set of predictors.
Results: The study included 230 women. Cases diagnosed with PE during all the follow up are 37 cases (16.1%). Five factors were not significant; maternal age (P=0.154, OR=1.076), consanguinity (P=0.821, OR=1.104), age at marriage (P=0.266, OR=1.404), age at first pregnancy (P=0.319, OR=0.735) and order of pregnancy (3rd or more) (P=0.951, OR=0.984). Only two factors significant; a history of diabetes mellitus (P=0.010, OR=5.923) and history of hypertension (P=0.045, OR=7.838). Probability of PE based on the finding of the nomogram was 68% with good discrimination.
Conclusion: History of diabetes mellitus and hypertension were the predictors in the final model among pregnant women for the development of preeclampsia.
Keywords: Prediction; Preeclampsia; Risk factors; Nomogram
Introduction
Hypertensive disorders of pregnancy are one of the leading causes of maternal and infant morbidity and mortality. Worldwide, hypertensive disorders of pregnancy affect 5-10% of all pregnancies and cause approximately 50,000 deaths among women every year [1]. The incidence of preeclampsia (PE) is influenced by parity, racial, genetic predisposition, and environmental factors may also have a role. The incidence of PE varies greatly worldwide. World Health Organization (WHO) estimates the incidence of PE to be seven times higher in developing countries (2.8% of live births) than in developed countries (0.4%) which is due to poor healthseeking behaviours and un-availability of health care facilities and personnel [2,3].
Maternal mortality due to PE varies between (2-30%) and is much higher in rural areas. In Egypt, the prevalence of PE is (10.7%) in a community-based study while, in hospital-based studies ranged from (9.1-12.5%) of all deliveries [1,4,5].
Prevention of PE may be primary, secondary. Primary prevention involves avoiding pregnancy in women at high risk for PE, modifying lifestyles or improving nutrients intake in the whole population to decrease the incidence of the disease. Therefore, probably most of the cases of PE are unpreventable. Secondary prevention is based on interruption of known pathophysiological mechanisms of the disease before its establishment. Recent efforts have focused on the selection of high-risk women and have proposed an effective intervention, as early as it is possible, to avoid the disease or its severe complications [6].
The aim of the study was to create a nomogram for prediction of PE causing risk factors during antenatal care at a tertiary maternity hospital.
Patients and Methods
A cross-sectional study was conducted at Antenatal Care Clinic (ANC) in Assiut Women Health Hospital. This clinic is the main largest clinic in Assiut Governorate which provides antenatal care services for pregnant women.
A convenience sampling of pregnant women who attended at ANC for six months period from the beginning of May 2016 till the end of December 2016 and follow up waves ended in (March 2017). The total number of the study sample composed of 230 pregnant women was included and continued until the end of the study. All pregnant women who agree to participate in the study were included if gestational age was from 4th to 18th weeks and without mental disorders.
Two tools were utilized in the current study:
Tool 1
Structured interview questionnaire developed after reviewing the literature and previous research which were relevant to the present study, it included the following (3) parts:
Part 1: included the following:
1. Personal data scale which included: Age, name, telephone number, level of education, occupation …etc.
2. Family history of risk factors for PE such as previous PE, a family history of (diabetes mellitus, chronic hypertension, chronic kidney disease, cardiovascular diseases, thrombophilia, lupus, and smoking).
3. Maternal medical and obstetric history such as Gestational age at the beginning of the current study, consanguinity, age at marriage, age at first pregnancy, history of (preeclampsia, hypertension, diabetes mellitus.etc.).
Part 2
Physical examination of pregnant women, including (blood pressure, weight, signs of edema, and urine analysis).
During the first contact with the women that were enrolled, the physical examination (blood pressure, urine analysis, and signs of edema) was done.
Part 3
It included following up the pregnant women through three waves after the first contact with the pregnant women (4:18 weeks):
1st wave of follow up:
At the (24th) weeks of gestation, the pregnant women conducted phone calling to know the result of (blood pressure, urine analysis) and know if she diagnosed with PE or not through the physician. If the pregnant woman is diagnosed with PE will not be followed through the second wave.
2nd wave of follow up:
Before the (32th) weeks of gestation, the pregnant women conducted phone calling to know the result of (blood pressure, urine analysis) and know if she diagnosed with PE or not through the physician. If the pregnant woman is diagnosed with PE will not be followed through the third wave.
