 Short Communication
Short Communication
Biomonitoring of Titanium Contamination by Moss Samples
Pierre Masson*, Patrice Soulé and Thierry Dalix
USRAVE, Centre de Recherches INRAE de Nouvelle Aquitaine - Bordeaux, France
Pierre Masson, USRAVE, Centre de Recherches INRAE de Nouvelle-Aquitaine Bordeaux, CS20032, 33882 Villenave d’Ornon Cedex, France.
Received Date: March 13, 2021; Published Date: March 23, 2021
Abstract
The large applications of titanium dioxide nanoparticles may shortly cause damage to the environment. There is a need to study their release into the ecosystems because the toxic effects of titanium have not been strongly investigated. To study the exposure of TiO2 nanoparticles to environmental media, mosses were used as bioindicators. An analytical procedure for the determination of titanium in moss samples by inductively coupled plasma-optical emission spectrometry (ICP-OES) was first developed. Two digestion procedures were applied to convert various solid moss samples to aqueous solution, namely wet digestion using hydrogen peroxide (H2O2) and nitric acid (HNO3) with and without hydrofluoric acid (HF). The obtained results showed that the use of HF was necessary for plant analysis. Finally, biomonitoring study of Ti was conducted on approximately 800 moss samples collected in several sites such as rural, forest or urban ecosystems. TiO2 is ubiquitous in the environment. In order, to distinguish its sources, natural or anthropogenic, further chemical quantification of Al, Fe and Zn was conducted and implemented in a multivariate statistic model.
Keywords: Moss; Plant analysis; Titanium dioxide
Introduction
Titanium dioxide nanoparticles (TiO2 NPs) are one of the most produced nanomaterials. They present very interesting properties such as white pigment with high UV absorbing properties, photocatalytic effects, and practical applications such as personal skincare product, water treatment agent or bactericidal agent. In consumer products, TiO2 NPs are introduced as E171 food additive. This extensive use of TiO2 NPs leads to industrial emissions and uncontrolled deposition of NPs into the environment [1]. Soil is a major compartment of accumulation for TiO2 NPs due to the agricultural application of sewage sludge, which accumulates these nanomaterials. Introduced into ecosystems, TiO2 NPs are involved in the exchange of matter between soil and plants. In these conditions, special attention must be given to Ti because it may present risks to human health and cause damage in living organisms [2]. Toxic effects have been reported trough a decrease of growth and photosynthesis activity of plants and algae [3-4]. Symptoms of Ti toxicity were chlorosis, necrotic spots on leaves, and stunting [5].
To assess environmental quality, methods using bio indicators are increasingly being used. Biomonitoring may be defined as the use of organisms to obtain data on the impact of a pollution source on environment. The relevant information is commonly deduced from changes in the behavior of the monitor organism (species composition, physiological performance, morphology) or from the chemical composition in the monitor tissues [6].This implies that the monitor should concentrate the elements of interest according to its ambient conditions. Various bio monitor materials have been applied in trace element air monitoring programs such as lichens, mosses, ferns, grass, tree bark, tree rings or pine needles [6-7]. The elemental composition of vegetation generally reflects the bioavailable and mobilized metals present in the soils.
Among these plant species, mosses are the most frequently used bio monitors for atmospheric depositions. Due to their anatomical structure and the way they absorb nutrients, these organisms effectively accumulate atmospheric pollutants, especially metals. Their morphology does not change with seasons and accumulation can occur throughout the year. They are tolerant to high metal levels, which allow them to be good witnesses of pollution. Many species are geographically widespread and grow under different environmental conditions, even in industrial or urban areas [8]. Mosses represent then a nice way to account for the exposure of living organisms to pollutants.
The present study aims to better understand TiO2 NPs contamination with a comparison of the Ti concentrations moss samples originating of rural, forest and urban areas. However, TiO2 is naturally present in soils. To identify a possible contamination, it was necessary to distinguish natural TiO2 from anthropogenic inputs and the natural background. The use of a discriminant model was tested using a multivariate characterization based on Al, Fe, Ti and Zn concentrations [9]. This work was supported by the analysis of Ti in 800 mosses from different European countries associated with a project of biomonitoring of metal deposition by moss. For elemental determination, inductively coupled plasma optical emission spectrometry (ICP-OES) was used. The aim of the study was also to determine the best digestion method. For this, moss samples were put in solution according to two methods, using or not hydrofluoric acid, to retrieve all the elements in a quantitative way. Estimation of the sensitivity of measurements was also carried out.
