Research Article
Research Article
Clinical-Epidemiological Profile of Patients Diagnosed with Hidradenitis Supurativa: Investigation in an Outpatient Clinic
Gustavo Moreira Amorim1*, Ingrid Reuwsaat Paul2, Mariá de O Gonçalves3, Igor H Bisello3, Renata Grudtner3 and Timotio V Dorn4
1Coordinator of Dermatology, Santa Teresa Hospital, Professor, Faculty of Medicine, University of Southern Santa Catarina - UNISUL, São Pedro de Alcântara,SC, Brazil
2Dermatology Resident, Santa Teresa Hospital, São Pedro de Alcântara - SC, Brazil
3Undergraduate student, Faculty of Medicine, University of Southern Santa Catarina - UNISUL, Brazil
4Preceptor, Dermatology Resident, Santa Teresa Hospital, São Pedro de Alcântara - SC, Brazil
Gustavo Moreira Amorim, Professor, Faculty of Medicine, University of Southern Santa Catarina – UNISUL, São Pedro de Alcântara-SC, Brazil
Received Date:November 09, 2023; Published Date:January 25, 2024
Abstract
Background: Hidradenitis suppurativa (HS) is a chronic inflammatory disease that affects approximately 1% of the population, causing
significant morbidity and impaired quality of life.
Methods: Observational, cross-sectional, retrospective study with exploratory analysis; performed based on medical records. Non-probabilistic
sample, for convenience.
Results: 75 patients were included, with a predominance of women (78.7%), white skin color (86.7%), with a mean age of 33 years. There
was an average delay of 8.68 years between the onset of symptoms and the diagnosis. The inflammatory clinical subtype predominated, with more
frequent involvement in the armpits (80%), followed by inguinal region (68%). Most patients had a Hurley score of II (II and III: 79.4%), with an
average IHS4 of 12.
Discussion: We have a classic clinical profile, with a predominance of middle- aged women, with an important relationship with overweight/
obesity and smoking.
Conclusions: In view of the average delay for the correct diagnosis, linked to the fact that the average score points to a severe disease (IHS4>11),
HS remains an unattended disease. Greater awareness and greater understanding of the disease are needed at all levels of health care, with a view
to establishing effective treatment strategies in phases of less accumulated damage caused by the disease.
Keywords: Hidradenitis; hidradenitis suppurativa; inflammation; immunity; autoimmunity; tumor necrosis factor alpha; adalimumab
Background
Hidradenitis Suppurativa (HS) is a chronic, immune-mediated inflammatory disease, clinically manifested by inflammatory, painful nodules; abscesses; evolving with fistulas along with scarring and deforming tissue; predominating in flexural regions. It is also called acne inversa or “Verneuil’s disease”. For a long time it was understood as a disorder of the apocrine glands, due to its topography of involvement, in addition to being included in diseases related to follicular occlusion [1-5]. The fact is that with the better understanding of the immunological mechanism involved, from a starting process of infundibulitis, it occurs the rupture of the follicular epithelium, presentation of antigens and recruitment of mono and polymorphonuclear cells, generating a mixed inflammatory reaction, including chronic, granulomatous. So, inflammation of the apocrine glands is secondary in the disease process. There is innate immune and memory hyperactivation, with an important participation of inflammatory cytokines from the Th1 and Th17 axis [6-9]. An average prevalence of 1% in the general population is estimated, predominantly in healthy post-pubescent women [10-14].
In Brazil, a telephone survey study evaluated around 17,000 patients from 85 municipalities, finding a prevalence of 0.41% [15]. Obesity and mechanical friction, in addition to smoking, are known risk factors for HS. In parallel, the disease is associated with other immune-mediated diseases, such as inflammatory bowel disease and spondyloarthritis [10-14]. There is a serious impairment of life quality, comparable to other immune-mediated inflammatory systemic skin diseases, such as psoriasis, atopic dermatitis and chronic urticaria [7,8]. Despite advances in understanding, including allowing to combine surgical treatment with specific target immunobiologicals, controlling symptoms and reducing patients’ suffering, there is still a long delay in the correct diagnosis of these patients, ending up delaying their access to adequate care. Adding to the knowledge of this highly stigmatizing disease is the contribution this work aims to make. With this objective, we primarily sought to outline a clinical epidemiological profile of patients diagnosed and treated with HS in our outpatient clinic. Secondarily, we aimed to identify the average time between the onset of signs/symptoms and the correct diagnosis, in addition to the prevalence of obesity, metabolic syndrome and smoking associated with HS in the studied sample.
