 Research Article
Research Article
Biofertilizer Impacts on Cassava (Manihot Esculenta Crantz) Rhizosphere: Soil Microbiome Engineering, Genetic and Sustainable Agroecosystems, Igbariam, Nigeria
Otaiku AA1*, Mmom PC2 and Ano AO3
1Doctoral student, Faculty of Social science, Department of Geography and Environmental Management, University of Port Harcourt, Choba, Port Harcourt, Rivers states, Nigeria
2Faculty of Social science, Department of Geography and Environmental Management, University of Port Harcourt, Choba, Port Harcourt, Rivers states, Nigeria
3National Root Crops Research Institute, Umudike Umuahia, Abia state, Nigeria
Otaiku AA, Doctoral student, Faculty of Social science, Department of Geography and Environmental Management, University of Port Harcourt, Choba, Port Harcourt, Rivers states, Nigeria.
Received Date: July 03, 2020; Published Date: November 20, 2020
Abstract
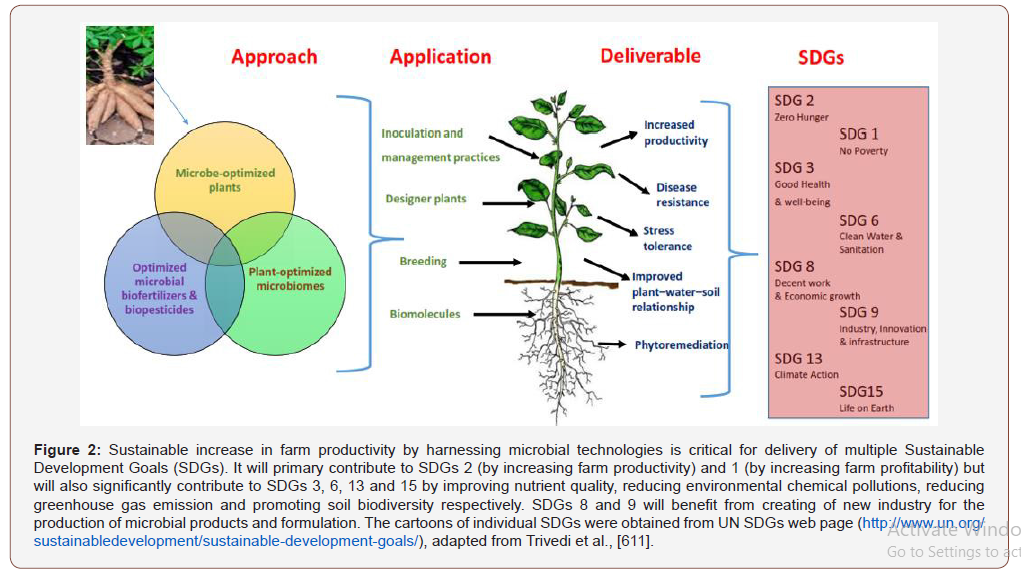
There is a wealth of unexplored knowledge about microbe’s ecosystem functioning impacts on food security, climate change mitigation and sustainable agriculture with focus on cassava cultivation in the tropics using biofertilizer. The rhizosphere modified by biofertilizer as agronomic management during cassava cultivation by the inoculants for microbiome engineering for integrated soil fertility management by the release of plant root exudates and microbial metabolites. Microbiome and the cassava crop are highly dependent on each other as the microbiome contributes a significant portion of the secondary genome of the host plant like quorum quenching strategies that suppress the virulence of pathogens, enhance high yielding cultivars and favorable environments for development. Rhizosphere engineering using biofertilizer can reduce and improves chemical dependant agriculture for resilience agriculture. Cassava genetic engineering integrated with biofertilizer can transforms the use of cassava cultivar for polluted soils phytoremediation and bio-energy crop cultivation in the tropics as re-generative agriculture technique for xenobiotic pollution management. The economic impacts will be development of remediation-to-biofuel economy of restorated polluted soils by conversion of the bioenergy crop cultivated into bioethanol. Cassava cultivation with biofertilizer accentuate regenerative agriculture as an integral potential for food security and sustainable development goals (SDG).
