Research Article
Research Article
Topical Transient Heat by The Medical Device Epiivo® Immediately Reduces Pruritus in Atopic Dermatitis, So Improves Quality of Life Significantly
Dominik Schmaltz1 and Tim Mentel2*
1Independent Researcher Muenster, Germany
2mibeTec GmbH, Sandersdorf-Brehna, Germany
Tim Mentel, mibeTec GmbH, Sandersdorf-Brehna, Germany.
Received Date: March 19, 2024; Published Date: April 01, 2024
Abstract
Atopic dermatitis (AD) represents a chronic inflammatory skin disorder affecting millions of people as children but also while adolescence and adulthood. Next to erythema and dry skin particularly at all flexural surfaces of extremities is chronic pruritus the dominating symptom, impairing quality of life (QoL) drastically. Aim was to reduce the annoying itching by topical transient heat using the medical product epiivo® (mibeTec, Germany). Its active principle succeeded already to relieve itching from insect bites or experimentally induced pruritus. As single-arm cohort 25 volunteers with mild to moderate AD applied epiivo® on itching skin ad libitum frequently over 4 weeks in everyday life. The portable, handheld epiivo® transfers transiently 47 or 49 oC by direct skin contact. Participants answered every second day an online questionnaire about position and frequency of applied epiivo® next to felt pruritus intensity. Dermatology Life Quality Index (DLQI) and severity of atopic dermatitis (SCORing AD, SCORAD) were assessed too. Participants applied epiivo® twice daily on arm/crook, leg/bend, and other skin areas (e.g. décolleté, back), 3 times daily on face/neck and hands. This intervention resulted in significant reduction of itching intensity. In consequence severity of AD was significantly reduced after 4 weeks using epiivo®, confirmed by DLQI indicating significantly decreased influence of AD on QoL. Application of the epiivo® was dermatologically tolerated well. This for the first-time evidenced efficacy of counter stimulatory heat in the context of AD can enrich the antipruritic treatment arsenal. The principle could unfold its beneficial effect also on other pruritus dominated skin disorders.
Keywords: Atopic dermatitis; Pruritus treatment.
Introduction
Atopic dermatitis (AD) stands as a prominent global health concern, representing the most prevalent chronic inflammatory skin disorder in developed nations [1]. Epidemiological data from Europe and the USA indicate AD prevalence rates of approximately 20% among children and 7-14% among adults, with notable variations across regions [2]. In 2019 the prevalence of AD in Germany was 4,2%, with 3,2% in the age group ˂ 20 years and 8,4% in the age group ≥ 20 years [3]. Lifetime prevalence of doctor-diagnosed AD ranges for adults from 18 – 20%. [2] Although the pathophysiology of AD remains under active investigation, genetic and environmental factors leading to skin barrier dysfunction, along with cutaneous and systemic immune dysregulation and alterations in the skin’s bacterial microbiota, have emerged as key causative factors [4-9]. Obvious complex interaction of these effects lead while adolescence and adulthood to erythema, edema, excoriations, all else belonging to superficial disruption and wounds next to lichenification, dry skin especially at flexural surfaces of extremities, hands and feet [10]. Independent of age pruritus accompanies AD generally throughout almost every day, worsening at night resulting in sleep loss [11]. The presence of superficial, visible symptoms on the skin as well as palpable inside reduces affected peoples’ quality of life (QoL) [12,13]. Restoring of skin integrity and improving of dry skin by application of topical emollients constitutes still the basic therapy of AD at all stages of expression [14]. Next to avoiding of itching triggers, topical corticosteroids, immune-modulators and antihistamines are used currently against pruritus [15-17]. Even so-called biologicals comprising a small army of orally or subcutaneously applied human antibodies against an interleukin, interleukin receptors and a signal transduction enzyme are under investigation in clinical trials up to approval already achieved [18]. However, convincing reduction of pruritus on application of such antibodies was detected earliest on day 2 respectively day 1 [19- 21].
This study aimed to directly and immediately alleviate ADrelated pruritus by employing topically applied local concentrated heat, utilizing the medical device epiivo® (mibeTec, Sandersdorf- Brehna, Germany). The antipruritic efficacy of such hyperthermia, around 51°C, has been previously reported for alleviating pruritus from mosquito bites and experimentally induced itch from histamine or cowhage [22,23]. Herein, we present the first report of in vivo application of concentrated heat against AD-related pruritus.
