 Mini Review
Mini Review
Haematological and Iatrical Response of Clarias Gariepinus (Burchell, 1822) Fed Different Commercial Feed and Farm-Made Feed
Mogaji Oluwaseyi Yanmife1*, Robert, Emem Aniete1 and Mogaji Naomi Oluwaseyi2
1National Institute for Freshwater Fisheries Research, New-Bussa, Niger State, Nigeria
2Ladoke Akintola University of Technology, Ogbomosho, Oyo State, Nigeria
Mogaji Oluwaseyi Yanmife, National Institute for Freshwater Fisheries Research, NewBussa, Niger State, Nigeria.
Received Date: June 24, 2021; Published Date: July 07, 2021
Abstract
The C. gariepinus juveniles were stocked with a mean average weight of 15 grams in nine 1m×1m×1m concrete tanks at a density of 60 juveniles/fish and fed twice daily to satiation. There was an increase in the platelet (166×103/μL), red blood cell (2.05×106/μL), and haemoglobin concentration (9.3 g/dL) in the treatment fed the farm-made diet with a significant difference (p<0.05) compared to the concentration of the fish before the experiment. However, there was a corresponding decrease in the treatment fed the “S” diet with a significant difference (p<0.05) compared to the initial value. There was no significant difference (p>0.05) in the serum biological values of total protein, albumin and aminotransferase concentration of the treatment fed the “S” and “B” diets compared to the initial values. The treatment fed farm-made diet “L” showed relative reduction in total protein (4.7 g/dL), albumin (0.8g/dL), aminotransferase (180μL), and alanine transaminase concentration (18 μL) and significantly different (p<0.05) as compared to the initial value of total protein (6.3 g/dL), albumin (1.3 g/dL), aminotransferase (192.67 μL), and alanine transaminase concentration (30.33 μL). The composition of the feed diet could impact the haematological and iatrical responses of C. gariepinus juvenile.
Keywords: Nutrition; Haematology; Fish feed; Immunity
Introduction
As aquaculture production becomes more intensive, fish feed is a significant factor in increasing the productivity and profitability of aquaculture [1]. Fish has continued to be a source of hope towards solving the global problem of protein malnutrition due to its nutritive value above other animal proteins. Moreover, about 50% of the world fish harvest is captured by the less developed countries and a large proportion of this catch is consumed internally [2]. For productive and sustainable aquaculture, a reliable supply of nutritionally balanced feed containing adequate amounts of all essential nutrients such as protein, fat, carbohydrate, vitamins, and minerals is a necessity [3]. The deficiency or excess of one or more nutrients in the diet may lead to reduced growth and pathological conditions. Dietary requirements for optimum growth and prevention of various deficiency signs are important for aquatic species. Haematological indices such as minerals, protein and nutrients reflect the overall well-being of fish. Iron (Fe) is an essential mineral for all animals including fish due to its vital role as a functional constituent of proteins [3]. It is involved in a wide range of biological processes such as oxygen transport, DNA synthesis and energy production [4,5]. It is against this backdrop that this study was done.
Materials and Methods
Experimental procedure
The experiment was a completely randomised design of 3 treatments in triplicate. A total of 540 Clarias gariepinus juvenile was bred at the fish farm of the University of Ibadan and acclimated for 14 days before the commencement of the study. The fish was sampled averagely within the weight of 15-17 grams and randomly stocked at a density of 60 juveniles per tank in triplicate in experimental concrete tanks of 1m×1m×1m in 750 litres of fresh water. The fishes were hand-fed twice daily (9:00 am and 5:00 pm) to satiation for 12 weeks.
The feed ingredients for the locally made fish feed “L” were compounded according to the formulation of the University of Ibadan fish farm unit. These ingredients were sourced and purchased in consideration of their similarities to the ingredients commonly used as catfish diet in Nigeria. Fish offal was procured from the fish farm unit of the University of Ibadan. The offal was cleaned and cooked gradually to a 100 ºC for 15 minutes before blending with other ingredients. The mixture was further ground with a Unitech hammer mill to homogenous size, mixed in an appropriate ratio, made into dough, pelleted into 2 mm size and sun-dried for 24 hours. The dried feed was packaged in an air-tight polythene bags and stored in a container at room temperature. The commercial 2 mm size feed was purchased at a feed depot in Ibadan. The two commercial diets connoted by “S” and “B” were used in this experiment. The gross composition of ingredients in the local feed is shown in Table 1. The feed formulation of the commercial diets was not available from the producers.