3rd wave of follow up:
After (32th) weeks, the pregnant women conducted phone calling to the result of (blood pressure, urine analysis) and know if she diagnosed with PE or not through the physician.
Tool 2
Included nomogram, which was built based on the data of regression analysis, was used to predict the value of one or more responses from a set of predictors.
The collected data were reviewed, prepared for computer entry, coded categorized, analyzed and tabulated. Data entry and data analysis were done using STATA version 12 and SPSS (Statistical Package for Social Science) version 19. Data were presented as a number, percentage, mean, standard deviation. Chi-square and Fisher Exact Tests were used to compare qualitative variables. Mann-Whitney test was used to compare quantitative variables between groups in case of non-parametric data. Regression analysis was done to rank the different risk factors of preeclampsia. P-value considered statistically significant when P < 0.05. Based on the regression analysis the probability of PE calculated through nomogram.
Results
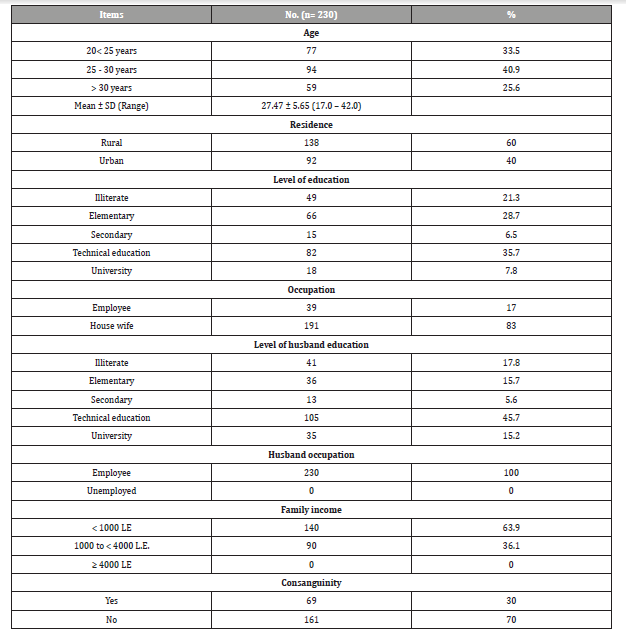
As shown in Table 1 more than two fifths (40.9%) of the study participants aged from 25-30 years, three fifths (60%) of them were from rural area and more than one third (35.7%) of them had technical education, while (83%) of pregnant women were housewives, more than three fifths (63.9%) of them had income less than 1000 L.E and less than one third (3%) of them had consanguinity marriage.
Table 1: Socio-demographic characteristics of the study participants (n=230).
The current study found that (10%) of the study participants had chronic disease, (47.8%) of them had DM and more than quarter (26.1% and 26.1%) respectively of the pregnant women were had chronic hypertension and cardiovascular disease, while (4.3%, 4.3%, and 4.3%) respectively of them had thrombophilia, lupus, and anti-thrombin deficiency
Additionally, 5.2% of the study participants had a family history of PE. Concerning women history of PE in the previous pregnancy, this table shows that (7.4%) of the study participants had a history of preeclampsia, (76.5%) of them had a history of preeclampsia for one time.
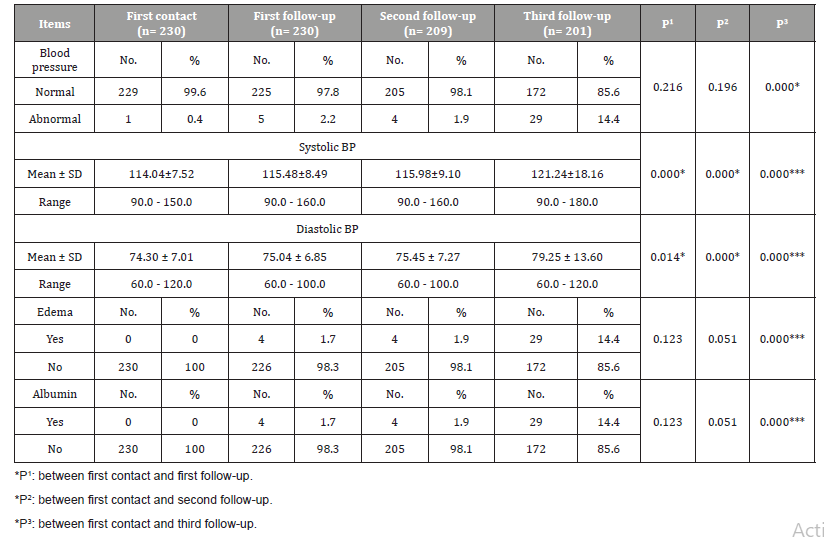
Table 2 shows that there was a statistical significant difference (P=0.000) between waves of first contact and 1st, 2nd and 3rd followup and physical examination of the study participants (Blood pressure, edema, and albumin). There was a statistically significant difference between following up of blood pressure of the pregnant women during the three waves of follow-up and developing of PE.