Materials and Methods
Trial design
The present study was conducted as a dermatological, singlecentre, home-based, single-arm cohort [10]. trial involving [25] volunteers diagnosed with mild to moderate atopic dermatitis (AD). Participants with AD severity requiring medical intervention were excluded from the study. In the context of AD-related pruritus, participants applied the medical device epiivo® to affected areas of itching skin, as needed. The epiivo® was utilized over a period of 4 weeks at home, integrated into participants’ daily routines during September and October 2021. The trial was conducted by Dermatest GmbH, Germany, in accordance with the principles outlined in the Declaration of Helsinki. All participants provided written informed consent prior to participation. The trial also included a 30-days follow-up period to assess the safety and tolerability of the intervention on the skin. Medical devices used in the trial were provided by mibeTec (Sandersdorf-Brehna, Germany).
Volunteers
20 female and 5 male volunteers in the age of 16 – 61 years (mean 37,4 +/- 11,7; median 37,0 years), SCORing AD (SCORAD) < 50, suffering under AD related pruritus participated in this trial. Volunteers had to report in their diary about administration of topicals or pharmaceuticals applied in parallel while usage period of the epiivo® (Table S1).
Table 1: Demographic volunteers’ data, topicals with active substance or pharmaceuticals applied in parallel while epiivo® usage period.

Trial intervention
Participants received their epiivo® devices at the trial site, where they familiarized themselves with the device manual and underwent initial application under the supervision of a study nurse. Any instances of improper device usage were promptly corrected as needed. Volunteers who were concurrently using topical corticosteroids at the onset of the trial were excluded from participation. All participants were instructed not to alter their regular skincare routines involving emollients without active substances throughout the duration of epiivo® usage. The last application of such an emollient was permitted no later than 8 hours before the trial initiation at the trial site. The epiivo® device, developed by mibeTec GmbH (Sandersdorf-Brehna, Germany), is a rechargeable, portable, handheld medical device equipped with a ceramic, electrically heatable plate measuring 8 cm². This plate conducts heat through direct skin contacts and is manually applied by the user. At the adjustable level 1 setting, the ceramic plate elevates its temperature to 47°C, maintains this temperature for 5 seconds and then automatically deactivates. Alternatively, at the selectable level 2 setting, the epiivo® device heats the ceramic plate to 49°C, maintains this temperature for 5 seconds, and then shuts down automatically.
Trial outcomes
The primary objective of the study was to ascertain the efficacy of reducing pruritus intensity associated with atopic dermatitis (AD) by administering localized, short-duration heat impulses using the epiivo® device. To achieve this, volunteers were required to complete an online questionnaire (diary) every second day. This questionnaire inquired about the location of epiivo® application, the frequency of daily usage, and the perceived intensity of pruritus both before and after epiivo® application.
participants could select options about location of application, including “arm/crook of arm,” “leg/knee bend,” “face/neck,” “hand,” and were also provided with a free-text option for specifying other areas. Additionally, they were asked to rate the intensity of pruritus using a four-point verbal rating scale (VRS) consisting of the following indices: 0 = no, 1 = slight, 2 = moderate, 3 = large intensity of pruritus [24].
Prior to commencement and following 2 and 4 weeks of epiivo® usage, all participants completed an online questionnaire assessing the Dermatology Life Quality Index (DLQI) in accordance with the methodology outlined by Finlay and Khan [12]. Original scoring of answers was applied for calculation of DLQI score [12]. Additionally, upon completing 4 weeks of epiivo® usage, participants responded to a concluding questionnaire regarding their experiences with the device, its compatibility and any alterations in scratching behaviour.
In accordance with the consensus report of the European Task Force (ETF) on Atopic Dermatitis (AD) from 1993 [25] participants’ presence was required at the trial site for the assessment of SCORAD (SCORing Atopic Dermatitis) scores at the beginning of the study and after 4 weeks of epiivo® usage. The grading of the six intensity items—erythema, edema/papulation, oozing/crusts, excoriations, lichenification, and dryness—was conducted on a scale ranging from 0 to 3 [25]. Subjective items such as pruritus and sleep loss were evaluated by participants using a numerical rating scale ranging from 0 to 10, rather than the traditional 100 mm paper scale. The extent of inflammatory lesions was determined using the rule of nine [25] implementing 2 more detailing modifications. For the assessment of the dorsal lower limbs, the femoral and lower leg regions were assigned 3.5% each, while 1% was allocated for the knee bend and plantar foot regions. Similarly, the arm was subdivided into the upper arm (2%), forearm (1%), crook of the arm (0.25%), and wrist (0.25%), totalling 3.5% per arm instead of the previously recommended 4.5%. However, the inclusion of these two modifications resulted in a total body surface area of 100%, as opposed to the potential 102% as per ETF guidelines [25]. The SCORAD was calculated using the standard formula: A/5 + 7B/2 + C, as outlined by the ETF [25].
