 Research Article
Research Article
Influence of Agricultural Lime in Alleviating Acidity Level of Various Acid Soils
Dipendra Chaudhari1, Shree Prasad Vista2, Prabin Ghimire3 and Chakra Devkota3
1Agriculture and Forestry University, Rampur Chitwan, Nepal
2Soil Science Division, Nepal Agricultural Research Council, Khumaltar, Lalitpur, Nepal
3Institute of Agriculture and Animal Science, Post Graduate Campus, Tribhuvan University Kritipur, Nepal
Dipendra Chaudhari, Agriculture and Forestry University, Rampur Chitwan, Nepal.
Received Date: October 14, 2019; Published Date: October 21, 2019
Abstract
Influence of agricultural lime in acidity level, macro-nutrients (P&K) mineralization and plant growth in various acid soils were investigated inside glass house in French bean (Phaseolus vulgare). The experiment underwent from 24th July to 8th October 2017. Liming of five different acid soils (Pine Forest Soil, Black Soil, Red Soil, Swampy Soil and Cultivated Soil) were done as per the doses recommended by SMP buffer method. Plant height was measured, and the soil was sampled to 15 to 25cm depth, initially and then at interval of 15 days till 75th day of treatment application. The experiment was carried under Completely Randomized design (CRD). Data of soil parameters (soil pH, P & K availability) and plant parameter (plant height) at different times were critically analyzed for significance test at 5% level of significance. Desirable pH range for crop cultivation was achieved after 30 days, however desirable soil pH range was only achieved after 45-60 days of lime application. Most significant increase in pH of Red soil was observed after 15 days of treatment application (from pH 4.9 to 6.2) and gradually increased up to 7.3 in 75 days, highest among all other soils. mineralization of soil K in swampy soil was statistically significant and superior among all types of soil/treatment considered for study followed by black soil cultivated soil as most superior in terms of P-mineralization for all cases followed by black soil French bean seemed much responsive to Normally Cultivated Soil while the least responsive to Pine Forest Soil.
Keywords:Soil Acidity; Liming; Available soil potash; Available soil phosphorus
Introduction
Soil acidity and alkalinity is the state of soil representing the concentration of hydrogen and hydroxyl ions i.e. soil pH indicates the acidity or alkalinity level of soils. Precipitation and leaching are the dominant processes that lead towards soil acidification. Soil acidity and alkalinity problems are becoming the concerning issue throughout the world as plant growth, its health and production are influences to a greater extent
Soil pH is an indicative chemical property of soil and governs the nutrient availability to and production of crops. It is essentially the measure of hydrogen ion concentration in soil. The substance that donates hydrogen ions (H+) to others in aqueous solution is called acid [1] and so the soil will be acidic if it has higher concentration of hydrogen. Acidity of soil can either be active (H+ ions concentration in soil solution) or reserved (H+ ions concentration adsorbed in soil particles). However, the active acidity is quite lesser than reserve acidity [2].
Soil pH indicates the extent of acidity or basicity in soil. Its value ranges from 0-14. Soil Survey Division Staff [3] has divided this pH range into different categories like, ultra-acid (< 3.5), extremely acid (3.5-4.4), very strongly acid (4.5-5.0), strongly acid (5.1-5.5), moderately acid (5.6-6.0), slightly acid (6.1-6.5), neutral (6.6-7.3), slightly alkaline (7.4-7.8), moderately alkaline (7.9-8.4), strongly alkaline (8.5-9) and very strongly alkaline (> 9).
According to Queensland Department of Environment and Heritage Protection (retrieved on 2017), optimum pH range for crop production is 5.5- 7.5 because most of the plant nutrients are available to crops in this pH range. More acid and more alkaline soils, both decrease the crop productivity, but soil acidity problem is more pronounced. Human practices that induce soil acidity are excessive use of nitrogenous fertilizer, bases removed by crop harvesting, acid deposition by fossil fuel consumption, pine forest plantation and less use of organic manures. Acid soils impairs crop production and also human health due to its harmful effects like aluminum toxicity, water logging, unavailability of plant nutrient, declining of microbial population, herbicide leaching and nitrous oxide emission. For the proper management of soil acidity for optimum crop production, it is necessary to understand the sources, mechanism of soil acidification and the methods of ameliorating acid soils.
The negative effects of soil acidity on physical and chemical soil conditions can be partly compensated by ensuring high organic matter content but heavy application of compost is not feasible and only effective alternate way to control acid soil is limestone application [4].
