 Research Article
Research Article
Chemical Drying of Nematodes for Scanning Electron Microscopy Observations
Fawzia H Abdel Rahman*
Department of Biology, Texas Southern University. Houston, TX 77004, USA
Fawzia H Abdel-Rahman, Department of Biology, Texas Southern University. Houston, TX 77004, USA.
Received Date: May 10, 2021; Published Date: May 26, 2021
Abstract
Use of the Scanning Electron Microscopy (SEM) to study nematode morphology and taxonomy is extremely important tool and it has become an essential component of new species description. However, the general preparation procedures for SEM can frequently result in specimen distortion during fixation and other different drying procedures such as critical point dry. Here, a chemical preparation method using hexamethyldisilazane (HMDS) was employed to dry nematodes for SEM. The plant parasitic nematode Globodera sp., and the soil free-living nematode Caenorhabditis elegans were dried without any deformities. Adult females and Cyst stages of Globodera, and the synchronized adults of C. elegans hermaphrodites were used in this test. Globodera adults and cysts were obtained in FAA, and the synchronized C. elegans hermaphrodites were killed, and fixed in FAA for 48 hours. All nematode specimens were dehydrated through an acetone series to 100% acetone. Nematodes then were transferred gradually to 100% hexamethyldisilazane (HMDS). All nematode specimens in 100% HMDS were left overnight under the hood to dry. Dried nematodes were mounted on SEM aluminum stubs, sputter coated with gold and viewed with SEM using accelerated voltage of 10, 15, or 20 KV. SEM Images of C. elegans and Globodera sp., proved that drying of nematodes using HMDS were as good/or better than those regularly dried by critical-point dryer. Using HMDS to prepare nematodes for SEM is efficient, safe and easy to use.
Keywords: C. elegans; Chemical drying; Globodera; Hexamethyldisilazane; HMDS; Method; Nematodes; SEM; Technique
Introduction
The scanning electron microscope has been available to nematologists for long time; it is a useful tool to study nematodes morphology and taxonomy. The SEM has the advantage of obtaining magnifications higher than 50,000X; it has a greater depth of field and much higher resolution than the light microscope, which enables the maximum useful magnification with good resolution of 1,000X. Also, specimens viewed with SEM can also be orientated at various angles that allow many important cuticular and morphological structures to be viewed and imaged easily [1]. The SEM is significantly different from the light microscope, and thus successful observation of nematodes requires specialized preparative procedures [2]. Therefor results from SEM vary greatly in quality, being much affected by the type of fixation, processing and mount ing involved. In SEM observation, morphological details are often obscured due to poor preparation, which makes the specimens preparation for SEM observation still the most important limiting factor in the quality of information obtained by SEM; in addition the specimens are easily lost or damaged during the long preparation process [1].
To observe nematode specimens using the SEM, specimens have to be viewed in a vacuum and they need to be completely dried to withstand the vacuum and the electron beam [1-3]. Successful preparation of nematodes for SEM includes several procedures such as killing, fixation, dehydration and drying the animals. Various methods of fixation and processing specimens for observation with the SEM have been proposed and several authors reviewed techniques for preparing nematodes for Scanning Electron Microscopy [1-5]. Drying nematodes could be accomplished by several methods including air drying [14-16]; with or without fixation, for tough structures such as style’s, spicules, and cysts, also include processing nematodes to glycerol, drying via acetone, impregnation with Spur resin [9-12]. Critical point drying (CPD), via ethanol and/or amyl acetate, and freeze drying [1-3,12-14]. Critica1 point drying [2,3,5,12,15-18] has been used extensively but results are variable, and the pressure apparatus is expensive and requires skilled users. Eisenback [2,3] reviewed the various methods for drying nematodes and concluded that freeze-drying, gave satisfactory results for many but not all specimens and also concluded that equipment for freeze-drying and particularly a freezing stage for use with the SEM are very expensive. Therefore, although there are various satisfactory methods for preparing nematodes for observation with the SEM, the most accurate techniques require expensive equipment and all procedures require technical expertise. As a consequence, examination of specimens with the SEM may not be routinely available. Because after fixation, the nematodes must be dehydrated and possibly infiltrated with a fluid that is miscible with the CPD solution in order to dry nematodes. Nematodes that have been fixed and dehydrated are still in a liquid environment, and because successful observation with the scanning electron microscope, requires that the specimens be completely dry using different processes such as CPD. Severe distortions and artifacts often result when nematodes are dried because evaporating liquids exert pressure on the surface of the tissue [1-3]. Here in this technique nematodes prepared for SEM observation were chemically dried using hexamethyldisilazane (HMDS, Sigma) to eliminate and replace other complicated drying techniques such as the Critical point drying.