Statistical analysis
Statistical analysis was conducted using GraphPad Prism version 9.1 (GraphPad Software, San Diego, USA). Prior to analysis, the normal distribution of data for each parameter and time point was assessed using the Shapiro-Wilk Test and Kolmogorov-Smirnov Test. If the data exhibited a normal distribution, a two-tailed, paired t-test was utilized to compare data at the start versus after 2 or 4 weeks of epiivo® usage, or before versus after application of epiivo®. In instances where the pairing of the t-test was deemed ineffective (determined by a Pearson correlation coefficient R and corresponding P-value > 0.05), the two-tailed t-test with Welch’s correction was employed. For data not adhering to a normal distribution, the two-tailed Wilcoxon matched-pairs signed rank test was applied. A significance level of P < 0.05 was required for all statistical analyses.
Results
Frequency and area of epiivo® application
Participants were instructed to complete an online questionnaire (diary) every second day throughout their 4-week application period of the epiivo® device. Analysis of responses revealed that, on average, the 25 participants reported experiencing pruritus on 10 days during this period (Table 1).
Table 2: Frequency and area of epiivo® application (n = 25).

Among the participants, 15 individuals applied the epiivo® device to itching skin areas on their arm/crook of arm with a median frequency of twice daily, while an equal number of volunteers (15) applied it to their leg/knee bend. Additionally, 6 participants chose to apply the epiivo® to other areas such as the décolleté (refer to Table S2), also with a median frequency of twice per day. Notably, 13 volunteers experiencing pruritus on their hands and 3 on their face/neck applied the epiivo® more frequently, with a median frequency of 3 times daily.
Table 3: Average frequencies of applied epiivo® per reporting day with pruritus.

Pruritus
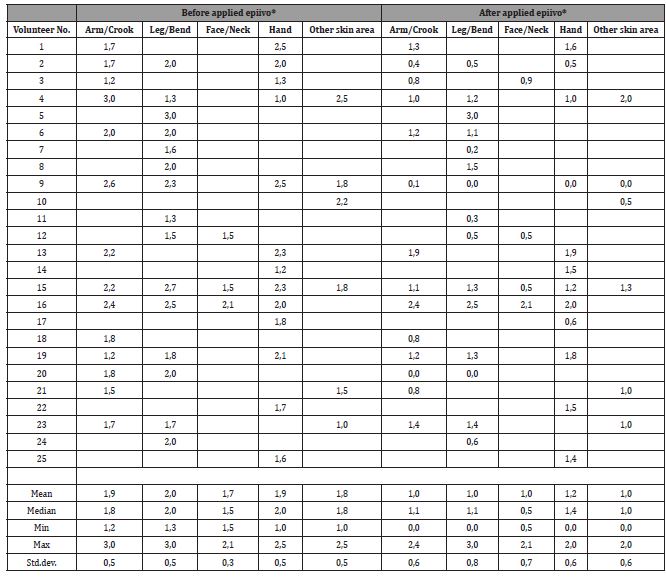
Based on the selected verbal rating scale (VRS) options for assessing pruritus before and after epiivo® application over a 4-week period, a median of 14 volunteers reported moderate pruritus on their arm/crook of arm before treatment, which significantly decreased to slight intensity following epiivo® application Figure 1. Similarly, a significant reduction in pruritus intensity from moderate to slight was observed in a median of 15 participants who applied epiivo® to their leg/knee bend. Among the 13 volunteers who applied epiivo® to their itching hands, the reduction in pruritus was slightly less pronounced compared to the leg/knee bend but still significantly improved compared to pretreatment itch intensities.

Reductions in pruritus intensity (from moderate to slight) were also reported by 2 participants who applied epiivo® to their face/ neck, while one participant noted no change in pruritus intensity (Table S3).
Additionally, 6 volunteers who applied epiivo® to areas such as the décolleté and back experienced a median reduction in pruritus intensity from moderate to slight. Importantly, the reduction in pruritus was not confined to the immediate post-application period. On average, participants reported a reduction in pruritus lasting between 2 to 3 hours, during which they did not experience the urge or impulse to scratch on their arm/crook of arm, leg/knee bend, face/neck, or hand (Table S4).
Table 4: Intensity of pruritus (No = 0; Slight = 1; Moderate = 2; Large = 3) [Mean of reporting days with pruritus per volunteer].

Table 5: Average time [h] without pruritus after applied epiivo® (Mean of reporting days with pruritus per volunteer) according to answers in online questionnaire (diary).
However, the duration of pruritus-free time varied individually among participants, ranging from a minimum of 1 hour up to 1 day. In the final questionnaire, 76% of volunteers reported a change in their scratching behavior over the 4-week period of epiivo® usage. Representative comments from volunteers included statements such as “Pruritus ceased for periods of up to half a day” and “Applied the device immediately upon feeling pruritus, preventing the onset of scratching.”