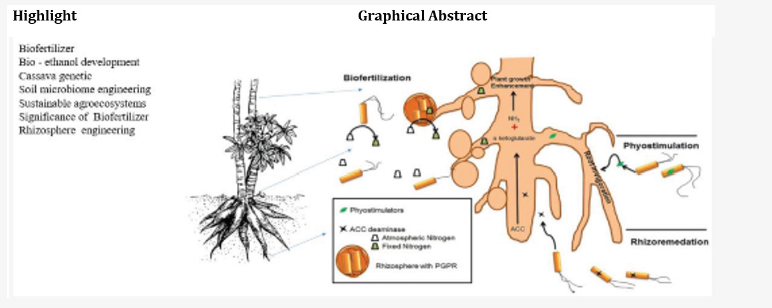
Keywords: Biofertilizer; Rhizosphere engineering; Cassava genetics; Niger-Delta; Sustainable development goals (SDG); Biodegradation; Remediation-to-biofuel development; Re-generative agriculture; Bio-ethanol
Introduction
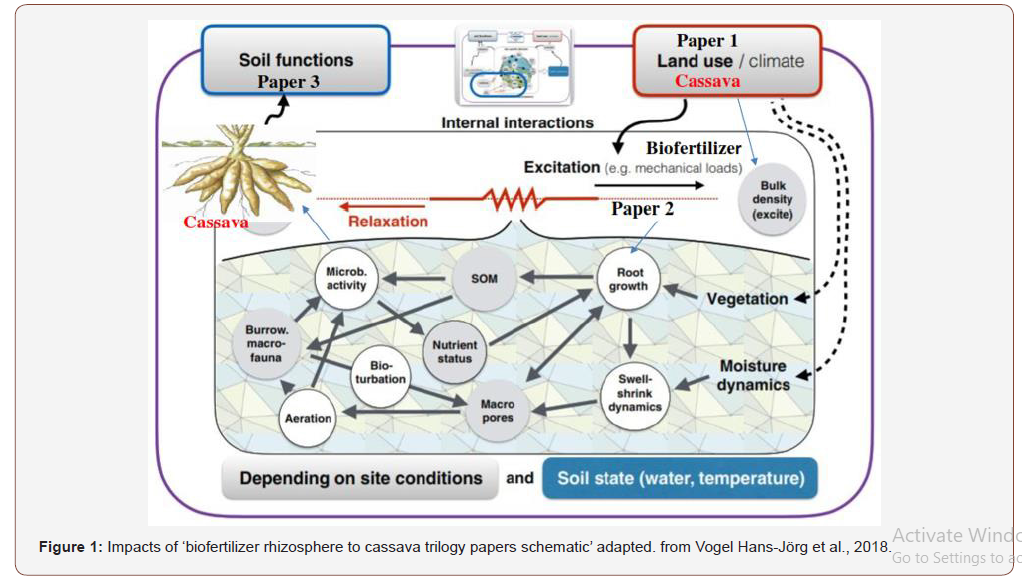
Currently, researchers round the globe are facing challenges of maximizing the functions of microbiome under the limitation of the natural and anthropogenic activities including new strains of pests and pathogens, climate change, use of chemical fertilizers, as these activities are continuously menacing stable agricultural productions [96]. The agriculture paradigm shifts in 21st century is re-generative agriculture techniques by deploying the soil microbiome for integrated soil nutrient management as affirmed by scholars [97,627,703] and to enhance plant nutrient uptake. Soil microbiome represent an unexploited pool of opportunities to face the sustainability issues of agriculture [69,93,143,257,507] under climate change. The intricate interactions between the plant genotypes, microbiome structures and different environmental factors offer indispensable information in sustainable agriculture. The research articles are trilogy: Biofertilizer Impacts on Cassava (Manihot esculenta Crantz) Rhizosphere: crop yield and growth components (paper 1); improved soil health and quality (paper 2); soil microbiome engineering, genetic and sustainable agroecosystems (paper 3). The crux of the paper is that ‘’the agriculture production and sustainability is under threat’’ from various natural and anthropogenic factors, farm inputs, agronomic practice and plant-microbe’s interactions that sustains the earth biodiversity’’. Biofertilizer enhances soil microbiome techniques of plant-microbe interactions.