Liming is the practice of increasing the soil pH through the incorporation of lime materials into the upper cultivable soil layer for amelioration of acid soils, thus its deleterious effects on soil physical, chemical and chemical properties can be eliminated. Various liming materials are calcic limestone (CaCO3), dolomitic limestone (CaCO3.MgCO3), hydrated lime [Ca(OH)2], magnesite (MgO) and so on. Calcium carbonate, also known as agricultural lime, is most widely used liming material around the world. Nutrient availability, more symbiotic nitrogen fixation, precipitation of toxic elements and soil physical properties improvement are the important benefits of liming practice.
Amount of lime requirement is based on estimated initial pH value of soil solution, more specifically the pH value of soil solution plus buffer solution. There are four buffer methods for determining the lime requirement. The Shoemaker, McLean, and Pratt (SMP) buffer is used for soils with high OM alfisols, Woodruff buffer method for mollisols, Adams-Evans buffer method for low CEC ultisols, and Mehlich buffer method for highly weathered ultisols [5]. However, SMP method is considered efficient as it is quick, relatively low cost and presents good results. It is one of most employed method to evaluate potential acidity of soil [6].
Materials and Methodology
The experiment was conducted at glasshouse of Khumaltar Lalitpur. Generally, sites and so the soils were chosen which tend to be acidic in nature. Red soil, pine forest soil and swampy soils were collected from Godavari forest, were virgin and covered by evergreen natural forests. Black soil was collected form Dadhikot. Initial chemical properties of experimental soils were assessed in soil laboratory on the basis which research was to be preceded. Initial status of soil acidity was analyzed to set-up treatments while available P and K were to analyze the result after experimentation and organic matter percentage was assessed as it plays important role in reducing acidity and mineralization of P & K after liming.
There were altogether five treatments The set-up of experiment followed Completely Randomized Design. Each soil of 2.7 kg was weighed and thoroughly mixed with recommended lime dose. Then the mixture was kept in earthen pot for experimental setup. Each treatment was replicated 3 times. French bean Phaseolus vulgaris was taken as the sample plant for response determination.
Calculation of Ag-lime amount
On the basis of SMP (Shoemaker, McLean and Pratt) Buffer method, the amount of lime to be applied in each type of soil (per 2.7 kg) was determined as shown in the following Table 1.
Table 1:Soil type and lime dose details
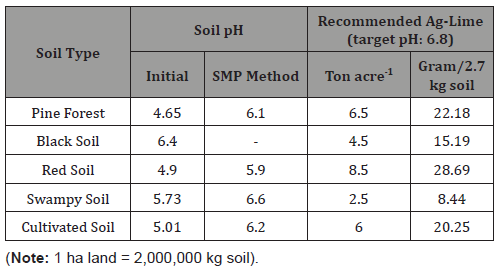
Details about treatment are as follows:
• T1: Pine Forest Soil + 22.18 g Ag-lime
• T2: Black Soil + 15.15 g Ag-lime
• T3: Red Soil+ 28.69 g Ag-lime
• T4: Swampy Soil + 8.44 g Ag-lime
• T5: Cultivated Soil + 20.25 g Ag-lime
Soil pH was measured by using Pen pH Meter which was first calibrated by using buffer solution of pH 4.0 and 7.0. Available soil phosphorus was determined by Modified Olsen’s Bicarbonate Method and available soil potassium was measured by Flame Photometric Method.
The data regarding soil chemical properties and plant height taken at different time were collected, sorted, tabulated and subjected to Analysis of Variance (ANOVA) test using SPSS, a computer software program. Various statistical parameters like p-value, F-value, grand mean, least significant difference, coefficient of variation and standard error mean were calculated using SPSS to find the reliability of the experiment. The statistical significance between the treatments was analyzed at 95% confidence interval i.e. p-value of 0.05. Duncan’s Multiple Range Test was also carried out to find the most significant treatment.
Results and Discussions
Influence of Ag-lime on acidity (pH) status of different types of soil
Data in Table 2 revealed that the application of Ag-lime decreased the soil acidity level in all types of adopted treatments and overall liming effect on reducing soil acidity was found statistically significant when compared between different soils (i.e. treatments) with estimated p-value 0.006 (i.e. <0.05). In two cases of 30 & 60 days after lime application the liming effect was found insignificant with respective p-values of 0.053 and 0.078 while in rest cases the effect was significant as shown in the following table.