Scorad
With the exception of one volunteer, the SCORAD (SCORing Atopic Dermatitis) scores of participants were reduced after 4 weeks of using epiivo® (Table S5).
Table 6: Scorad Index.

The median value of SCORAD index change rates across all 25 volunteers was calculated as -10, with a smaller standard deviation of 7. Similarly, the median percentage change in SCORAD scores, presented as -35%, was greater than the corresponding standard deviation of 22%. Consequently, the reduction in SCORAD scores from a median of 27 to 17 after 4 weeks of epiivo® usage was statistically significant (Figure 2).

This reduction aligns with the European guideline for the treatment of AD, indicating a shift from moderate severity (SCORAD 25 – 50) to mild severity (SCORAD < 25) [26]. Regarding the varying frequencies of epiivo® application Table S5 and the corresponding differences in the total number of epiivo® applications over the 4-week period among all participants, no clear relationship between the quantity of epiivo® applications and the magnitude of change in SCORAD scores was discernible based on the data from this study.
DLQI
In terms of numerical data, DLQI (Dermatology Life Quality Index) scores exhibited a reduction in all volunteers except for one after 4 weeks of using epiivo® Table S6. Prior to the application of epiivo®, a median DLQI score of 7 was indicative of a moderate effect of atopic dermatitis (AD) on quality of life (QoL), falling within the DLQI range of 6-10 [27]. This score significantly decreased to a median score of 5 after 2 weeks of epiivo® usage, reflecting a small effect on QoL (DLQI 2-5) [27]. Subsequently, this DLQI score, initially at the upper end of the ‘minor impact’ range, was further reduced to a more pronounced score of 2 Figure 3, nearing the threshold denoting ‘no impact’ on QoL after the fourth week of treatment.

The change rates of DLQI scores after 4 weeks of epiivo® usage averaged -4 in both mean and median across all 25 participants, surpassing the corresponding standard deviation of 3 (Table S6).
Table 7: DLQI (0-1=no; 2-5=small; 6-10=moderate; 11-20=very large effect of AD on QoL) [27].

Safety
The medical product demonstrated excellent dermatological tolerance among all test subjects. Notably, no side effects or adverse reactions, such as skin irritations or allergic responses (contact dermatitis), were observed. In the final questionnaire, 96% of participants rated the dermal compatibility of epiivo® as either “very well” (n=15) or “well” (n=9). Only one volunteer rated the tolerability as “sufficient”, with no instances of ratings indicating “insufficient”, “poor”, or “very poor” compatibility observed. Furthermore, throughout the 1, 2, or 3 weeks of epiivo® usage, no participant reported insufficient or worse tolerability. However, some participants provided comments such as “very hot”, “occasionally challenging to apply the device”, or “one becomes accustomed to the heat”.
A median value of “very well compatible” or a mean value of “well compatible” was calculated across all mean values per volunteer, as indicated in Table S7 of the supporting information. Additionally, volunteers assessed the compatibility of epiivo® on a daily basis when reporting pruritus using a 6-point verbal rating scale (VRS). Mean values of all assessments per volunteer are presented in (Table S7).
Table 8: Volunteer’s assessment of epiivo®’s compatibility mean value per volunteer over all reporting days (Very well = 1; Well = 2; Sufficient = 3; Insufficient = 4; Bad = 5; Very bad = 6).

Discussion
The application of the medical device epiivo® resulted in a significant reduction of AD-related pruritus, shifting from a median intensity of moderate to slight in 93% of volunteers experiencing symptoms on their arm/crook of arm. Similarly, 100% of participants noted a significant reduction in pruritus on their itching leg/knee bend, with the severity decreasing from median moderate to slight. This pattern of substantial reduction was also observed in itching hands among all volunteers. This consistent antipruritic effect, which lasted an average of 2-3 hours post-application of epiivo®, was assessed by volunteers using a four-point verbal rating scale (VRS) across various manifestations of AD.
Counter stimuli inhibit itch sensation
It is a well-established phenomenon that the sensation of itch can be alleviated by painful stimuli, such as scratching. Although itch and pain are distinct sensory experiences, they appear to share common neural pathways. One notable difference between itch and pain lies in the effects of counter stimuli on each sensation. While scratching and other noxious stimuli effectively inhibit itch, they do not have the same inhibitory effect on pain. This observation has been validated through numerous human psychophysical studies, wherein individuals were subjected to itchy stimuli on their skin and asked to rate the intensity of itch while various counter stimuli were applied [28].