Material and Methods
Samples and preparation
Approximately 800 samples of moss were analyzed for this study. Five moss species, Pseudoscleropodium purum (Pp), Hypnum cupressiforme (Hc), Thuidium tamariscinum (Tt), Pleurozium schreberi (Ps) and Hylocomium splendens (Hs), are sampled on 663 forest sites and rural sites representative of background pollution, which are, a priori, not influenced by the proximity of a local source of pollution. Mosses species are also sampled on 176 urban areas, which are influenced by local source of anthropogenic pollution. The overall samples represented several available ecosystems. Thus, the random sampling was done according to the geographical origin of samples in order to balance the class distributions within the splits (stratified sampling). Among these species, Hc, Pp and Tt were the most sampled and represented 96% of sites.
Samples were put in solution according to two methods, using or not hydrofluoric acid (HF), to extract Ti in a quantitative way. This last reagent allows knowing if the elements of interest remain adsorbed on silica often present in plants (between 0.1 and 10% of dry matter). All reagents were analytical grade. All vessels were rinsed with purified water before use. Water purified (18 MΩ) with an ELGA (Bucks, UK) Pure lab ultra-water purification system was used for all solutions. For the digestion of plant samples, the reagents were hydrogen peroxide (H2O2 30% Baker analyzed), concentrated nitric acid (HNO3 65% Baker analyzed), and concentrated hydrofluoric acid (HF 48% Baker analyzed). The samples were digested with blanks.
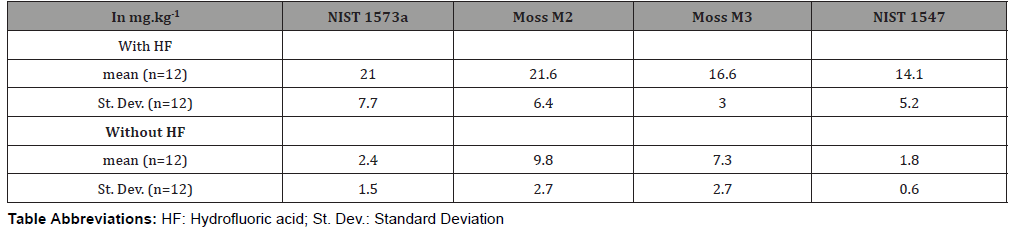
For both digestion methods, approximately 0.25 g (0.25 ± 0.01 g) of dried plant material was weighed into a 50 mL polyethylene tube. Then 3.5 mL of HNO3 and 7 mL of H2O2 were added. After 16 hours (overnight) of cold digestion, the tubes were heated on a hot block SC861 from Environmental Express (Mt. Pleasant, SC, USA) during a period of 2 h. The heating block was raised to 95°C. Next, after evaporation, the residue was taken up with 2.5 mL HNO3 and adjusted to 50 ml with purified water. For the digestion using HF, 2.5 mL of HF were added after the evaporation. Next, after a new evaporation to 125°C, the residue was taken up with 2.5 mL HNO3 and adjusted to 50 ml with purified water. For the study of the digestion, two samples of NIST (National Institute of Standard and Technology, Gathersburg, MD, U.S.A.) were used: NIST 1573a (Tomato leaves) and NIST 1547 (Peach leaves). Two samples of mosses, named respectively M2 and M3 were also used. However, all these samples present no certified reference concentrations concerning Ti.