Materials and Methods
Chemicals: hexamethyldisilazane (HMDS, Sigma- Aldrich) Milwaukee, WI, USA
Wild-type (N2) Caenorhabditis elegans were maintained in the laboratory at 20 °C on Nematode Growth Medium (NGM) agar plates (3g NaCl, 17g agar, and 2.5g peptone, 975ml H2O). All NGM plates were seeded with E. coli OP50 as the food source. Synchronized C. elegans hermaphrodite adults were washed off NGM plates and collected in M9 buffer (3g KH2PO4, 6g Na2HPO4, 5g NaCl, 1ml 1M MgSO4, H2O to 1 liter) or water. C. elegans adult hermaphrodites were killed by dipping the nematodes in a test tube in hot water (65 °C) for two minutes. Globodera sp. cysts and adult females were obtained fixed in FAA and/or glycerin. All Nematode samples were ultrasonicated and fixed further in FAA at least for 48 hours. Samples were dehydrated in a graded series of acetone in FAA (ethanol could be used also), until it reached 100% acetone [19-25]. The nematode specimens were dehydrated through an acetone series as follows: 25, 50, 75, 95%, this was followed with 3 changes of 100% acetone (30 minutes for each change). In the same way graded ethanol series could be used as a dehydrating agent to 100% absolute ethanol. All dehydrated nematodes were transferred gradually to 100% hexamethyldisilazane (HMDS). This was done by transferring the acetone dehydrated nematodes gradually through a graded series of mixtures of acetone and HMDS to 100% HMDS (75% acetone and 25% HMDS, then 50% acetone and 50% HMDS, then 25% acetone and 75% HMDS, and finally 3 changes of 100% HMDS). Nematode specimens in 100% HMDS were left overnight under the hood of the gas chamber for the chemical evaporation and complete dry of the nematodes. After the evaporation of HMDS, dry nematode specimens were observed using the stereomicroscope. Dried nematodes were kept in a desiccator for 24 hours and then mounted on SEM aluminum stubs using a double-coated carbon tape. All nematode specimens on stubs were sputter coated with gold and viewed with EVEX Global SEM using accelerated voltage of 10, 15, or 20 KV.
Results and Discussion
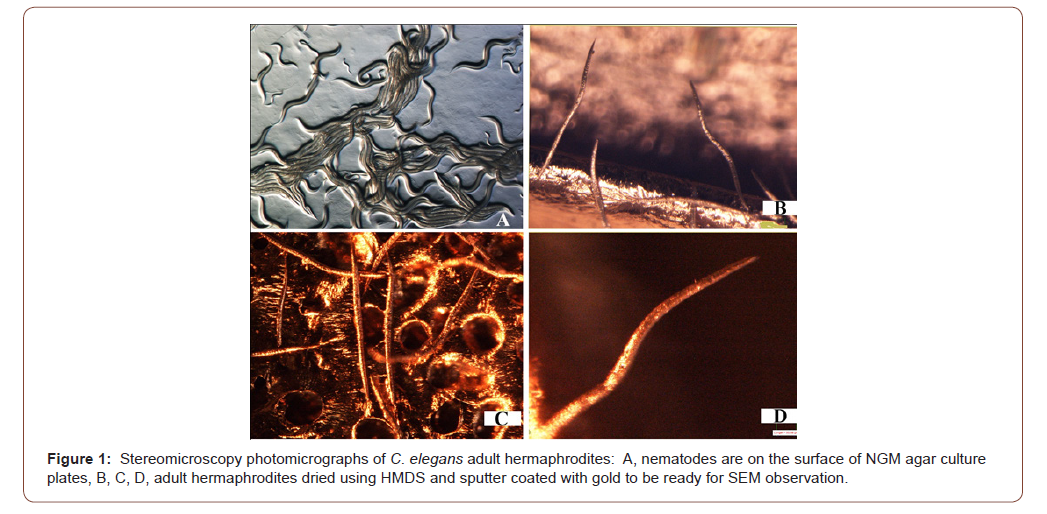

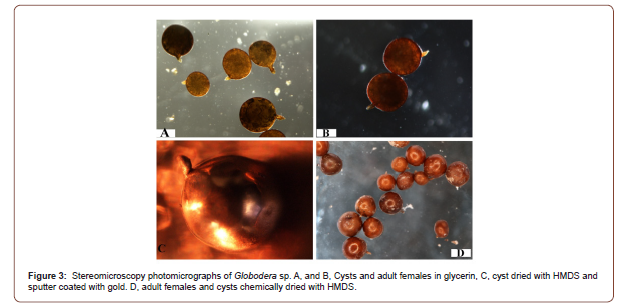
Stereomicroscopy observations of the nematode specimens proved that the chemically dried nematodes were in good shape and condition and that they are ready for sputtering for SEM observation (Figures 1 & 3). Furthermore the stereomicroscope and SEM images for both C. elegans and Globodera sp., proved that using hexamethyldisilazane (HMDS) as a drying agent to completely dry nematodes in order to be ready for SEM observation is safe and reliable and that it did not show any distortion, shrinkage or damage due to surface tension that is associated with other drying techniques.