These counter stimuli, employed in psychophysical studies, include scratching [29-30] pinpricks or pinching with forceps [31,32], electrical stimulation [31,33,34], noxious heat [29-33], noxious cold [29,35-39] and capsaicin application [40]. These stimuli span a range of modalities, including mechanical, thermal, electrical, and chemical, all capable of activating nociceptors and inducing pain. However, innocuous stimuli have generally not been demonstrated to reliably inhibit itch [28].
Previous research has demonstrated the significance of glycine and gamma-aminobutyric acid (GABA) activity within the dorsal horn in attenuating the activity of neurons evoked by pruritogens, induced by counter stimuli. This indicates that inhibitory interneurons play a crucial role in mediating the inhibition of itch in response to various sensory modalities such as scratching, noxious input, heat, and cold in humans [41].
One of the earliest documented instances or recommendations regarding the beneficial application of noxious heat in treating itch dates back to 1969 [42] as documented in a report available on PubMed. In this brief report, various case studies described the advantageous use of heat application in alleviating itch caused by mosquito bites, atopic dermatitis, and contact dermatoses. However, the literature concerning the use of heat therapy for atopic dermatitis exhibits heterogeneity. For instance, a study conducted by Fruhstofer in 1986 found that warm water application up to 45°C reduced itch in two-thirds of participants, while exacerbating itch in the remaining one-third [35]. Conversely, a more recent study by Ishiuji et al. (2008) [43] failed failed to observe a reduction in histamine-induced itch in patients with atopic dermatitis [43]. This discrepancy in findings may be attributed to the fact that, in the latter study, itch was induced using histamine iontophoresis in patients with atopic dermatitis, whereas in the study by Fruhstofer, patients experienced spontaneous itch episodes.
Repeated noxious heat-induced modulation of itch.
Akiyama and colleagues [44] demonstrated in an animal model that scratching provides relief from itch. They explored the potential involvement of inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA) and glycine in scratch-induced inhibition of spinal itch-signalling neurons using a mouse model of chronic dry skin itch. Neurons in the superficial dorsal horn ipsilateral to dry skin treatment on the hind paw exhibited elevated spontaneous firing rates, which were significantly reduced by cutaneous scratching, pinching, and exposure to noxious heat.
In a recent study by Riccio, et al. [23], the effects of transient heat stimuli on histaminergic and non-histaminergic itch responses were investigated. Itch was induced using either histamine (1% solution) or cowhage (35-40 spicules), and 5-second homotopic heat stimuli at temperatures of 32, 40, 45, or 50°C were applied. The study revealed that homotopic counter stimuli inhibited histaminergic itch by 76.66% at 50°C (p<0.0001), and this inhibition due to counter-stimulation was less effective against cowhage-induced itch at lower temperatures.
Moreover, the efficacy of localized heat application in reducing pruritus has been documented in a clinical study involving the medical device Bite Away® (mibeTec GmbH, Sandersdorf-Brehna, Germany). This device employs a short heat pulse of 51°Cover 3 or 5 seconds to treat insect bites or stings, resulting in alleviation of symptoms.
Transient Receptor Potential (TRP) channels serve as widely expressed poly-modal “cellular sensors,” sensitive to changes and stimuli in the physicochemical environment such as temperature, pH, osmolarity, ionic concentrations, endogenous mediators, and external chemical irritants [45]. Among the TRP subtypes, the Vanilloid subtype (TRPV), particularly TRPV1, was initially identified on C-type nociceptive sensory neurons as a molecular target for capsaicin, the active component of hot chili peppers [46]. TRPV1 appears to play a central role in the genesis of pruriceptive itch, acting on both sensory neurons and non-neuronal cutaneous structures [47].
Emerging evidence suggests that TRP ion channels not only function as polymodal cellular sensors in sensory neurons but are also functionally expressed in various non-neuronal cell types, notably in human skin epidermal keratinocytes, dermal Merkel cells, dendritic cells, and various keratinocyte populations within hair follicles [46,48-50]. This expression pattern is particularly significant in the skin, where TRP channels play a critical role in regulating various cutaneous functions under both physiological and pathological conditions [45].
Thermo TRPs possess a unique property in that they can be activated solely by changes in temperature. Specifically, TRPV1 channels respond to temperature elevations with a pronounced increase in current, driven by enhanced open probability and singlechannel conductance, with the former being more temperaturedependent. The precise mechanism by which absorbed heat induces a conformational change leading to channel opening remains largely unresolved [51].
Therefore, targeting TRPV1 to mitigate its activity emerges as a promising therapeutic approach for treating itch. It has been observed that desensitization with capsaicin follows a dosedependent pattern, where repeated application of low doses or a single high dose, whether administered topically or via injection, leads to immediate desensitization, while lower single doses may induce activation [52].