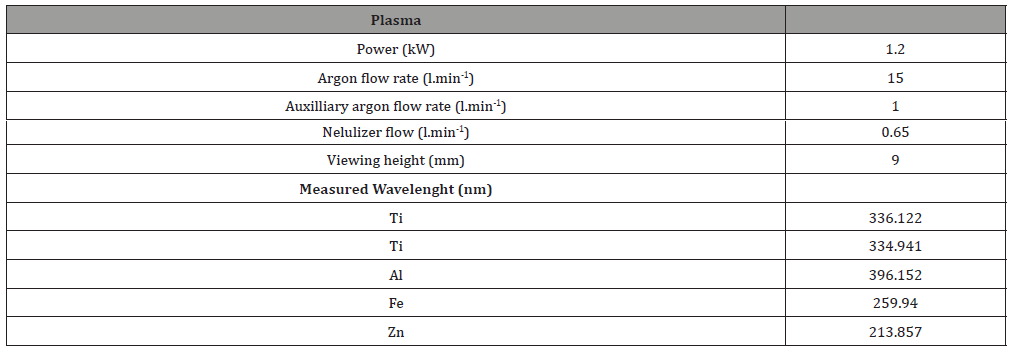
Table 1: Operating parameters of the ICP-OES spectrometer.
Chimiometry
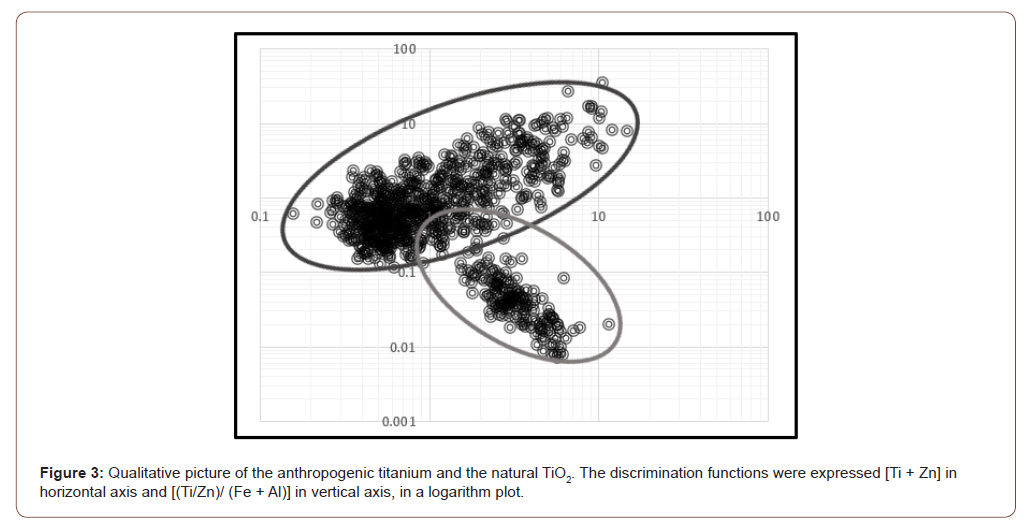
To understand the impact of engineered nanoparticles to environmental media, it was important to identify the presence of TiO2 NPs in the natural bioavailable Ti. Distinguishing between these two forms of TiO2 requires chemometric method. Kim proved the possible use of discrimination functions based on the elemental concentrations of target elements (Ti, Zn) compared to background elements (Al and Fe): In a logarithm scale, the elemental ratio of {[Ti] + [Zn]} to {[Ti]/[Zn]}/{[Fe] + [Al]} were plotted for all samples [9]. In this study, we tested the feasibility of this chemometric multivariate characterization method to distinguish between NPs and natural TiO2 in the investigated moss samples.
Results and Discussion
Digestion methods
The aim of the study was first to determine the best digestion method, with or without HF to retrieve the overall Ti presents in plant samples. Table 2 shows the experimental results obtained on various materials. These materials are not certified for their Ti concentrations but the results show that it was necessary to investigate the use of HF. In fact, significant differences in Ti concentrations were observed between the two digestion methods on the two reference materials (NIST 1547 peach leaves and NIST 1573a tomato leaves) and on the two moss samples M2 and M3. These four samples were used in each batch of samples to guarantee the quality of measurement concerning the other elements. The recoveries obtained for the wet digestion method with the mixture HNO3-H2O2-HF were largely higher than those obtained with only the mixture HNO3-H2O2. This last method suffered then from losses of Ti. The mixture HNO3-H2O2 is a strongly oxidizing environment, which participates in the destruction of organic matter with oxidative and hydrolysis reactions [10] but it is known that silica generated some insolubility problems in wet digestion. These results can be then interpreted by the formation of insoluble compounds of Ti with silica, which are insoluble without HF [11]. A chemical compound as titanium silicide TiSi2 is only soluble in HF [12].