Soil is the most diverse and complex habitat that consists of millions of fungi, billions of bacteria and other macro organisms [55]. Microorganisms present in soil play important roles in nutrient cycling and shielding plant from harmful effects of abiotic and biotic stresses [12,13,247,310,317]. Intensive agriculture practices lead to increase in crop production but in the same time, it poses detrimental effects on the biological and physical properties of soils. In agricultural systems, macronutrients are generally provided through chemical fertilizers and a treat to modern agriculture and compared to applications of biofertilizer and biopesticide that improve the growth of plants health, crop productivity and enhance biodiversity. Plant growth promoting rhizobacteria, a group of diverse rhizosphere microbe, produce a variety of bioactive chemical substances that besides promoting the growth of plants, protect the plants from pathogens [157]. Microbes, the most diverse and profuse group of organisms constitute more than 60% of the Earth’s biomass [57] sustains the vibrant integrity and equilibrium of biosphere is imperative, as the subsistence of life is reliant upon the sustained and microbial arbitrated transformation of matter, both in the aquatic as well as terrestrial environments [652]. Microbes have a myriad of functions, and they play an imperative role in sustainability and biogeochemical cycling [132]. This cycling of elements besides shaping the structure and function of ecosystems also enriches the soil with the abilities that can provide varied services to the people [15]. Soil microbiome along with their allied functions determines the productivity of agro-ecosystems [625], sustainable agriculture relies on soil health, diversity of microbes for soil biodiversity. It is estimated that majority (≥ 90%) of the microbial diversity still remains to be explored. These novel unexplored diversities correspond to treasure troves of improved and innovative biotechnological developments with applications in the fields of energy, agriculture, chemicals, mining, materials, food, pharmaceuticals and environmental protection. Identification of the key ecosystem driving elements is one of the challenging tasks and manipulating these drivers to produce appropriate benefits is even more demanding for modern agriculture today.
Soil microbiome interactions involving plants and roots in the rhizosphere include root–root, root-insect, and root-microbe interactions. The root microbiome is the main determinant for the plant growth and health; and does so, by assisting the host plant in nutrient uptake, protection against pathogen attack and by supporting abiotic stress tolerance [69,90,157,527].
Synchronized interactions between the microbes and their host plants encompass a supreme importance and significance for improving the plant growth and in maintaining appropriate soil conditions. There is now overwhelming evidence which supports the fact that plant can shape their microbiome by the belowground plant microbe interaction [103].
Even the most ancient lineages of plants show a strong ability to alter the relative abundance of microbial groups in the soils surrounding the rhizosphere [621]. The close symbiosis of plants and microbes can be viewed as an integrated ecological unit known as a halobiont [632]. These contrasting microbiomes have been attributed to differences in root exudate chemistry [46,498] and in plant nutrient uptake rates [62]. Genotypic and phenotypic variations in plant traits that support microbiomes that increase plant nutrient availability prevent pathogens or otherwise enhance plant health, growth and performance incur a fitness advantage, now affirmed by omics tools of biotechnology for modern agriculture.
Cassava Nutritional Health
The exponential growth in human population has demanded a concurrent production and supply of food, particularly from plants. Consequently, a highly productive and intensive agricultural system has been mostly accomplished by the use of synthetic chemical fertilizers of nitrogen and phosphorus [534] resulting to environmental pollution problems by emissions of greenhouse gases like nitrous oxide (N2O) from fertilizer production and application [411]. A biofertilizer of selected efficient living microbial cultures, when applied to plant surfaces, seed or soil, can colonize the rhizosphere or the interior of the host plant and then promote plant growth by increasing the availability, supply, or uptake of primary nutrients to the host. Biofertilizers are mostly supplied as conventional carrier-based inoculants in liquid or solid forms. The mass production of biofertilizers involves culturing of microorganisms, processing of carrier material, mixing of carrier material with the broth culture, and packing (focus of paper 1) and it is predicted that market share of biofertilizers will reach US$1.66 billion by 2022 and will be compounding the annual growth rate of 13.2% during the years of 2015–2022 [396,602] SCOPE 65 reported. Okon & Labandera-Gonzalez [435] were firstly arguing that rhizospheric organisms which improve soil nutrients utilization. Fuentes-Ramirez & Caballero-Mellado [199] defined biofertilizer as “a product that contains living microorganisms, which exert direct or indirect beneficial effects on plant growth and crop yield through different mechanisms”. Vessey [651] affirmed, biofertilizer are formulated product containing the microorganisms that is applied to the plant or soil. Indeed, it has been proved that consortia of species normally improving nutrient efficiency (e.g. double inoculants with bacteria and fungi in one gel formulation) can show plant protection properties [639-641,651].