Table 2: Distribution of the study participants according to results of physical examination during current pregnancy.
Risk factors of PE according to nomogram prediction among study participants
As shown in Table 3 Regression analysis included seven factors: five factors were not significant [maternal age (P=0.154, OR=1.076 and 95%CI=0.973-1.191), consanguinity (P=0.821, OR=1.104 and 95%CI= 0.469-2.600), age at marriage (P=0.266, OR=1.404 and 95%CI=0.772-2.555), Age at first pregnancy (P=0.319, OR=0.735 and 95%CI=0.401-1.348), order of pregnancy (3rd or more) (P=0.951, OR=0.984 and 95%CI=0.583-1.659)]. On the other hand, two factors were significant [history of DM (P=0.010, OR=5.923 and 95%CI=1.519-23.091) and history of hypertension (P=0.045, OR=7.838 and 95%CI=1.048-58.368)].
Table 3: Regressions analysis for prediction of spreeclampsia risk factors among study participants.

The nomogram used by finding predictor points indicated by the uppermost point scales that correspond to each patient variable value; the sum of the points (seven variables) was projected to the probability of PE scale (Figure 1).
Figure 2 shows the discrimination of the nomogram model, the nomogram based on the variables included in the model had good discrimination: the concordance indices before and after bootstrapping was 0.68 (P < 0.001).


Discussion
PE has remained a significant public health threat in both developed and developing countries, contributing to maternal and perinatal morbidity and mortality globally. It affects approximately 3-14% of all pregnancies worldwide. The identification of predisposing risk factors for the development of PE could lead to a better understanding of the causality and pathogenesis of this challenging and high-risk disorder. Such knowledge is crucial for the development of an evaluation and management algorithm for the prevention of PE and its associated complications [7-9].
The socio-demographic findings of this study showed that 40.9% of the study participants aged 25-30 years, 60% of them were from a rural area, 35.7% of them had technical education, 83% of them were housewives and 72.6% of the pregnant women were in the middle class of socioeconomic level. This agreed with Jašović-Siveska, et al. [10] who conducted a study in Macedonia and reported that the age group of participated women ranged from 25-30 years old was two-fifths of the total study sample. Additionally, the study was in the same line with Endeshaw, et al. [9] and supported by Essam, et al. [11], who recorded that more than one-fifth of the participant’s age group were ranged from 26- 30 years old.
As regarding family history of chronic disease among the participants, this study reflected that more than two fifths (42.2%) of the study sample had DM. This result is disagreement with Chrelias, et al. [8], who reported that the DM was only (8.2%) among the participated women. The current study also reflected that less than one half (47.8%) of the studied sample had DM, this result in contrast with Budhram [12] who reported the DM was only present in 13.4% of the study participants.
The present study showed that more than one quarter (26.1%) had a history of hypertension, in contrast with Macdonald-Wallis, et al. [13] who reported that (15.3%) of the participants had a history of gestational hypertension.
The result of the current study recorded that 5.2% of pregnant women had a family history of PE. This agrees with Essam, et al. [11] who reported that only 4% of women had a family history of PE. In the light of the current result, 7.4% of women had a previous history of PE, and this disagreed with Luo, et al. [14] who found that 11.1% of their study participants had a history of PE.