Table 2: Ti concentration measured on some samples after digestion with and without HF.
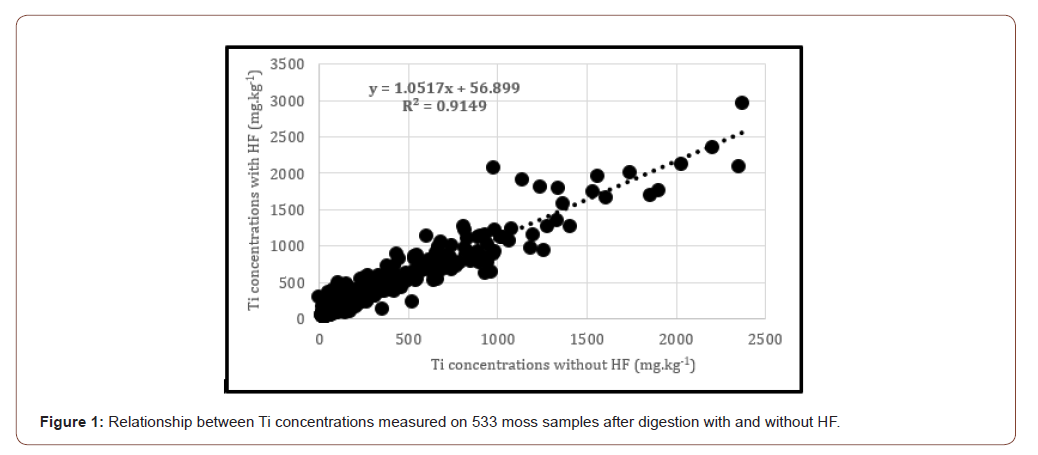
The Relationship between Ti concentrations measured on moss samples after digestion with and without HF (n=533) are presented in Figure 1. The slope was close to 1 and it appeared no significant difference between the two digestion methods concerning only these moss samples. Mosses were probably poor in silica. However, in the case of samples rich in silica, the use of HF is preferable for Ti determination regardless of the nature of plant samples.
Analyse of moss samples
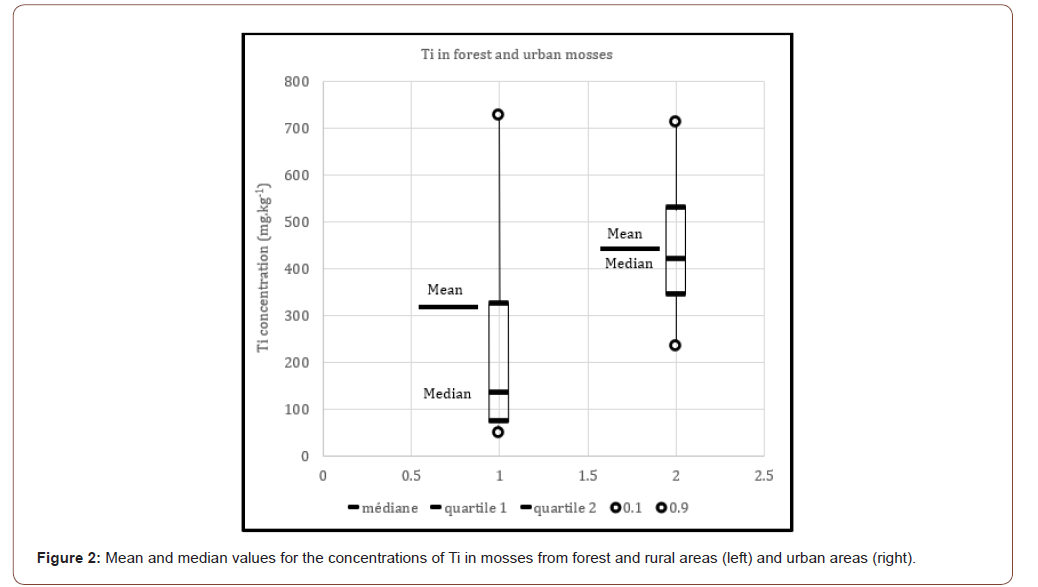
Mosses, because of their morphological and physiological properties, may accumulate metals and are recognized as excellent sensors of atmospheric depositions. The mean and median values for each area were taken into consideration and are presented in Figure 2. Average concentration of Ti in mosses from forest and rural ecosystems was 325 mg.kg-1 (median 134 mg.kg-1) and range from 17 to 2900 mg.kg-1. However, 90% of samples range from 17 to 726 mg.kg-1. These values are slightly higher than those reported in mosses from Norway, which average 53 mg.kg-1 and range from 12 to 310 mg.kg-1 [13] but remain comparable. Concerning urban samples, concentrations of Ti in mosses average 450 mg.kg-1 (median 421 mg.kg-1) and range from 45 to 1745 mg.kg-1. 90% of samples range from 234 to 711 mg.kg-1. These values are higher than those reported in mosses from forest or rural areas. Comparison of the median values indicated that, regardless of the study area, more Ti is accumulated in urban mosses. The reason is probably a large accumulation of Ti nanoparticles in the environment due to atmospheric falls. Natural variabilities in ambient climate such as acidity, temperature, humidity may also cause variable behavior for the bio monitors.
Soils can be a natural source of Ti for plant samples in various ecosystems and Ti concentrations found in mosses could originate from this primary source local through uptake of metals from soil solution or from natural cycling processes such as leaching from dead or living plant material. However, Ti could also originate from mineral-engineered nanoparticles of TiO2. The accumulation of anthropogenic titanium can be distinguished from natural TiO2 using discrimination functions. A recent study showed a distinct clustering of natural and engineered TiO2-NPS when plotting {[Ti] + [Zn]} to {[Ti]/ [Zn]}/{[Fe] + [Al]} on a log scale [9]. These results were