The stereomicroscope examination of C. elegans adult hermaphrodites that were dried by HMDS and sputter coated with gold, did not show any distortion or damage due to the procedure of chemical drying (Figure 1). When those nematodes observed using SEM with low or high magnification again the nematode body and morphological structures did not display any distortion or any artifacts (Figure 2). Also it was evident using the stereomicroscopy examination for Globodera stages that were dried by HMDS as a drying agent to dry the adult females and the cysts, that the nematodes sustained the treatment and appeared in very good shape, did not suffer from any damage or shrinkage (Figure 3). Also, this was proved further by the SEM observation for the HMDS dried Globodera adult and cyst stages, that the chemical dried nematodes did not sustain any damage or any obstruction of morphological characteristics (Figure 4).

Chemical drying of nematodes for SEM observation using hexamethyldisilazane (HMDS) is an easy and quick to use procedure. It does not require any equipment or expertise other than being carful as using any toxic and hazardous chemical. Chemical drying of nematodes using HMDS did not cause any harm, shrinkage or distortion in the two different species used C. elegans, and Globodera sp . It is a very efficient and reliable procedure in addition it is inexpensive, quick, and easy to use comparing to other available procedures of drying nematodes, such as air dry, impregnation with resin, Critical point drying and freeze drying. Specimens after fixation, must be dehydrated and possibly infiltrated with a fluid that is miscible with the CPD solution. Severe distortions often result when nematodes are dried because evaporating liquids exert pressure on the surface of the tissue [2]. In addition, the pressure apparatus is expensive and requires skil1ed users, also, specimens are easily lost or damaged during preparation [1-3]. Freeze drying of nematodes for SEM observation results are also variable in addition to the cost involved [2,3]. Air-drying of nematodes is only possible with certain structures such as stylets, spicules, or the toughened cuticle of the cyst stages of certain nematodes [2,3]. The simple drying technique reported in this study, using the chemical HMDS to dry two different species of nematodes, C. elegans, and the toughened cysts of Globodera sp., in addition drying of the soft boded adult females of Globodera sp., yielded very good results without any artifacts or damage to the dried specimens. It is an excellent alternative for other complicated and expensive methods such as critical point drying [26-29].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Hooper DJ (1988) Use of the Scanning Electron Microscope for the Identification of Nematodes. in R. Fortuner, ed. Nematode Identification and Expert System Technology, vol. 7. NATO Scientific Affairs Division (Series A: Life Sciences), Springer, Boston, MA.
- Eisenback JD (1985) Techniques for preparing nematodes for scanning e1ectron microscopy. Pp 79-105 in KR Barker CC Carter, and JN Sasser, Eds an advanced treatise on Me1oidogyne, Vo1ume II: Methodology. North Caro1ina State University, Ra1eigh, USA.
- Eisenback JD (1986) A comparison of techniques usefu1 for preparing nematodes for scanning e1ectron microscopy. J Nematol 18: 479-487.
- Shepherd AM, C1ark SA (1986) Preparation of nematodes for e1ectron microscopy. in JF Southey, ed. Laboratory methods for work with plant and soil nematodes. London, Her Majesty's Stationery Office, pp. 121-131.
- Wergin WP (1981) Scanning electron microscope technique an application for use in nematology. in BM Zuckerman RA Rhode, eds. Plant parasitic nematodes, vol 3. New York: Academic Press, pp. 175-204.
- Eisenback JD, Hirschmann H (1982) Morphological comparison of stylets of male root-knot nematodes (Meloidogyne spp.). Scanning Electron Miscroscopy III: 837-843.
- Eisenback JD, Hirschmann H, Triantaphyllou AC (1980) Morphological comparison of Meloidogyne female head structures, perineal patterns, and stylets. J Nematol 12: 300-313.
- Ellenby C, Wilson EM (1969) Scanning electron microscope observations on the spear of the second-stage larva of Heterodera rostochiensis. Nematologica 15: 290-291.