In the present study, the evaluation of blood pressure started as early as 14 weeks of gestation, which is very important in discovering PE in the early stages. Measuring the blood pressure from the beginning of the pregnancy and knowing the values of the artery pressure in the period within 20 weeks. The values of mean arterial blood pressure over 85-90mmHg and values of diastolic blood pressure over 75mmHg are an important predictive indicator for determining the risk of hypertensive disorders in pregnancy, especially PE [15].
On the other hand, concerning to measuring of the systolic blood pressure and diastolic blood pressure during following up, the current study revealed that there is a statistical significant difference between the same groups in each wave (P=0.000). Likewise, the other finding same recorded by Jašović-Siveska, et al. [10] who found a statistical significant difference between the group of hypertensive and development of PE during pregnancy.
Cnossen, et al. [16] worked on 34 studies and observed 5.5% of women had PE. Their hypothesis was based on the diastolic average, but not on the systolic pressure. They concluded that the diastolic blood pressure over 75mmHg in the period of 13-20- week gestation has predictive importance. They also concluded that blood pressure values over 85-90mmHg also has predictive importance for the development of PE, which was also like the previous research done by Duckitt, et al. [15].
Moreover, a study was done by Hassan, et al. [17] confirmed that pre-eclamptic women in their study had significantly higher systolic and diastolic blood pressure measurements in the studied group than those in the control group. This was consistent with the findings of Siddiqui, et al. [18] who reported that a mean systolic BP was 143.1±7.8 mmHg versus 125.1±19.6 mmHg and mean diastolic BP was 94.3±4.9 mmHg versus 78±13.3 mmHg in the preeclamptic and control groups, respectively (P < 0.05).
The present study revealed to 16.1% of pregnant women has had PE. This result agrees with Guerrier, et al. [19] who reported that (16%) of pregnant women had PE.
Major risk factors for PE are: nulliparity, maternal age >40, prior preeclampsia, antiphospholipid antibody syndrome, family history of PE, renal disease, chronic hypertension, diabetes mellitus, multiple gestations, strong family history of CV disease (heart disease or stroke in ≥2 first-degree relatives), obesity [20].
The current study revealed that DM and hypertension were the most significant risk factors among study participants diagnosed with PE. This was consistent with Shiozaki, et al. [21] in Japan who found that DM in that study was one of the risk factors for developing PE. Additionally, a history of hypertension was a risk factor for PE in a previous study done by Bartsch, et al. [22].
In the present study, PE occurred in 37 patients (16.1%). In a multivariate model, history of DM (P=0.010) and history of hypertension (P=0.045) were associated with occurrence of PE and were informative in the model, while maternal age, consanguinity, age at marriage, age at first pregnancy and order of pregnancy (3rd or more) were not, this result disagreed with Deis, et al. [23] who found that PE occurred in 110 patients (2.4%). In a multivariate model, nulliparity (P=0.0021), previous PE (P=0.0039), DBP at the first visit (P< 0.0021), UARI (P=0.08), biparietal diameter (P=0.011) and twin pregnancy (P=0.0007) were associated with occurrence of PE and were informative in the model, while chronic hypertension, body mass index, ethnicity, history of first-trimester miscarriage, previous intra-uterine fetal death, auto-immune disease, tobacco use, urinary proteinuria at first prenatal visit, abdominal perimeter and femur length were not.
Conclusions
In conclusion, history of DM and hypertension were the predictor in the final model among pregnant women who diagnosed with PE. Probability of PE based on the finding of the nomogram was 68% with good discrimination.
Acknowledgement
None.
Conflict of Interest
Authors declare no conflict of interest.
References
- Munirathnamma M, Lakshmamma T (2013) Knowledge of Staff Nurses Regarding Management of Pregnancy Induced Hypertension (PIH). International Journal of Humanities and Social Science Invention 2(11): 8-12.
- Abubakar A, Abdullahi R, Jibril H, Dauda M, Poopola M (2009) Maternal ethnicity and severity of preeclampsia in Northern Nigeria. Asian J Med Sci 1(3): 104-107.
- (2005) World Health Organization (WHO) Make every mother and child count, the world health report, Geneva, Switzerland.
- El-Moselhy E, Khalifa H, Amer S, Mohammad K, El-Aal H (2011) Risk factors and impacts of preeclampsia: An epidemiological study among pregnant mothers in Cairo, Egypt. Journal of American Science 7(5): 311-323.