Table 2:
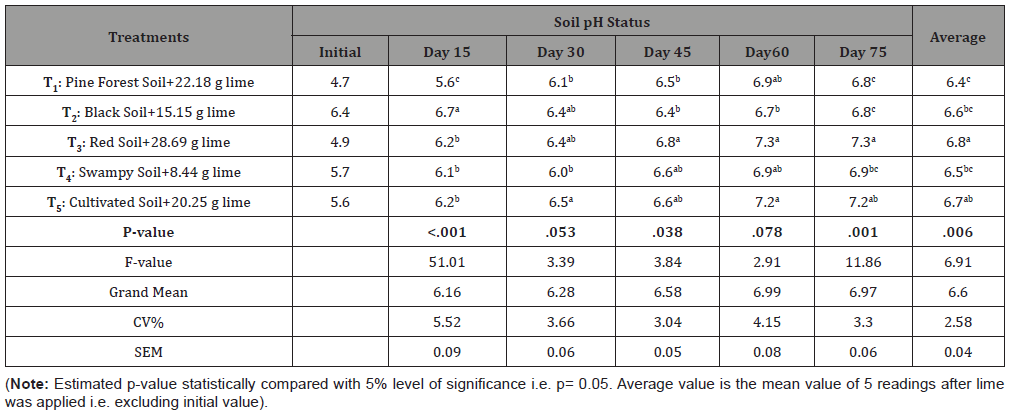
The average pH values observed during experiment in different soil at different time intervals were examined in accord with DMRT test and found similar results in most cases except for that first 15- day case in which black soil was significantly much responsive to lime application. It was due to its higher initial pH level. But in other cases, red soil was statistically most significant towards liming response followed by normally cultivated soil. But in terms of least responsiveness mixed results were found. However, analyzing the average acidity reduction in soil till 75 days of lime application found the red soil as most responsive followed by respectively cultivated soil, black soil, swampy soil and pine forest soil, as shown in the table above. The data in Table 3 also revealed that in general, liming increased the soil pH up to intended level till 60 days after lime application and in most cases, it was stabilized. However, there was variation in time with soil type to achieve the intended pH level. For example, in red soil intended pH (6.8) was achieved in 45 days while for black soil it was 60 days and for remaining soils it was between 45-60 days of lime application. The pH of red soil increased throughout the time of investigation (up to 75 days of liming) but in rest four different soil, the reaction was found to be stable after 2 months of lime application. In case of red and cultivated soil, the pH level at 75 days of lime application was found to be greater than intended level (6.8) i.e. 7.3 & 7.2 respectively.
Effect of Ag-lime on availability of soil Potassium (K)
Regarding mineralization of soil K, five readings were observed at 15 days interval in each type of soil after the lime was applied. Average values of available soil K recorded during the experiment are tabulated in Table 3.
Table 3:Influence of Ag-lime in the availability of Soil Potash (K).
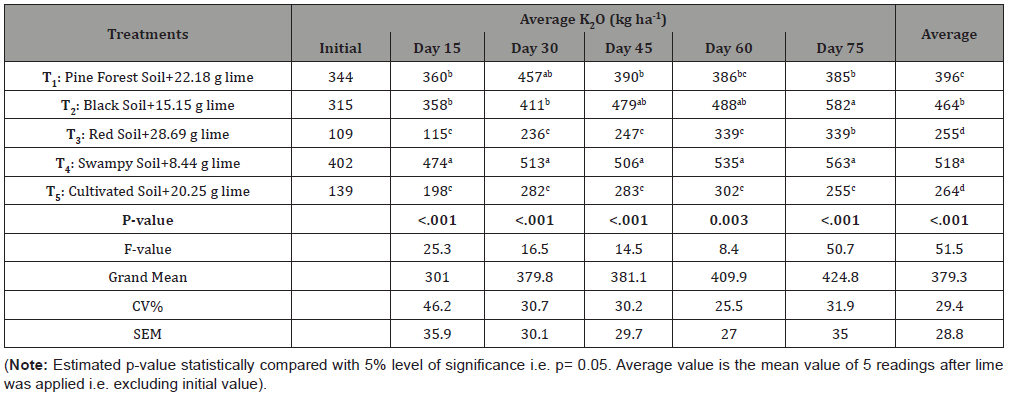
Critical analysis of the following data in Table claims that the type of soil i.e. physical and chemical properties of played significant role in the release of soil K after the lime was being applied. Each case had estimated p-values of much lesser than .05 and meant that the readings were statistically significant while compared between treatments.