Methods
This is a retrospective cross-sectional observational study, with
exploratory analysis; carried out based on the review of medical
records. The studied population was made up with patients
diagnosed with HS, attended and treated by Board Certified
Dermatologists; in a referenced outpatient clinic as part of a
Dermatology Service in a Public Hospital with a Medical Residency
Program in Dermatology. All patients treated with HS between
January 2021 and August 2022 were included (non-probabilistic
sampling, for convenience). A patient who presented the following
3 criteria (clinical diagnosis) was considered a case of HS [10].
a) Comedones (two comedones close together, over a papule
or nodule) and/or Nodules and/or Abscesses and/or Fistulas,
associated or not with scar defects (single or multiple, intercommunicating
fistulas); pruritic, painful, exudative lesions.
b) Typical regions: armpits, inter- and inframammary folds,
groin, perineal and perianal region, and buttocks.
c) Chronic evolution (at least 6 months), recurrent in nature.
Some patients had their diagnosis complemented by imaging
(ultrasound) or histopathological examination as a result of
resection of lesions or lesional areas, but not as a mandatory
inclusion criteria. Patients with at least 1 of the following criteria
were excluded from the analysis:
a) Insufficient data in the medical records consulted.
b) Follow-up time of less than 6 months.
The evaluated variables were:
a) Gender, assessed in a qualitative, nominal, dichotomous
manner, considering male or female.
b) Skin color, broken down in a qualitative, nominal manner,
according to the self-declaration of color contained in the
identification form in the medical record, distinguishing white,
brown or black.
c) Age at diagnosis, time of disease evolution until diagnosis
were analyzed in a quantitative, continuous way, both in years.
d) Age range at onset of the condition, operationalized in a
qualitative, ordinal way, broken down into pediatric (6 to 11
years and 11 months), puberty (12-18 years) and adult (> 18
years).
e) Clinical phenotype, in a simplified classification
determined by Martorel A et al. in 2015, analyzed qualitatively,
nominally, as follicular, inflammatory or mixed [16].
f) Lesion topography, operated in a nominal dichotomous
qualitative way: armpits (yes or no), chest (yes or no), abdomen
(yes or no), inguinal regions (yes or no), perineal/perianal
regions (yes or no) and buttocks (yes or no).
g) Facial involvement, studied in a nominal dichotomous
qualitative way (yes or no).
h) Hurley clinical staging, qualitatively, nominally, being stage
I (isolated nodules and abscesses, in small numbers, without
scar defects), II (single or multiple abscesses, with formation
of fistulas or scars) or III (multiple interconnected fistulas, in
addition to abscesses, covering a complete anatomical area)
i) Counting the number of lesions: number of nodules,
abscesses and fistulas, in a quantitative, discrete way (in order
to fill in the activity score).
j) International Hidradenitis Suppurativa Severity Score
System (IHS4), in a continuous quantitative manner and
subsequently stratified according to severity, as proposed by
Zouboulis CC et al. [17] (number of nodules x 1 + number of
abscesses x 2 + number of fistulas x 4): ≤ 3 (mild disease); 4–10
(moderate); ≥ 11 (severe).
k) Body Mass Index, in continuous quantitative form, in
square meters per kilogram of weight.
l) Abdominal circumference, in continuous quantitative
form, in centimeters.
m) Obesity, Metabolic Syndrome, Smoking, assessed in a
qualitative, nominal, dichotomous manner (yes or no). In an
exploratory manner, Obesity will be stratified according to the
current classification.
Other comorbidities and ongoing medications, along with current treatment strategies were analyzed in a descriptive way. The selected data were gathered into printed spreadsheets and digitized, using the Excel 2011 program (Microsoft® Excel® for Mac 2011 / Version: 14.2.0). The data were analyzed using SPSS statistical software, version 24.0. (Statistical Package for the Social Sciences, Version 20.0, [Computer program]. Chicago: SPSS Inc 2009). The qualitative variables were presented in absolute and relative frequencies, and the quantitative variables in measures of central tendency (mean or median) and their respective measures of variability/dispersion (Amplitude [maximum and minimum] and standard deviation). The study is in accordance with resolution 466/12 of the National Health Council, is registered on Plataforma Brazil and was approved by the responsible Research Ethics Committee (CAAE: 56630622.5.0000.0113).