In an in vitro study by Benham, et al. [53], it was demonstrated that repetitive stimulation of TRPV1 channels with stimuli at 48°C for 500 milliseconds resulted in a significant reduction of inward current, leading to cellular desensitization [53]. Another electrophysiological study showed in vitro that heat ramps lasting 4 and 6 seconds, reaching temperatures up to 50°C, decreased the activity of TRPV1 channels51. Additionally, this study illustrated that repeated heat ramps caused irreversible inhibition of the channels. However, the precise mechanism underlying the temperaturedependent inactivation process and thus desensitization remains largely unknown [51].
The underlying mechanism behind the reduction of itch by noxious stimuli remains an area requiring further investigation. However, one proposed mode of action is the inhibition of TRPV1, a dermal receptor responsible for both itch and pain, through sustained or repetitive activation. Recently, Wohlrab, et al. [54] corroborated in an ex vivo study that the application of temperatures ranging from 47 to 49°C for 5 seconds, administered via direct skin contact using an electric heatable ceramic plate, is adequate to reach the activation threshold of TRPV1 within the epidermis.
However, scratching, as a noxious stimulus, can provide temporary relief of pruritus. In atopic dermatitis (AD), however, chronic itch persists even after scratching. Repeated scratching by individuals affected by AD leads to disruption of the skin barrier, which, in turn, exacerbates scratching-associated inflammation, perpetuating the cycle of pruritus known as the itch-scratch cycle [55-59]. Breaking this cycle of itch and scratching is a primary objective in the treatment of AD, as it is expected to improve the appearance of the skin and enhance the quality of sleep, among other benefits [60].
In this regard, the efficacy of epiivo® in reducing pruritus consequently led to a significant decrease in AD severity. Accordingly, the 4-week use of epiivo® resulted in a reduction of the SCORAD score from a median value of 27 (indicative of moderate AD severity) to 17 (indicating mild severity). This reduction suggests, at the very least, an interruption of the itch-scratch cycle.
A reduction in pruritus is anticipated to enhance Quality of Life (QoL), as evidenced by a significant decrease in Dermatology Life Quality Index (DLQI) scores from a median value of 7 (indicating a moderate effect of AD on QoL) before using epiivo® to a median of 2 (reflecting a small effect) after 4 weeks of epiivo® use. This change underscores the beneficial impact of epiivo® on another symptomatic parameter related to AD severity
However, further trials are warranted to thoroughly investigate the efficacy of epiivo®. Moreover, the potential of epiivo® to be extended to other conditions characterized by itch, such as prurigo, urticaria, and psoriasis, should be explored in such trials.
Conclusion
Acute and chronic itch represent significant conditions that accompany a broad spectrum of both non-dermatological and dermatological disorders, such as atopic dermatitis, where pruritus is a predominant symptom. Counter stimuli, such as noxious heat or scratching the skin, can provide relief from itch, although the latter carries the risk of perpetuating a vicious cycle known as the itch-scratch cycle. While systemic or topical application of pharmaceutical formulations can lead to a reduction in pruritus, they often entail a shorter or more prolonged onset before achieving alleviation.
In contrast, the application of transient heat stimuli ranging from 47 to 49°C has been shown to produce an immediate and clinically significant reduction in itch. Therefore, such transient heat stimuli may serve as a valuable adjunct to the array of therapies aimed at reducing itch. Further research is necessary to gain a more comprehensive understanding of the efficacy of transient heat stimuli in treating pruritic conditions, thereby confirming the potential benefits for patients afflicted with such conditions.
Data Availability
The authors confirm that the data supporting the results of this article/study are presented within this article and its attached supporting information.
Ethics Statement
Only volunteers affected by mild to moderate atopic dermatitis participated in this 4-week dermatological home in-use study. Volunteers, which exhibited atopic dermatitis with severity necessary to be medically treated, were excluded. The trial was carried out by Dermatest GmbH, Germany in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Funding Statement
The investigation was funded by mibeTec GmbH, Brehna, Germany.
Acknowledgments
Both authors contributed equally to protocol development. Dominik Schmaltz supervised the study as principal study manager as well as performed data analysis and statistics. We thank all volunteers participated in this study and great thanks to all the best study nurses Stella Fersch, Andrea Lechler, Stephanie Neuhaus, Natalie Peters, Carmen Rupp, Michelle Spier,
Conflict of Interest
None to declare.
References
- Torres T, Ferreira EO, Goncalo M (2019) Update on Atopic Dermatitis. Acta Med Port 32: 606-13.