- Kharaghani R, Cheraghi Z, Okhovat Esfahani B, Mohammadian Z, Nooreldinc R (2016) Prevalence of Preeclampsia and Eclampsia in Iran. Arch Iran Med 19(1): 64-71.
- Leslie K, Thilaganathan B, Papageorghiou A (2011) Early prediction and prevention of pre-eclampsia. Best Practice Res Clin Obstet Gynecol 25(3): 343-354.
- Cheng P, Huang S, Su S, Hsiao C, Peng H, et al. (2016) Prognostic value of cardiovascular disease risk factors measured in the first-trimester on the severity of preeclampsia. Medicine 95(5): e2653.
- Chrelias G, Makris G, Papanota AM, Spathis A, Salamalekis G, et al. (2016) Serum inhibin and leptin: Risk factors for pre-eclampsia? Clinica Chimica Acta 463: 84-87.
- Endeshaw M, Ambaw F, AragawA, Ayalew A (2014) Effect of maternal nutrition and dietary habits on preeclampsia: a case-control study. Int J Clin Med 5(21):1405.
- Jasović-Siveska E, Jasović V (2011) Prediction of mild and severe preeclampsia with blood pressure measurements in first and second trimester of pregnancy. Ginekol pol 82(11): 845-850.
- Essam A, Khalifa H, Amer S, Mohammed K (2011) Risk Factors and Impacts of Pre-Eclampsia: An Epidemiological Study among Pregnant Mother in Egypt. J Am Sci 7(5): 311.
- Budhram S (2015) A prospective study evaluating the association of specific risk factors with the development of preeclampsia (Doctoral dissertation, Stellenbosch: Stellenbosch University).
- Macdonald-Wallis C, Silverwood R, De Stavola B, Inskip H, Cooper C, et al. (2015) Antenatal blood pressure for prediction of preeclampsia, preterm birth, and small for gestational age babies: development and validation in two general population cohorts. BMJ 351: h5948.
- Luo B, Ma X (2013) Risk factors for preeclampsia: a case–control study. Hypertens Pregnancy. 32(4):432-438.
- Duckitt K, Harrington D (2011) Risk factors for preeclampsia at antenatal booking: systematic review of controlled studies. BMJ 330(7491): 565.
- Cnossen JS, Vollebergt KC, de Vrieze N, ter Riet G, Mol BW, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. BMJ 336: 1117-1120.
- Hassan B, Almushait M, Mubashar H, Zia S (2016) The Role of risk assessment at antenatal care clinics in the prediction of preeclampsia in a High Altitude Area. Int J Clin Med 7:10-15.
- 18. Siddiqui I, Jaleel A, Kadri H, Saeed W, TamimiW (2011) Iron Status Parameters in Preeclamptic Women. Arch Gynecol Obstet 284(3): 587-591.
- Guerrier G, Oluyide B, Keramarou M, Grais R (2013) Factors associated with severe preeclampsia and eclampsia in Jahun, Nigeria. Int J Women’s Health 5:509-513.
- Maynard S, Karumanchi S, Thadhani R (2007) Hypertension and kidney disease in pregnancy. In: Brenner BM (Eds), Brenner and Rector’s The Kidney, (8thedn), Philadelphia, PA: WB Saunders, USA.
- Shiozaki A, Tanaka T, Ito M, Sameshima A, Inada K, et al. (2017) Prenatal risk assessment of gestational hypertension and preeclampsia using clinical information. Hypertens Res Pregnancy 4(2): 74-87.
- Bartsch E, Medcalf K, Park A, Ray J (2016) Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353: i1753.
- Deis S, Rouzier R, Kayem G, Masson C, Haddad B (2008) Development of a nomogram to predict occurrence of preeclampsia. Eur J Obstet Gynecol Reprod Biol 137(2): 146-151.
-
SA Khalaf, Elham MA, RH Hassanen, AM Abbas, Ahmed AY. A Nomogram for Prediction of Risk Factors for Preeclampsia during Antenatal Care at a Tertiary Maternity Hospital. W J Gynecol Women’s Health. 3(4): 2020. WJGWH.MS.ID.000569.
Prediction, Preeclampsia, Risk factors, Nomogram, Mortality
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