Result
A total of 75 patients with HS were selected for the study, according to the inclusion and exclusion described criteria. Table 1 shows clinical epidemiological profile. According to the proposed methodology, Table 2 presents objective classifications regarding the staging and severity classification of the sample studied. Table 3 shows the comorbidities associated with HS. Finally, the treatment proposals for the studied patients were outlined in general terms on Table 4.
Table 1:Clinical Epidemiological Profile.
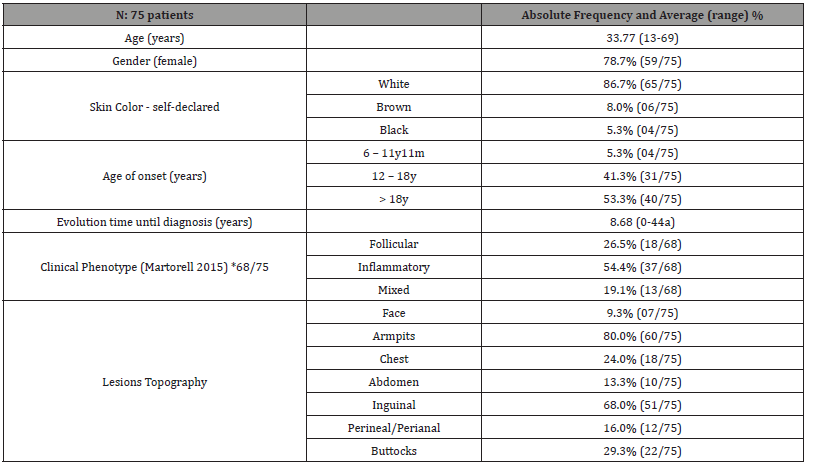
*There was a loss of data regarding clinical phenotype in 7 of the 75 patients.
Table 2:Clinical Staging / Severity of disease.
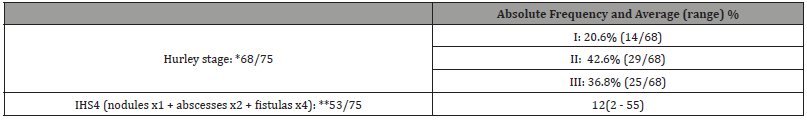
IHS4: International Hidradenitis Suppurativa Severity Score System.
*There was a loss of data regarding staging in 7 of the 75 patients and a ** 18 loss for IHS4.
Table 3:Comorbities.
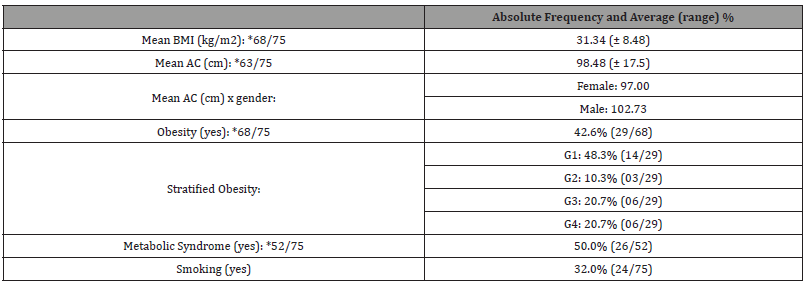
BMI: Body Mass Index / AC: Abdominal Circumference.
Table 4:Treatment.
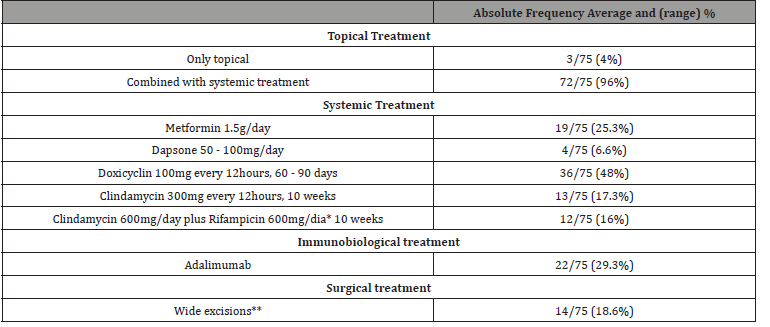
*It is noteworthy that in the macro-region where we operate, despite the use of a combination of clindamycin and rifampicin being included in the
clinical guidelines protocol, the user does not always have access to the medication, limiting the use of Rifampicin.