based on the analysis of bulk samples after digestion (without prior separation of the TiO2-NPs). The elements Al, Fe and Zn were regarded as the characteristic elements in the TiO2 NPs and may be treated as the most powerful referents to identify origins Ti in samples [14]. This technique shows the natural grouping of the studied samples as well as the variables in a multidimensional space.
It was then possible to establish a two-dimensional principal component analysis model providing a qualitative picture. The results given in Figure 3 show that the moss samples can be accurately separated in two different groups according to their geographical origin. The first group consisting of forest and rural samples, and the second group of urban samples. This distribution can be interpreted from the loading plot by the higher contents of Fe and Al in the samples from the urban sites. The most common natural source of Ti is soil, which is constituted of silicate clay minerals, aluminum and iron oxides/hydroxides or humic organic matter. NPs have a distinct contrast with natural Ti. Compared to bulk materials, nanoparticles have physical properties such as uniform particle size, hierarchical nanostructure, well-defined crystalline structure, and high surface area. Transformation processes such as dissolution, aggregation, complexation in environmental media tend to alter the biogeochemical cycle of natural Ti. In these conditions, the atmospheric deposition of these particles can also affect the metal content in moss through the transport of soluble compounds from the soil to the moss tissue during periods of soil/ water contact. The elemental ratio between Ti and Al or Fe is then different with engineered nanoparticles.
Interference studies
The aim of the study was also to estimate the sensitivity of measurements of Ti and the presence, or not, of spectral interferences on the measured wavelengths. To our understanding, there is no significant overlapping between the wavelength of titanium used and the wavelengths of other elements potentially present in samples [12]. For the wavelength of 334.941 nm, the most influent element could be Cu with an emission wavelength at 334.924 nm. However, the very low sensitivity of this wavelength cannot be taken into account. However, it remains difficult to obtain reference samples with certified Ti concentrations. To our knowledge, only few samples exist in China [15]. The Limit of detection calculated as 3 times the standard deviation of blanks was estimated at Ti ≤ 2 μg. l-1.