- Clark SA, Stone AR (1975) Asimple method for preparing nematodes for scanning electron microscopy, using Spurr's low-viscosity epoxy resin. Nematologica 21: 256-266.
- DeGrisse AT (1974) A method for preparing nematodes and other soft tissues for scanning electron microscopy. Meded Fac Landbouwwet Rijksuniv Gent 38: 1685-1695.
- DeGrisse AT (1979) Modification of themini-sieve and prepolymerized plate techniques for use in electron microscopy. J Nematol 11: 196-199.
- Green CD, Stone AR, Turner RH, Clark SA (1975) Preparation of nematodes for scanning electron microscopy. Journal of Microscopy 103: 89-99.
- Hammon RA (1969) The preparation of nematode material for scanning electron microscopy. J Micros 90: 273-274.
- MacKenzie AP (1976) Principles of freeze drying. Transplant Proc (2. Suppl 1): 181-188.
- Anderson TF (1951) Techniques for the preservation of three-dimensional structure in preparing specimens for the electron microscope. Transactions of the New York Academy of 13: 130-134.
- Boyde A (1978) Pros and cons of critical point drying and freeze drying for SEM. Scanning Electron Microscopy 11: 303-314.
- Cohen AL (1979) Critical point drying principles and procedures. Scanning Electron Microscopy 11: 303-324.
- Wergin WP, Stone AR (1981) Techniques for preparation and examination of plant parasitic nematodes in the scanning electron microscope. Scanning Electron Microscopy III: 169-176.
- Abdel Rahman, FH, Maggenti AR (1987a) Hirschmanniella pomponiensis n. sp. (Nemata: Pratylenchidae), Parasitic on Bulrush, Scirpus robustus Pursh. J Nematol 19: 147-151.
- Abdel Rahman FH, Maggenti AR (1987b) Meloidogyne californiensis. n.sp (Nemata: Meloidogyninae) Parasitic on Bulrush, Scirpus robustus Pursh. J Nematol 19: 207-2 17.
- Abdel Rahman F H, Maggenti AR (1988) Gracilacus elongata n. sp. (Nemata: Criconematoidea) parasitic on Juncus ensilfolius from Mendocino, California. Revue de Nematologie 11(3): 303-306.
- Abdel Rahman FH (1993) SEM Observations and Description of Plectus cylindricus sp. n. (Nematoda: Plectidae), from California, USA. Nematologia Mediterranea 21: 59-62.
- Maggenti PA, Maggenti AR, Abdel Rahman F (1990) Description of a new species of Plectus Bastain 1865 (Nemata: Plectidae) from Mendocino County, California, USA with SEM observations. Revue de Nematologie, 13(1): 89-927.
- Maggenti AR, Abdel Rahman FH, Del Perado Vera IC (1992) Three new species of Rhabdochona Railliet, 1916, (Nemata: Rhabdochonidae) from Rainbow Trout in California Streams. J Nematol 24(3): 379-390.
- Massoud SI, Abdel Rahman FH, Ghorab AI (1988) Studies on Heterodera Daverti on Egyptian clover Trifolium Alexandrium. Nematologia Mediterranea 16: 7-11.
- Green CD, Stone AR, Turner RH, Clark SA (1975) Preparation of nematodes for scanning electron microscopy. Journal of Microscopy-Oxford 103: 89-99.
- Green CD, Stone AR, Turner RH, C1ark SA (1975) Preparation of nematodes for scanning e1ectron microscopy. Journal of Microscopy 103: 89-99.
- Murphy JA (1978) Noncoating techniques to render biological specimens conductive. Scanning Electron Microsc II: 175-193.
- Wergin WP (1981) Scanning e1ectron microscopic techniques and app1ications for use in nemato1ogy. in BM Zuckerman, and RA Rohde, eds. Plant Parasitic Nematodes. Volume III. New York, Academic Press, pp. 175-204.
-
Fawzia H Abdel Rahman. Chemical Drying of Nematodes for Scanning Electron Microscopy Observations. World J Agri & Soil Sci. 7(2): 2021. WJASS.MS.ID.000656.
-
C. elegans, Chemical drying, Globodera, Hexamethyldisilazane, HMDS, Method, Nematodes, SEM, Technique
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Abstract
- Introduction
- Scope of GM Crops and Genetic Engineering
- Biotechnology Adoption
- Property Rights and Privatization
- Role of the European Union
- Adoption of GM Crops
- Genome Editing
- Diet and Nutrition
- Climate Change
- Feed the Future African Countries
- Conclusion
- Acknowledgement
- Conflict of Interest
- References