**Were included in this description only extended/wide resections, covering an entire anatomical unit, for example, the entire axillary cavity. Localized
surgical interventions, such as abscess drainage, corticosteroid infiltration or even fistulectomy, were not counted.
Discussion
An observational, cross-sectional research study was carried out, which aimed to characterize the clinical epidemiological profile of patients diagnosed with HS treated at a reference outpatient clinic. In parallel, the average time between the onset of symptoms and diagnosis was evaluated, in addition to the prevalence of comorbidities associated with the disease. Hidradenitis suppurativa is an inflammatory disease characterized by skin nodules, abscesses, winding paths and scars in the folds, particularly the armpits, intermammary, inguinal and intergluteal regions [18]. It is a chronic and recurrent disease, which causes physical pain and major negative effect in quality of life [14]. It most commonly develops after puberty [18]. The prevalence of HS in the general population remains uncertain, varying between 0.005 and 4% depending on the study design carried out - the highest estimates come from prospective and self-reported studies, and the lowest from observational studies [5,18,19]. A retrospective analysis conducted in the United States of America with 48 million patients found a prevalence of 0.1%, with women being almost three times more affected than men, the majority of whom were of African- american. The same study found an annual incidence of 11.4 cases per 100,000 inhabitants [20]. The most affected age group were women, between 20 and 40 years old [21,22]. Despite being a chronic disease, there is a tendency for reduction of flares after menopause, and may even reach clinical remission [23]. In Europe, it is understood that approximately 1% of the population carries the disease [11,24,25].
Our findings regarding the clinical aspects of patients corroborate with the presentation of HS, previously described in the literature: the majority of patients had nodules, absecess and fistulas, located in fold areas, predominantly in the armpits (81.0%). Furthermore, lesions were identified in the groin (69.8%), buttocks (31.7%) and infra-mammary fold [1,2,14]. HS is a chronic disease related to multiple comorbidities: metabolic syndrome and its components (diabetes, high blood pressure, dyslipidemia, obesity), sexual dysfunction, polycystic ovary syndrome, inflammatory bowel disease, depression, anxiety, among others.1 In addition to these, patients with HS have an increased risk of death from cardiovascular causes and suicide [26]. The majority of patients studied had associated comorbidities. The most identified changes were obesity (44.6%), metabolic syndrome (48.9%) and smoking (31.7%). It is worth highlighting that the average BMI of our sample was 31.77, which is an aggravating factor for metabolic disorders and consequently contributing to the emergence of new lesions. Our findings corroborate the results obtained by Goldburg and collaborators in 2019, linking HS with metabolic disorders and obesity [14].
Obesity is the comorbidity most associated with HS, affecting between 18% and 88% of patients depending on the study [24,27- 30]. Obesity determines chronic inflammation and hyperinsulinism, stimulating a greater expression of pro-inflammatory cytokines. Another possible contribution of hyperinsulinism to the pathogenesis of HS is due to hyperandrogenism - the appearance at puberty and some clinical improvement with antiandrogen therapy suggest hormonal changes may play a role in this disease [18]. Among our HS patients studied, 40% were obese and 45% were at least overweight. Obesity is considered a comorbidity related to HS, but with no established prevalence. A retrospective study analyzing 249 patients before and after undergoing bariatric surgery found a prevalence of 18.1% of HS in patients with a BMI above 30, with a significant reduction in symptoms in a third of these after losing 15% of body weight or more [20]. Diabetes Mellitus is three times more prevalent in patients with HS when compared to the general population, with data ranging from 7.1% to 24.8% of patients [31,32]. The prevalence of dyslipidemia varies between 3.3% and 45.3% of the patients studied, and its screening is important in patients with HS [32]. The presence of atherosclerosis, as well as the occurrence of cardiovascular events such as acute myocardial infarction, ischemic stroke and deaths from all causes are significantly elevated in patients with HS [33,34]. Active smoking is considered a trigger of the disease, as well as its history [27,34].