Conclusion
Exposure to bioavailable Ti tended to impact plant ionome. It has been shown that the use of mosses is a good tool in biomonitoring atmospheric deposition of TiO2 nanoparticles. These organisms effectively accumulate atmospheric pollutants and can allow the assessment of the potential contamination of the environment by TiO2 NPs through an increasing transfer of Ti in bioindicators. Although TiO2 is naturally present in soils, anthropogenic TiO2 may be distinguished from natural TiO2 because urban mosses were significantly enriched in Al and Fe. These concentrations could be a preliminary screening tool to differentiate between NPs and natural Ti. This tendency of groupings between samples clearly shows the systematic separation of samples according to their geographical origin. However, the use of HF in the digestion method is necessary for Ti determination in plant samples.
Acknowledgement
Thanks to the National Museum of Natural History (MNHN, Paris, France) for the sampling and the preparation of moss samples.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Sun TY, F Gottschalk, K Hungerbühler, B Nowack (2014) Comprehensive probalistic modelling of environmental elissions of engineered nanomaterials. Environ Pollu 185: 69-76.
- Vijayaraj V, C Liné, S Cadarsi, C Salvagnac, D Baqué, et al. (2018) Transfer and ecotoxicity of titanium dioxide nanoparticles in terrestrial and aquatic ecosystems: A microcosm study. Environ Sci Technol 52: 12757-12764.
- Jing Hou, Luyao Wang, Chunjie Wang, Songlin Zhang, Haiqiang Liu, et al. (2019) Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J Environ Sci 75: 40-53.
- Dev A, AK Srivastava, S Karmakar (2018) Nanomaterial toxicity for plants. Environ Chem Let 16: 85-100.
- Wallace A, GV Alexander, FM Chaudhry (1977) Phytotoxicity of cobalt, vanadium, titanium, silver, and chromium. Commun Soil Sci Plant Anal 8: 751-756.
- Szczepaniak K, M Biziuk (2003) Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environ Res 93: 221-230.
- Klos A, Z Ziembik, M Rajfur, A Dolhanczuk Srodka, Z Bochenek, et al. (2018) Using moss and lichens in biomonitoring of heavy-metal contamination of forest areas in southern and north-eastern Poland. Sci Total Environ 627: 438-449.
- Onianwa PC (2001) Monitoring atmospheric metal pollution: A review of the use of mosses as indicators. Environ Monit Assess 71: 13-50.
- Kim W, C Yeom, H Lee, H Sung, E Jo, et al. (2017) Feasibility study on the differentiation between engineered and natural nanoparticles based on the elemental ratios. Korean J Chem Eng 34: 3208-3213.
- Gorsuch TT (1970) Destruction of organic matter. Pregamon Press, Oxford. United Kingdom. pp. 19-27.
- Hoenig M, H Beaten, S Vanhentenrijk, E Vassileva, Ph Quevauviller (1998) Critical discussion on the need for an efficient mineralization procedure for the analysis of plant material by atomic spectrometric methods. Anal Chim Acta 358: 85-94.
- Lide DR (2004) Handbook of Chemistry and Physics. CRC Press, Boca Raton. Florida, USA. pp. 10-1 to 87.
- Kabata Pendias A (2011) Elements of group 4. In: A Kabata Pendias (Editor), Trace Elements in Soils and Plant. (4th), CRC Press, Boca Raton, Florida, USA, pp. 167-169.
- Pradas del Real AE, H Castillo Michel, R Kaegi, C Larue, W de Nolf, et al. (2018) Searching for relevant criteria to distinguish natural vs. anthropogenic TiO2 nanoparticles in soils. Environ Sci Nano 5: 2853-2863.
- Ihnat M (1998) Plant and related reference materials for data quality control and elemental content, In: YP Kalra (Editor), Handbook of Reference Methods for Plant Analysis, CRC Press, Boca Raton, Florida, USA, pp. 235-284.
-
Pierre Masson, Patrice Soulé, Thierry Dalix. Biomonitoring of Titanium Contamination by Moss Samples. World J Agri & Soil Sci. 6(4): 2021. WJASS.MS.ID.000641.
-
Moss, Plant analysis, Titanium dioxide, Toxicity, Urban areas
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