- Bylund S, Kobyletzki LB, Svalstedt M (2020) Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm Venereol Pp: 100.
- Augustin M HK, Glaeske G (2021) Neurodermitis report in: Techniker Krankenkasse.
- Palmer CN, Irvine AD, Terron Kwiatkowski A, Zhao Y, Liao H, et al. (2006) Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 38: 441-446.
- Takai T, Ikeda S (2011) Barrier dysfunction caused by environmental proteases in the pathogenesis of allergic diseases. Allergol Int 60: 25-35.
- Kezic S, O'Regan GM, Lutter R, Jakasa I, Koster ES, et al. (2012) Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol 129: 1031-1039.
- Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, et al. (2009) Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol 124: 496-506.
- Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, et al. (2006) Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol 155: 680-7.
- Jinnestal CL, Belfrage E, Back O, Schmidtchen A, Sonesson A, et al. (2014) Skin barrier impairment correlates with cutaneous Staphylococcus aureus colonization and sensitization to skin-associated microbial antigens in adult patients with atopic dermatitis. Int J Dermatol 53: 27-33.
- Weidinger S, Novak N (2016) Atopic dermatitis. Lancet 387: 1109-22.
- Kapur S, Watson W, Carr S (2018) Atopic dermatitis. Allergy Asthma Clin Immunol 14: 52.
- Finlay AY, Khan GK (1994) Dermatology Life Quality Index (DLQI) a simple practical measure for routine clinical use. Clin Exp Dermatol 19: 210-216.
- Ali F, Vyas J, Finlay AY (2020) Counting the Burden: Atopic Dermatitis and Health-related Quality of Life. Acta Derm Venereol 100: 12.
- Van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM, et al. (2017) Emollients and moisturisers for eczema. Cochrane Database Syst Rev 6: 2-2.
- Patel T, Yosipovitch G (2010) Therapy of pruritus. Expert Opin Pharmacother 11: 1673-1682.
- Pavlis J, Yosipovitch G (2018) Management of Itch in Atopic Dermatitis. Am J Clin Dermatol 19: 319-332.
- Stander S, Yosipovitch G (2019) Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br J Dermatol 181: 932-938.
- Bonnekoh H, Butze M, Metz M (2022) Characterization of the effects on pruritus by novel treatments for atopic dermatitis. J Dtsch Dermatol Ges 20: 150-156.
- Silverberg JI, Yosipovitch G, Simpson EL, Kim BM, Wu JJ, et al. (2020) Dupilumab treatment results in early and sustained improvements in itch in adolescents and adults with moderate to severe atopic dermatitis: Analysis of the randomized phase 3 studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J Am Acad Dermatol 82: 1328-36.
- Guttman Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, et al. (2020) Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults with Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol 156: 411-420.
- Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, et al. (2020) Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 396: 255-266.
- Muller C, Grossjohann B, Fischer L (2011) The use of concentrated heat after insect bites/stings as an alternative to reduce swelling, pain, and pruritus: an open cohort-study at German beaches and bathing-lakes. Clin Cosmet Investig Dermatol 4: 191-6.
- Riccio D, Andersen HH, Arendt-Nielsen L (2019) Antipruritic effects of transient heat stimulation on histaminergic and nonhistaminergic itch. Br J Dermatol 181: 786-795.
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, et al. (2012) Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 92: 502-507.
- Severity scoring of atopic dermatitis: the SCORAD index (1993) Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 186: 23-31.
- Wollenberg A, Barbarot S, Bieber T, Christen Zaech S, Deleuran M, et al. (2018) Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 32: 657-682.
- Hongbo Y, Thomas CL, Harrison MA, Sam Salek M, Finlay AY, et al. (2005) Translating the science of quality of life into practice: What do dermatology life quality index scores mean. J Invest Dermatol 125: 659-664.
- Davidson S, Moser H, Giesler G (2014) Ascending Pathways for Itch. In: Itch: Mechanisms and Treatment (Carstens E, Akiyama T, (eds). Boca Raton (FL).
- Yosipovitch G, Duque MI, Fast K, Coghill RC (2007) Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol 156: 629-34.
- Kosteletzky F, Namer B, Forster C, Handwerker HO (2009) Impact of scratching on itch and sympathetic reflexes induced by cowhage (Mucuna pruriens) and histamine. Acta Derm Venereol 89: 271-277.
- Graham DT, Goodell H, Wolff HG (1951) Neural mechanisms involved in itch, itchy skin, and tickle sensations. J Clin Invest 30: 37-49.