Climate change also greatly impact upon overall quality of the crop and the dynamics of the associations that exist between crops, pests and diseases. Fluctuations in climatic factors like rainfall, solar radiation, and temperature have great potentials to influence crop production [120]. Various soil microbes interact with each other as well as with plants in a myriad of different ways that help in maintaining and shaping different components of ecosystem [70,249,477]. These interactions have great potential to mediate some very important processes like composition of plant community, mineralization, and shifts in ecological interaction related important functions [6,279]. Climate change alters plant phenology and distribution of microbes therefore, plant species distribution is affected in response to climate change [120]. The unsustainable use of chemical fertilizers is causing the disruption of Earth’s bio-geochemical cycles by altering the mechanism and are responsible for soil degradation, eutrophication, and greenhouse gas emissions [22,578].
Production of N-fertilizer using energy-intensive Haber– Bosch process relies upon fossil fuels and thus contributes to global warming and natural resource depletion which ultimately contributes to climate change [174]. Chemical fertilizers application induces severe consequences, alternative methods for sustaining soil health and plant nutrition with minimum input of mineral fertilizers is needed [192] - the paper objective for alternative resilience agriculture with beneficial and specific root-associated microbes that mineralize the bound organic nutrients for enhanced biodiversity. There is vast assemblage of microbes (fungi and PGPRs) that colonize the plants roots and provide ecological services for increment of plant diversity, enhancement of seedling recruitment and better nutrient acquisition. Biofertilizer supplies functional diversity of symbionts for root microbiome to complement each other each other in obtaining various limiting nutrients and in driving ecosystem functions and affirmed by scholars [626,659].
Rationale
Recently the field of plant biology has recognized the importance of root exudates in mediating these biological interactions [39]. Identification of the key ecosystem driving elements is one of the challenging tasks and manipulating these drivers to produce appropriate benefits is even more demanding. The impact of a warming climate on spring plant phenology is evident [20,78].
A longer growing season may increase carbon uptake and potentially mitigate climate change [78,154], leaf emergence [283], fruiting [684], and germination [441]. Abiotic stress factors include extreme temperature [357], drought, water logging, light, and salinity as major parameters that affect plant growth and detailed mechanisms are unclear. Plants are immobile, they have coevolved with microbes and acquired a number of mechanisms that modulate the outcome of their interactions [437]. Campbell et al., [98], observed that the density of microbes in the rhizosphere was 100 times greater than that in the bulk soil and plant root exudates shape the soil bacterial community [242,330]. How does the microbiome diversity function potentially affect host plant performance? The presence of microbial hubs in plant microbiome networks plays an important role between a plant and its microbial community [624] with key microbial metabolic processes related to plant nutrition in paper 1 [448]. The mechanism of action of Plant growth–promoting rhizobacteria (PGPR) in the biofertilizer applied to cassava cultivation (paper 1) can produce a complex blend of volatile substances, which are distinct between bacterial species and other closely related species [214,215,233] provides the cassava soil and microbiome engineering, Some of these bacterial volatiles can stimulate plant growth [464,516], suppress disease stimulating ISR [464] or antagonize phytopathogens [290,695], nematodes, or insects [84,87]. Soil resources can also be transferred by shared symbiotic fungi called common mycorrhizal networks (CMNs) affirmed by Simard et al., [555].