According to DMRT, mineralization of soil K in swampy soil was statistically significant and superior among all types of soil/ treatment considered for study followed by black soil. Similar mineralization result was also observed between these soils in some case. For example, at 75 days of lime application these two soils were insignificant with p-value 0.53 (i.e. >.05) and availability of K in black soil was even greater (582 kg ha-1) than swampy soil. Similarly, release of soil K in pine forest soil was found similar to black soil in most cases. Red soil and cultivated soil have least K availability, were insignificant with each other but statistically significant with remaining soils.
Availability of potash in soil is greatly determined by its texture, CEC and pH level [7]. Generally, clayey soils and organic matter rich soils possess highly negative charged (CEC) colloids that bounds more potash in ionic forms, thus showing high potash content during laboratory analysis. Similar, results were recorded in case of Black/Clayey Soil and Swampy Soil, which showed relatively higher K content. Red Soil, Pine Forest Soil and Cultivated Soil had relatively lower potash content but as the pH level raised, its availability increases. Because, lesser the acidity of soil more is the cations (Ca2+ and Mg2+) concentration, which displaces the K+ ions and makes more available [8]. It was also favored by Hargreaves [7] showing that 100% potash available at pH 6.0, 77% available at pH 5.5 and 52 % at pH 5.0. However, this is the case for short term i.e. when the pH exceeds above 6.5, the availability of potash decreases. Slaton, Roberts and Ross [9] showed the soil K (ppm) value as 123/108, 126/115, 108/103 and 89/90 for winter/ spring season at pH level 6.1, 6.4, 6.8 and 7.1 respectively. So, the decrement in potash level observed in case of Pine Forest Soil and Cultivated Soil might be due to increase in pH level, leaching (as water was supplied in daily basis) and uptake by the crops planted in the soils.
Effect of Ag-lime on availability of soil Phosphorus (P)
In this experiment, mineralization of phosphorus (P) was one of the considered parameters to be compared in different types of soil as a result of liming. The average but approximate quantity of released P2O5 during experiment are tabulated in the Table 4, which shows that P mineralization due to liming was statistically significant in different types to soil as estimated p-values in each case is <.001 (i.e. much lesser than 5% probability value). This generally means that availability of P increases in all types of soil when lime is applied but vary with soil type.
Table 4:
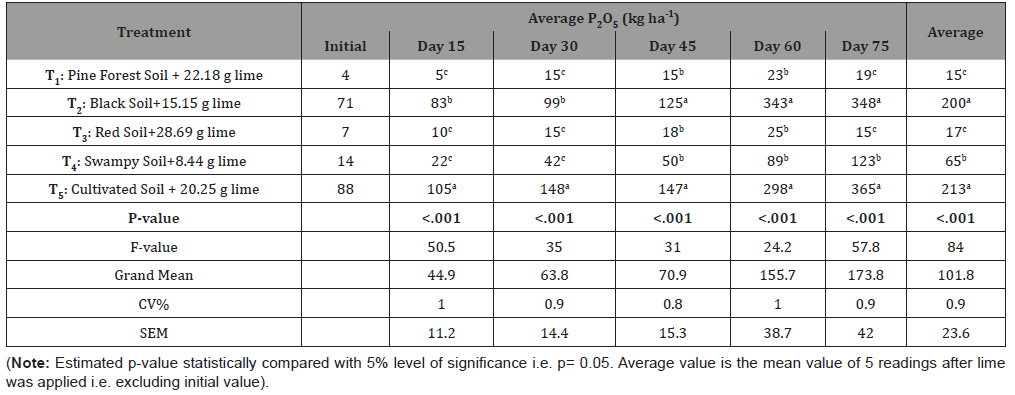
After the significance test of above data, it was further analyzed via Post-Hoc Duncan’s test and found that normally cultivated soil as most superior in terms of P-mineralization for all cases followed by black soil. In some cases, black soil was even insignificant i.e. similar to cultivated soil. Pine forest soil and red soil were also alike while swampy soil was initially similar with these two soils but at latter stage high mineralization was recorded and remained significant with two pairs of soils (i.e. Black soil- Cultivated soil & Red Soil- Pine forest soil).
Effect of different acidic soil plus lime in the growth of french bean
When the average plant’s height in each soil type and at different time interval were analyzed with Duncan Multiple Range Test (DMRT), French bean seemed much responsive to Normally Cultivated Soil while the least responsive to Pine Forest Soil. Swampy Soil was second most favorable soil for the growth of French bean among the treatments adopted. Similarly, Black Soil and Red Soil were insignificant in terms of plant growth although Black Soil was close to Swampy Soils while Red Soil was close to Pine Forest Soil in terms of performance (Table 5).