The average time for diagnosis delay was 9 years. Regarding the Hurley staging, it was noted that 41.3% had stage III at the time of data collection. Based on the IHSA4 classification, a total of 53 patients were evaluated, whose average result was equal to 13, corroborating the classification of severe disease (when result ≥ 11). It is clear that the diagnostic delay had an impact on the advanced conditions of the patients evaluated. Unlike what was found by other authors, in our outpatient clinic the majority of patients with HS are self-declared white (72.9%) [1,20]. This is possibly justified because the population of the state of Santa Catarina has its majority self-declared as white: according to data from the Brazilian Institute of Geography and Statistics (IBGE) collected in 2010, the percentage of people from Santa Catarina who declared themselves white was 83.9%, compared to 12.6% mixed race and 2.9% black, being the Brazilian state with the lowest proportion of mixed race and black people [35]. As limitations of the study, we identified sampling bias, due to the non- probabilistic sample. To this date, there are no robust epidemiological studies that define the population with HS in Brazil, which makes it difficult to even determine the number of patients needed for such a sample. This limitation may impact the generalizability of the study’s findings to the broader population of HS patients in the country.
Conclusion
Taking into account the prolonged delay between the appearance of signs and symptoms of HS and the correct diagnosis, which in our study was 9 years on average, it is clear that HS remains a neglected underrecognized disease. Greater awareness and greater understanding of the disease are needed, at all levels of health care, especially for professionals who make the first contact with patients in routine consultations (general practitioners). Once diagnosed, we consider imperative to stablish a multidisciplinary follow-up, improving HS care.
Conflict of Interest
No.
Funding
No Financing.
Ethical Aspects
Project was submitted to Plataforma Brazil, assessed by the responsible Ethics Committee and approved without any pending issues (CAAE: 56630622.5.0000.0113)..
References
- Alikhan A, Lynch PJ, Eisen DB (2009) Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 60(4): 539-563.
- Sabat R, Jemec GBM, Matusiak L, Kimbal L, Kimbal AB, et al. (2020) Hidradenitis suppurativa. Nat Rev Dis Primers 6(1): 18.
- Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, et al. (2015) European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 26(4): 619-644.
- Delany E, Gormley G, Hughes R, McCarthy S, Kirthi S, et al. (2018) A cross-sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol 32(8): 467-473.
- Jemec GB, Kimball AB (2015) Hidradenitis suppurativa: Epidemiology and scope of the problem. J Am Acad Dermatol 73(5 Suppl 1): S4-7.
- Vekic DA, Frew J, Cains GD (2018) Hidradenitis suppurativa, a review of pathogenesis, associations, and management. Part 1. Australas J Dermatol 59(4): 267-277.
- von der Werth JM, Jemec GB (2001) Morbidity in patients with hidradenitis suppurativa. Br J Dermatol 144(4): 809-813.
- Tzello T, Yang H, Mu F, Calimlim B, Signorovitch J (2019) Impact of hidradenitis suppurativa on work loss, indirect costs, and income. Br J Dermatol 18(1): 147-154.
- Obadia DL, Daxbacher ELR, Jeunnon T, Gripp AC (2009) Hidradenitis suppurativa treated with infliximab. An Bras Dermatol 84(6): 695-697.
- Magalhães RF, Rivitti-Machado MC, Duarte GV, Souto R, Nunes DH, et al. (2019) Consensus on the treatment of hidradenitis suppurativa Brazilian Society of Dermatology. An Bras Dermatol 94(2, suppl 1): 7-19.
- Imgran J, Jenkins-Jones S, Knipe DW, Morgan CLI (2018) Cannings-John D, Piguet V. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 178: 917-924.
- Martorell A, García-Martínez FJ, Jiménez-Gallo D, Pascual JC, Pereyra- Rodriguez J, et al. (2015) An Update on Hidradenitis Suppurativa (Part I): Epidemiology, Clinical Aspects, and Definition of Disease Severity. Actas Dermosifiliogr 106: 703-715.
