Case Report
Case Report
Coexistence of Morphea and Lichen Sclerosus Et Atrophicus in Pediatric Age
Annalucia Virdi MD1,2*, Bianca Maria Piraccini Prof1,2 and Iria Neri MD1,2
1Dermatology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Policlinico S.Orsola Malpighi, Bologna Italy
2Department of Medical and Surgical Sciences, University of Bologna, Italy
Annalucia Virdi, Dermatology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Policlinico S.Orsola Malpighi, Bologna Italy.
Received Date: June 25, 2024; Published Date: July 05, 2024
Abstract
There are few pediatric reports on the coexistence of genital lichen sclerosus (LS) and morphea. We found a higher prevalence of genital LS (15.4% corresponding to 8 girls) among 52 children presenting morphea compared to the healthy pediatric population. Therefore the inspection of anogenital region should be performed also in all girls with morphea, regardless of symptoms. We believe LS and morphea to be distinct diseases, but in 2 of our cases the detection of clinical and histological features of extragenital LS on morphea lesions may be due to a more superficial mechanism of atrophy and sclerosis.
Keywords: Morphea; Lichen sclerosus et atrophicus; Pediatric
Abbreviations: Lichen sclerosus et atrophicus; CS: Circumscribed superficial type; L: linear; ECDS: en coup de sabre; yrs: years; ANA: antinuclear antibodies; MTX: methotrexate; GVHD graft-versus-host-disease; ENA: extractable nuclear antigen; Ab: antibodies; AD: atopic dermatitis; Ig: immunoglobulins.
Introduction
Lichen sclerosus et atrophicus (LS) and morphea or localized scleroderma are inflammatory scarring dermatosis leading to cutaneous atrophy and sclerosis. There are few pediatric reports on the coexistence of genital LS and morphea.
The objective of this study was to determine the prevalence of genital LS in a group of patients < 18 years affected by morphea and the associated clinical and laboratory findings.
Case Presentation
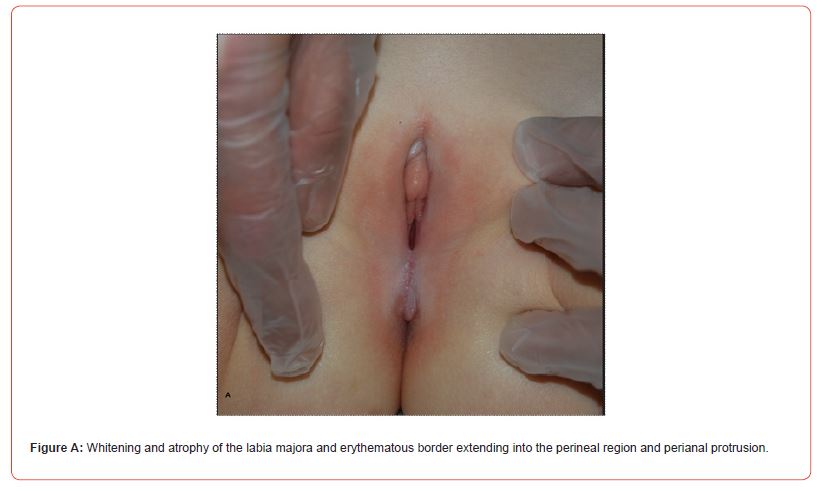
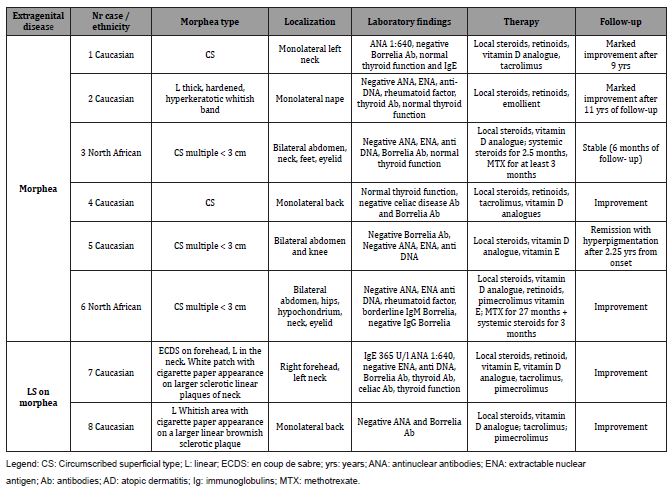
We made a retrospective observational cross-sectional study through a selection of 8 girls (6 Caucasians, 2 North Africans) with genital LS from 52 pediatric morphea cases (37 females, 15 males) diagnosed in our pediatric dermatology outpatient service between 2007 and 2020. In the same period children affected by LS were 137 (77 F, 60 M). Follow-up lasted 6.20 years on average (range 5 months -12.83 years). 7/8 presented genital LS and morphea at the first visit and in at least 3 cases (n° 1,6,8) there was no awareness of the concomitant genital problem. In these cases we arbitrarily considered the date of the first visit as the onset of the genital disease. The mean age at genital LS onset was 7.6 years (range 4 –12 years), the mean age at morphea onset was 6.14 years (range 3 –10.5 years). Only girl n° 1 complained of genital itch and had perianal protrusion (Figure A).