Despite of nutrient availability and favorable pH status, the plant growth did not perform well and died after 45 days of sowing, and somehow concluded that crop growth just after 15 days of lime application was not acceptable. It might also be due to the dry environment because, on one hand the experiment was conducted in summer season inside glasshouse and on other hand the Red Soils and Pine Forest Soils had soil drying properties, while the Black Soil was much clayey, heavy and hard that restricts both root and shoot growth. As a result, growth of French bean was much poorer in these three types of soils compared to Swampy and Cultivated Soils since these soils were relatively light and had better water retaining properties.
Table 5:Response of French bean (Phaseolus vulgaris) to different soil plus lime application.
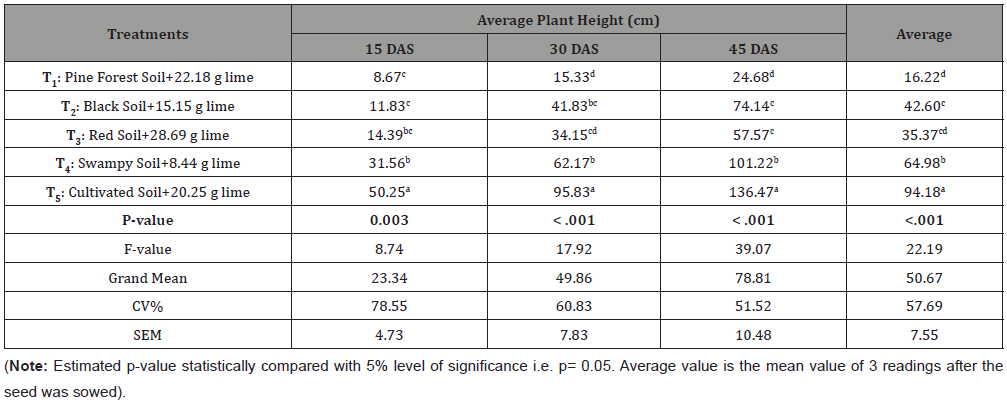
Several studies were done to relate the drought and plant growth. Whitmore and Whalley [10] found that drought as a complex stress affect plant growth in several interacting ways including root growth, water and nutrient absorption, root-shoot signaling and so on. When root perceives stress conditions in soils, it sends inhibitory signals to the shoot that may affect stomatal conductance, cell expansion, cell division and the rate of leaf appearance [11]. Similarly, the availability of some macro-nutrients and optimum pH do not favor the potential growth of plant because for better performance there should be balanced ratio of each essential nutrients [12].
The growth of French bean in each treatment (i.e. soil type) during experimental period is shown in the following bar diagram (Figure 1).
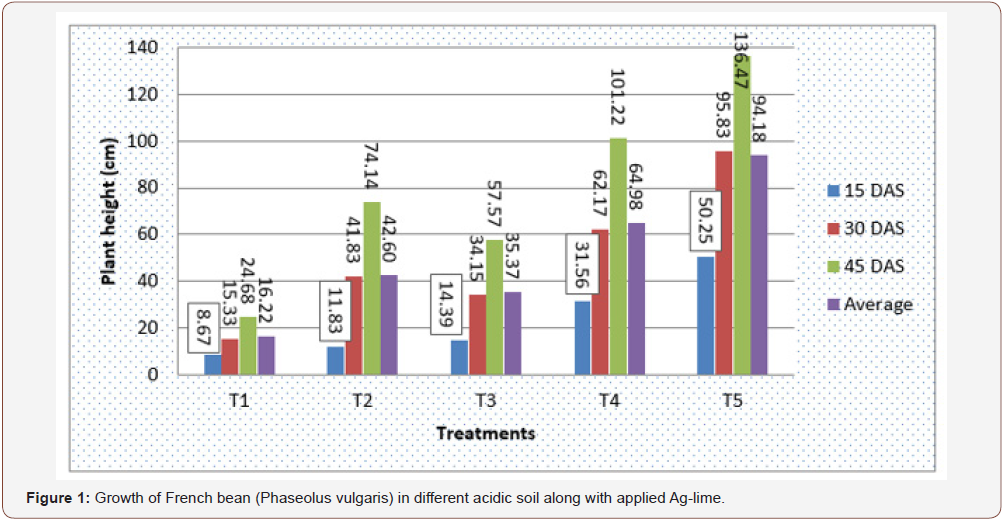
As shown in the above figure, among various treatments adopted T5 (Cultivated Soil + 20.25g lime) was superior at each case which had average plant height of 50.25cm, 95.8cm & 136.47cm at 15, 30 & 45 DAS, respectively. While, T1 (Pine Forest Soil + 22.18 g lime) supported least growth of plant with respective average plant height of 8.67cm, 15.33cm & 24.687cm at 15, 30 & 45 DAS. T4, T2, & T3 were in decreasing order of favoring plant growth. In terms of overall performance of French bean, cultivated soil was most favorable followed by swampy soil. Soil under pine forest was least favorable while Black and Red soils were similar. In first 15 days the height of plant in Red soil was 14.39cm and Black soil had 11.83cm but overall comparing these two, black soil was better for plant growth.