- Chapman LF, Goodell H, Wolff HG (1960) Chapter Viii - Structures and Processes Involved in the Sensation Of Itch**From the Study Program in Human Health and the Ecology of Man and the Departments of Medicine, (Neurology) and Psychiatry, New York Hospital, Cornell Medical Center, Cornell University, New York, New York. In: Cutaneous Innervation (Montagna W, (eds): Pergamon 161-188.
- Ward L, Wright E, McMahon SB (1996) A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain 64: 129-38.
- Nilsson HJ, Psouni E, Carstam R (2004) Profound inhibition of chronic itch induced by stimulation of thin cutaneous nerve fibres. J Eur Acad Dermatol Venereol 18: 37-43.
- Fruhstorfer H, Hermanns M, Latzke L (1986) The effects of thermal stimulation on clinical and experimental itch. Pain 24: 259-269.
- Melton FM, Shelley WB (1950) The effect of topical antipruritic therapy on experimentally induced pruritus in man. J Invest Dermatol 15: 325-32.
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, et al. (2003) Imaging of central itch modulation in the human brain using positron emission tomography. Pain 105: 339-46.
- Murray FS, Weaver MM (1975) Effects of ipsilateral and contralateral counterirritation on experimentally produced itch in human beings. J Comp Physiol Psychol 89: 819-26.
- Simone DA, Alreja M, LaMotte RH (1991) Psychophysical studies of the itch sensation and itchy skin ("alloknesis") produced by intracutaneous injection of histamine. Somatosens Mot Res 8: 271-279.
- Brull SJ, Atanasoff PG, Silverman DG, Zhang J, Lamotte RH, et al. (1999) Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens Mot Res 16: 299-303.
- Snyder LM, Ross SE (1969) Itch and its inhibition by counter stimuli. Handb Exp Pharmacol 226: 191-206.
- Hot water for itching (1969) Med Lett Drugs Ther 11: 60.
- Ishiuji Y, Coghill RC, Patel TS, Dawn A, Fountain J, et al. (2008) Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br J Dermatol 158: 78-83.
- Akiyama T, Iodi Carstens M, Carstens E (2011) Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One 6: 7.
- Toth B (2013) TRP Channels and Pruritus. The Open Pain Journal 6: 62-80.
- Steinhoff M, Bienenstock J, Schmelz M, Marcus Maurer, Wei E, et al. (2006) Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol 126: 1705-1718.
- Toth BI, Olah A, Szollosi AG (2014) TRP channels in the skin. Br J Pharmacol 171: 2568-2581.
- Bíró T, Tóth Bi Fau Marincsák R, Marincsák R Fau Dobrosi N TRP channels as novel players in the pathogenesis and therapy of itch.
- Boulais N, Misery L (2008) The epidermis: a sensory tissue. Eur J Dermatol 18: 119-27.
- Boulais N, Pereira U, Lebonvallet N (2007) The whole epidermis as the forefront of the sensory system. Exp Dermatol 16: 634-5.
- Sanchez Moreno A, Guevara Hernandez E, Contreras Cervera R, Rangel Yescas G, Ladrón-de-Guevara E, et al. (2018) Irreversible temperature gating in trpv1 sheds light on channel activation. Elife pp: 5-7.
- O'Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, et al. (2012) Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev 64: 939-71.
- Benham CD, Gunthorpe MJ, Davis JB (2003) TRPV channels as temperature sensors. Cell Calcium 33: 479-87.
- Wohlrab J, Mentel T, Eichner A (2023) Efficiency of cutaneous heat diffusion after local hyperthermia for the treatment of itch. Skin Research and Technology 29: 2.
- Ishiuji Y (2019) Addiction and the itch-scratch cycle. What do they have in common? Exp Dermatol 28: 1448-1454.
- Mack MR, Kim BS (2018) The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol 39: 980-991.
- Tominaga M, Takamori K (2022) Peripheral itch sensitization in atopic dermatitis. Allergol Int 71: 265-277.
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, et al. (2010) Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65: 886-98.
- Stander S, Grundmann SA (2012) Chronic pruritus. G Ital Dermatol Venereol 147: 161-169.
- Harrison IP, Spada F (2019) Breaking the Itch-Scratch Cycle: Topical Options for the Management of Chronic Cutaneous Itch in Atopic Dermatitis. Medicines (Basel) 18: 6-76.
-
Dominik Schmaltz and Tim Mentel*. Topical Transient Heat by The Medical Device Epiivo® Immediately Reduces Pruritus in Atopic Dermatitis, So Improves Quality of Life Significantly. World Journal of Dermatology & Cosmetics. 1(2): 2024. WJDC.MS.ID.000508.
-
Skin disorder, Adolescence, adulthood, Itching skin, Atopic Dermatitis, Pruritus intensity, Psychophysical studies, Heterogeneity, Heat stimuli
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.