We prescribed topical treatment for genital LS: potent to very potent steroids or tacrolimus and vitamin E. 6 /8 girls achieved remission of the genital disease* after 2.9 years on average (range 0.83 -5 years) from onset, and improvement was observed in the remaining cases.
Circumscribed superficial (CS) morphea affected 5/8 cases and linear (L) morphea 3/8 cases (Figure B) including patient n° 7 with linear morphea on the neck (Figure C) and en coupe de sabre variant (ECDS)** on the forehead (Table 1).
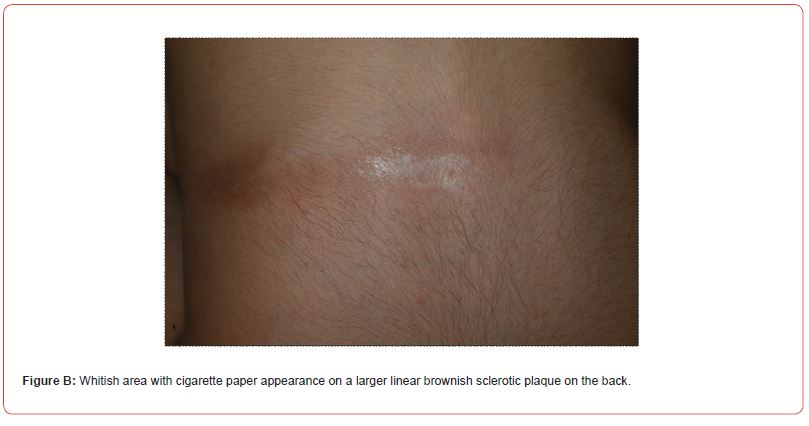
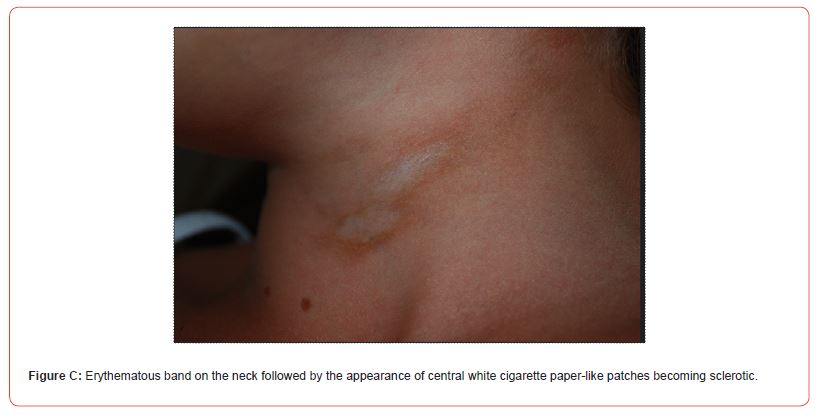
Table 1: clinical, anamnestic, laboratory data, treatment and follow-up of morphea.
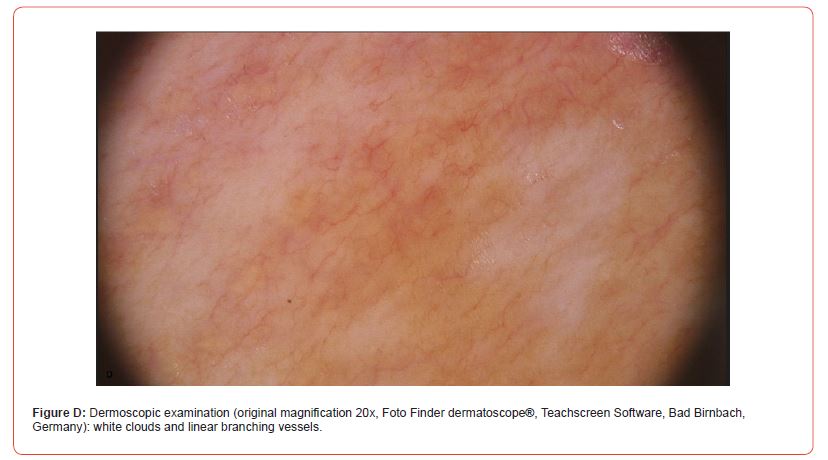
In cases n° 7 and n° 8 (Figure B), the onset of linear sclerotic plaques (respectively on the neck and on the back) was followed by overlying smaller white patches with cigarette paper appearance resembling extragenital LS. Dermoscopy of the neck lesions was reported (Figure D).
Skin biopsy was made only in case n° 7 from these smaller patches on the neck and revealed the histological features of LS.
Personal and familiar autoimmune diseases (respectively vitiligo and familiar thyroid disease and diabetes) were associated in case n° 6 and atopy was reported in cases n° 7 and 8.