Conclusion
The study was conducted in different soils to observe the effect of liming concluded that, there was significant effect of Ag-lime in the alleviation of soil acidity as well as for mineralization of macronutrients like Potash and Phosphorus [13]. Liming increased the soil pH level and mineralization of P & K in all soils, but the extent of increment varied accordingly due to difference in chemical properties as well as physical properties. Generalization of soil pH level, nutrient (P & K) availability and plant growth in different soils after liming were the positive aspects of the study. According to the experiment, the desired soil pH value was achieved after 45-60 days of liming, but the desirable pH range was achieved after 30 days. Liming increased the availability of soil P & K significantly and depends upon the nutrient potentiality plus properties of soil. The growth response of French bean was greatest in normally cultivated soil followed by swampy soil while applying lime.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Tisdale SL, Nelson WL, Beaton JD, Havlin JL (1993) Soil acidity and basicity. Soil fertility and fertilizers, Macmillan Publication, New York 5: 364-404.
- USDA (1998) Soil Quality Indicators: pH. Soil Quality Information Sheet, by National Soil Survey Center in cooperation with the Soil Quality Institute, NRCS, USDA, and the National Soil Tilth Laboratory, Agricultural Research Service, USDA.
- Soil Survey Division Staff (1993) Soil Survey Manual, USDA Handbook. U.S. Government Printing Office, Washington, DC.
- Carson B (1992) THE LAND, THE FARMER, AND THE FUTURE, A Soil Fertility Management Strategy for Nepal, Nepal, ICIMOD 21: 54-56.
- Westerman RL (1990) Soil Testing and Plant Analysis. Number 3: In: Soil Science Society of America Book Series. Inc Madison, Wisconsin, USA, pp. 1-27.
- Santanna MA, Kaminski J, Dos Santos DR, Dos Anjos J, Cella C, et al. (2010) A new buffer that imitates the SMP solution for determining potential acidity of Brazilian soils. 19th World Congress of Soil Science, Soil Solutions for a Changing World, pp. 13-16.
- Hargreaves P (2015) Soil Texture and pH Effects on Potash and Phosphorus Availability. Potash, Potash Development Association, UK.
- Mc Kenzie RH (2003) Soil pH and Plant Nutrients. Agriculture and Forestry, Alberta, Canada.
- Slaton N, Roberts T, Ross J (2013) Fertilization and Liming Practices. Arkansas Soybean Production Handbook - MP197, Division of Agriculture-University of Arkansas, pp. 22-38.
- Whitmore AP, Whalley WR (2009) Physical effects of soil drying on roots and crop growth. Journal of Experimental Botany 60(10): 2845–2857.
- Passioura JB (2002) Soil conditions and plant growth. Plant, Cell and Environment, CSIRO, Division of Plant Industry, Australia, 25(2): 311-318.
- Tavakoli MT, Chenar AI, Rezaie M, Tavakoli A, Shahsavari M, et al. (2014) The Importance of Micronutrients in Agricultural Production. Advances in Environmental Biology 8(10): 31-35.
- Webster MA, Dixon GR (1991) Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycological Research 95(1): 64-73.
-
Dipendra Chaudhari, Shree Prasad Vista, Prabin Ghimire, Chakra Devkota. Influence of Agricultural Lime in Alleviating Acidity Level of Various Acid Soils. World J Agri & Soil Sci. 3(3): 2019. WJASS.MS.ID.000565.
-
Soil Acidity, Liming, Available soil potash, Available soil phosphorus
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