Table 1: Gross composition of local diet “L”.

Haematological studies
The blood samples of Clarias gariepinus were collected from un-anaesthetised fish as described by Morgan and Iwama [6]. The blood samples were taken from the dorsal fin of the fish following Klontz & Smith [7] for haematological analysis according to Dacie and Lewis [8]. Plasma total protein was estimated through the biuret method [9] The creatinine, globulin, and albumin/globulin ratio by the standard methods described by Coles [10].
The fishes were taken out individually using a small hand net and placed belly upward on a solid platform. Blood samples of about 5 mL was collected from the caudal peduncle [11] with the aid of a 2 mL plastic syringe and dispensed into Ethylene Diamine Tetraacetic Acid (EDTA) anticoagulant. Furthermore, 3 mL was put into a tube containing Lithium Heparin (LH) anticoagulant to get the plasma for biochemical analysis. The Packed Cell Volume (PCV) and Haemoglobin (Hb) were determined using the method described by Mitruka & Rawnsley [12]. Erythrocyte count and Leukocyte count were determined using the improved Neubauer haemocytometer after dilution [13]. Differential leukocyte counts were determined by scanning Giemsaʼs stained slides in the classic manner [13]. Alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and cholesterol were determined by the spectrophotometric method. Blood urea nitrogen was determined by urease method and creatinine by Folin-Wu filtrate methods as described by Connors et al [14]. Total protein content was determined using the biuret method as described by Stoskopf [11] while albumin was determined using the Bromocresol green method as described by Peters et al., [15].
The statistical analysis of the data was performed with a statistical package (SPSS 20.0 for Windows, SPSS Inc., Richmond, CA, USA). Data obtained were subjected to One-way analysis of variance (ANOVA) to test between the means of treatments and Turkey’s tests to compare the variance amongst the mean at p < 0.05.
Results and Discussion
The blood parameters were evaluated and recorded in Table 2. The results of the haematological serum biochemistry of C. gariepinus fed different diets showed no major negative indication of diets in the parameters before and after the study. The PCV, haemoglobin and RBC levels in all the treatments indicated a well transfer of oxygen and nutrients through the body. The WBC, MCHC, MCV and platelet levels in all the treatments indicated the immunity of the fish against any diseases. This indicated the acceptability of the diets in the treatments during the study period.
Table 1: Hematological serum biochemistry parameters of African catfish fed different diet at the start and end of experiment.
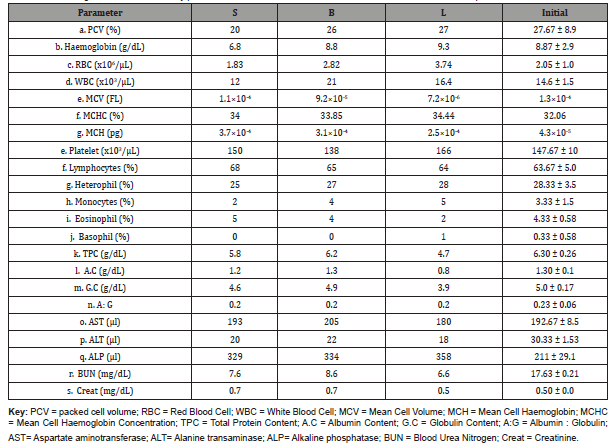
Changes in the haematology of fish in response to stressing agents are indicators of the stressful stage of fish, producing useful information to curb any unfavourable condition that may affect the fish health [16]. There were increase in the PCV, Hb, RBC, MCHC, platelet and ALP level of the treatments L compared to the treatment fed commercial diets. The study of George et al. [17] observed that when 50% fish meal was replaced by soybean meal in the diet for Clarias gariepinus, there was increase in PCV, Hb and RBC of the fish fed the diet which indicated high oxygen absorption and transportation capacity of the cells of the fish. The report of Fagbenro et al. [18] showed that decrease in haematological parameter with increasing level of incorporation of sesame meal might not be unconnected to the presence of tannin and phytate present in the seed meal. Though, there was no privy information on the formulations of the commercial diets. The study also showed a higher WBC, MCV, total protein content, albumin content, globumin content, AST and ALT levels in treatment fed commercial diet B compared to the treatment fed commercial diet S. This opined with the report of Akinwande et al. [19] that a measurable increase in white blood count of fish is a function of immunity and resistance to some vulnerable illness or disease. Though, no illness was recorded during the study period, the blood status of the fish is a valuable means of evaluating the physiological condition of cultured fish with respect to determining the effect of diets and other stress factors on fish health [18].