Discussion
The retrospective and small study confirmed a higher prevalence (15.4%) of genital LS in pediatric morphea compared to the suspected value (0.1%) reported in the 2018 LS European guidelines in the pediatric general population [1]. We found an exclusive female involvement.
Up to now in the literature the coexistence between genital LS and morphea has already been reported in larger adult casuistries (such as 38% of 76 patients [2], 3.7% of 735 cases [3], 1.7% of 472 patients (381 adults and 91 children) [4], but is poorly reported in children: 2 girls with genital LS among 136 cases with morphea [5], a girl with morphea among 15 children with genital and extragenital LS [6]. Two female monozygotic twins manifested respectively linear and guttate morphea with pediatric onset and genital LS at 19-20 years of age [7].
In our cases the trunk and the neck were the most common localizations of morphea. MTX was prescribed in 2 African girls because of the greater extension and a greater tendency to sclerosis, demonstrating a more severe morphea in this group. Only a small part of cases with genital LS disclosed symptoms like pruritus. Kreuter, et al. made an anogenital inspection only in patients with clinical symptoms [4], but it is well known that usually only a small part of cases with genital LS presented symptoms like pruritus. In fact only 20% of Lutz’s patients with genital LS disclosed symptoms but none spontaneously [2].
Therefore anogenital inspection should be carried out in all adults and children with morphea, particularly in females even if asymptomatic.
Clinical differentiation between extragenital LS and morphea is not always so clear and the coexistence of the two disease features in the same lesion makes the distinction more difficult. Similarly to 7 cases reported by Kreuter, et al. [4] two of our patients developed some smaller lesions clinically resembling extragenital LS on a previous linear plaque of morphea (Appendix).
In another study 59.2% of morphea patients with genital involvement also had extragenital lesions of LS typically overlying plaques of morphea [3]. Some authors think this it is due to koebnerization [4], and in fact in (our) case n° 8 the lesions developed in the dorsal waist area. The relationship between morphea and LS is still controversial in literature. On the basis of clinical, dermoscopic and histological difference and some overlapping features, including sclerosis and autoimmune background, we believe, like some authors, that LS and morphea are distinct diseases [8] affecting people with a basically sclerotic cutaneous immune response to some trigger. In our patients, the presence of clinical and histological features of LS on a part of the morphea lesion may be due to a more superficial mechanism of atrophy and sclerosis in the papillary dermis and favored by cutaneous friction (creases, waistband). This study highlights the importance of genital inspection also in children with morphea, as well as the overlaps between the latter and LS.
Acknowledgment
The parents of patients in this manuscript have given written informed consent to publication of son details.
Conflict of Interest Disclosure
The authors have no conflicts of interest to disclose.
References
- Kirtschig G, Cooper S, Aberer W, Günthert A, Becker k, et al. (2017) Evidence based (S3) Guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol 31(2): e81-e83.
- Lutz V, Frances C, Bessis D, Cosnes A, Kluger N, et al. (2012) High frequency of genital lichen sclerosus in a prospective series of 76 patients with morphea: toward a better understanding of the spectrum of morphea. Arch Dermatol 148: 24-28.
- Prasad S, Black S, Zhu JL, Sharma S, Jacobe H, et al. (2021) Morphea patients with mucocutaneous involvement: A cross-sectional study from the morphea in adults and children (MAC) cohort. J Am Acad Dermatol 85(1): 114-120.
- Kreuter A, Wischnewski J, Terras S, Altmeyer P, Stücker M, et al. (2012) Coexistence of lichen sclerosus and morphea: a retrospective analysis of 472 patients with localized scleroderma from a German tertiary referral center. J Am Acad Dermatol 67(6): 1157-1162.
- Christen Zaech S, Hakim MD, Afsar FS, Paller AS (2008) Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol 59: 385-396.
- Akbaş A, Kılınç F (2021) Clinic and demographic characteristics of pediatric patients with lichen sclerosus. The Turkish Journal of Pediatrics 63: 126-135.
- Lis Święty A, Mierzwińska K, Wodok Wieczorek K, Widuchowska M, Brzezińska Wcisło L, et al. (2014) Co existence of lichen sclerosus and localized scleroderma in female monozygotic twins. J Pediatr Adolesc Gynecol 27(6): e133-136.
- Patterson JA, Ackerman AB (1984) Lichen sclerosus et atrophicus is not related to morphea. A clinical and histologic. study of 24 patients in whom both conditions were reputed to be present simultaneously. Am J Dermatopathol 6(4): 323-335.
-
Annalucia Virdi MD*, Bianca Maria Piraccini Prof and Iria Neri MD. Coexistence of Morphea and Lichen Sclerosus Et Atrophicus in Pediatric Age. World Journal of Dermatology & Cosmetics. 1(3): 2024. WJDC.MS.ID.000511.
-
Lichen sclerosus, Pediatric morphea, Steroids, Skin biopsy, Immune diseases, Perianal protrusion, Pruritus
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.