- Goldburg SR, Strober BE, Payette MJ (2019) Part I. Hidradenitis Suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 82(5): 1045-1058.
- Ianhez M, Schmitt JV, Miot HA (2018) Prevalence of hidradenitis suppurativa in Brazil: a population survey. Int J Dermatol 57: 618-620.
- Martorell A, Sanz-Motilva V, Alfaro-Rubio A, et al. (2015) Phenotypic heterogeneity in hidradenitis supurativa: a prospective analysis of 50 cases. J Am Acad Dermatolol 72(5suppl1): AB105.
- Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, et al. (2017) Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 177(5): 1401-1409.
- Tchero H, Herlin C, Bekara F, Fluieraru S, Teot L (2019) Hidradenitis Suppurativa: A Systematic Review and Meta-analysis of Therapeutic Interventions. Indian J Dermatol Venereol Leprol 85(3): 248-257.
- Ergun T (2018) Hidradenitis suppurativa and the metabolic syndrome. Clin Dermatol 36(1): 41-47.
- Garg A, Kirby JS, Lavian J, Lin G, Strunk A (2017) Sex- and Age-Adjusted Population Analysis of Prevalence Estimates for Hidradenitis Suppurativa in the United States. JAMA Dermatol 153(8): 760-764.
- Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD (2013) Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol 133(1): 97-103.
- Slyper M, Strunk A, Garg A (2018) Incidence of sexual dysfunction among patients with hidradenitis suppurativa: a population-based retrospective analysis. Br J Dermatol 179(2): 502-503.
- Kromann CB, Deckers IE, Esmann S, Boer J, Prens EP, et al. (2014) Risk factors, clinical course, and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol 171(4): 819-824.
- Vossen ARJV, van der Zee HH, Prens EP (2018) Hidradenitis suppurativa: A systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol 9: 2965.
- Jemec GBE (2012) Clinical practice. Hidradenitis suppurativa. N Engl J Med 366(2): 158-164.
- Tzellos T, Zouboulis CC (2022) Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol 31(Suppl 1): 29-32.
- Sartorius K, Emtestam L, Jemec GBE, Lapins J (2009) Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 161(4): 831-839.
- Schmitt JV, Bombonatto G, Martin M, Miot HA (2012) Risk factors for hidradenitis suppurativa: a pilot study. An Bras Dermatol 87(6): 936-938.
- Kohorst JJ, Kimball AB, Davis MDP (2015) Systemic associations of hidradenitis suppurativa. J Am Acad Dermatol 73(5 Suppl 1): S27- 35.
- Kromann CB, Ibler KS, Kristiansen VB, Jemec GBE (2014) The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol 94(5): 553-557.
- Bui T-L, Silva-Hirschberg C, Torres J, Armstrong AW (2018) Hidradenitis suppurativa and diabetes mellitus: A systematic review and meta- analysis. J Am Acad Dermatol 78(2): 395-402.
- Garg A, Malviya N, Strunk A, Wright S, Alavi A, et al. (2022) Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol 86(5): 1092-1101.
- González-López MA, Hernández JL, Lacalle M, Mata C, López-Escobar M, et al. (2016) Increased prevalence of subclinical atherosclerosis in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol 75(2): 329-335.
- Egeberg A, Gislason GH, Hansen PR (2016) Risk of Major Adverse Cardiovascular Events and All-Cause Mortality in Patients with Hidradenitis Suppurativa. JAMA Dermatol 152(4): 429-434.
- König A, Lehmann C, Rompel R, Happle R (1999) Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology (Basel) 198(3): 261-264.
- Brazilian Institute of Geography and Statistics, Research on the Ethnic-Racial Characteristics of the Population.
-
Gustavo Moreira Amorim*, Ingrid Reuwsaat Paul, Mariá de O Gonçalves, Igor H Bisello, Renata Grudtner and Timotio V Dorn. Clinical- Epidemiological Profile of Patients Diagnosed with Hidradenitis Supurativa: Investigation in an Outpatient Clinic. World Journal of Dermatology & Cosmetics. 1(2): 2023. WJDC.MS.ID.000507.
-
Hidradenitis; hidradenitis suppurativa; inflammation; immunity; autoimmunity; tumor necrosis factor alpha; adalimumab
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.