Conclusion
This study shows variations in the haematological parameters of fish fed different diets. This study shows no detrimental issues to the health of African catfish. Though, fish feed could influence the haematological and iatrical factors of African catfish.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Mogaji O Y, IbiyoL MO (2016) Growth performance of Oreochromis Niloticus Fed Brewer’s dry grain (BDG) base NIFFR feed supplemented with xylanase enzyme. International Journal of Fisheries and Aquatic Studies 4(3): 220-222.
- Ibiyo LMO (2018) Chemical composition of clupeid fish as alternative replacement for foreign fish meal in fish feeds in West Africa region. Animal Husbandry, Dairy and Veterinary Science 2(4): 2-3.
- Zafar N, Khan MA (2020) Effects of dietary iron on growth, haematology, oxidative stress and hepatic ascorbic acid concentration of stinging catfish Heteropneustes fossilis. 516: 1-9.
- Luo Z, et al (2017) Effect of dietary iron (Fe) levels on growth performance, hepatic lipid metabolism and antioxidant responses in juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture Nutrition, 23: 1475 - 1482.
- Tarifeno Saldivia E et al (2018) Iron overload is associated with oxidative stress and nutritional immunity during viral infection in fish. Frontiers in Immunology 9: 1-14.
- MorganJD, IwamaG K (1997) Measurements of stressed states in the field. In: Iwama, G.W. et al. Fish stress and health in aquaculture. Cambridge, England: Cambridge University. Pp. 247-270.
- Klontz GW, SmithL S (1968) Methods of using fish biological research subjects. Methods of animal experimentation pp. 323-385.
- Dacie JV, Lewis S M (1984) Practical haematology, 11th edn. ELBS and Churchill, Livingston.
- Garry AW, WilliamsT Y (1977) Clinical methods for the assessment of the effects of environmental stress on fish health. Technical papers of the U.S. Fish and Wildlife services. Washington DC.
- Coles E H (1986) Veterinary Clinical Pathology, 3rd ED.; W.B. Sanders Company London. pp. 283-293.
- Stoskopf M K (1993) Clinical Pathology in Fish Medicine. WB Saunders Company, New York, USA. pp. 251.
- Mitruka B M, Rawnsley HM (1977) Clinical Biochemical and Haematological Reference Values in Normal Experimental Animals. Mass Publishing, New York, pp. 1-58.
- Schalam OW, Jain N C, Carroll E J (1975) Veterinary Haematology. 3rd Edn., Lea and Febiger, Philadelphia, USA, pp. 807.
- ConnorsW M, Pihl A, Dounce A L, Stotz E (1950) The determination of creatinine in plasma or serum and in urine: A critical examination. Journal of Biological Chemistry 184: 29-36.
- Peters T, Biamonte GT, Doumas BT (1982) Selected Method of Clinical Chemistry. AACC., Washington DC 9: 380.
- Bello Olusoji OA, Omoare VY, NwanaL C (2006) Comparative studies on the haematological characteristics of pond-cultured and wild tilapia (Oreochromis niloticus) Linnaeus, 1857 Nigerian Journal of Forestry 36: 134-141.
- George FOA, ObasaS O, Otubusin SO (2007) Growth response and carcass quality of African catfish, Clarias gariepinus (Burchell, 1822) fed multi-enzyme-supplemented soybean meal diets. Journal of Applied and Tropical Agriculture 12: 51-59.
- Fagbenro OA, Adeparusi EO, Jimoh WA (2013) Haematological profile of blood of African catfish (Clarias gariepinus, Burchell, 1822) fed sunflower and sesame meal-based diets. Journal of Fisheries and Aquatic Science 8: 80-86.
- Akinwande AA, Moody FO, Sogbesan OA, Ugwumba AAA, Ovie SO (2004) Haematological response of Heterobranchus longifilis fed varying dietary protein levels. Proceedings of the 19th annual conference of the fisheries society of Nigeria, Ilorin, Nigeria, pp. 715-718.
-
Mogaji Oluwaseyi Yanmife, Robert, Emem Aniete, Mogaji Naomi Oluwaseyi. Haematological and Iatrical Response of Clarias Gariepinus (Burchell, 1822) Fed Different Commercial Feed and Farm-Made Feed. World J Agri & Soil Sci. 7(2): 2021. WJASS. MS.ID.000660.
-
Nutrition, Haematology, Fish feed, Immunity, Diet, Blood, Globulin, Creatinine
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