How does cassava plant harbor unique microbial communities that shape a unique rhizosphere microbial community is the crux of paper 3? Fungi, PGPR and beneficial microbes in the biofertilizer applied was the methodology to study soils fiction when biofertilizer is applied to the cassava crop cultivation. Scholars affirms that microbes can enhance nutrient uptake, stimulating root, and shoot growth by producing indole acetic acid [260,452], 1-aminocyclopropane-1-carboxylate (ACC) deaminases [221,222], solubilizing phosphate [223], and enhancing the uptake of nutrients from the environment [53,162]. Microbes can enhance plant resistance to adverse environmental stresses such as drought, heavy metals, salts, and nutrient deficiency [705].
Biotic stress factors include interaction with other organisms and infection by pathogens or damage by insect pests, and some plant growth–promoting bacteria have been used as biocontrol agents against plant pathogens [262,265]. These are the questions that must be addressed. Modern genomic technologies (e.g., high throughput sequencing) can provide clues to the answer. The paper investigates the plant–microbe interactions at the soil functional level (what they are doing) in order to identify the signals involved in the interspecies interactions, food security and re-generative agriculture using cassava as phytoremediation plant for polluted soils and cultivation bioenergy crop for bio-ethanol production. In this context of doubling the global food demand by the year 2050, necessitates the expeditious and instant solutions [601] by deploying the soil microbiome to increase the resistance to various stresses (biotic and abiotic) reported by [97,627,703] and to enhance plant nutrient uptake. Hiruma et al., [257] present one of the few unexploited pools of opportunities to face the sustainability issues of agriculture [69,93,143,507] under climate change (Figure 1).
Methodology
Cassava crop and microbes can interact at soil aggregate scale, with substantial variations being noticed across soil aggregates [341]. Synchronized interactions between the microbes and their host plants encompass the role of soil microbiome in crop development and integral function of the microbial inocula in the formulation (PGPRs biosurfactants presents in OTAI AG® Inocula, Otaiku et al., [448]) of the biofertilizer applied during the cassava cultivation, PGPR gains unique and extraordinary attention due to their diverse functional characters such as production of hormone and certain beneficial enzymes, effective root colonization and solubilization of nutrients for sustainable agriculture. Knowledge and understanding regarding the ecology, growth promoting characters, mechanism of action as well as application of the naturally occurring microbial populations hold key importance for plant growth and soil microbiome as alternative to chemical fertilizer. Soil microbial community intimately relate to soil physical properties and immediately affect ecosystem processing, their presence, abundance and diversity have often been proposed as bioindicators of soil health reported by Lu et al., [346].
In Otaiku et al. [448] reported that biofertilizer characteristics (Paper 1 Table 5, pages 6 -7) and biosurfactants (Table 3 pages 8-9) applied in the filed cassava cultivation requires no chemical pesticide because cassava plant-microbes associated lifestyle requires adaptation to several niches, in which different metabolites act as signals for interaction (communication) with the plant and host specific plants nutrient and crop protection as narrated. Plant community dynamics are driven by the microbial mediation of soil resource partitioning and sharing in the “rhizosphere” because key microbial metabolic processes related to plant nutrition as executed using biofertilizer [448]. “Rhizosphere” defined as the soil compart-affected by plant roots [262]. Soil microbes are chemotactically attracted to plant root exudates, volatile organic carbon, and rhizodeposition, and then proliferate in this carbonrich environment [348]. Plant root exudates differ between plant species, so differences in rhizosphere microbiomes of different plant species are expected [514]; Plant species-specific microbiomes [274,300]. Plants can also shape the microbial community via root exudates.
Root exudates can be categorized as sugars, amino acids, organic acids, nucleotides, flavonoids, antimicrobial compounds, and enzymes [39,514]. The change in the microbial composition generates feedback on the plant relative performance that defines the long-term effects of the soil microbes on their coexistence with that plant species [71,73] and affirmed in Figure 2. The feedback can be of two types; positive plant-soil microbial feedback reinforces the spatial separation of the microbial communities [17], while negative feedback results in plant replacement, which necessitates recolonization of locally specific roots [72,660,664]. Systematic methods such as genome-wide association studies have enabled us to explore the relationships of plant loci and symbiotic communities in details [251,262].
Microbial hubs might be responsible for mediating defense signals among plants and the effectiveness of biological control agents [624]. The term “Endophytic bacteria” has been proposed for the presence of a kind of hub species that would be a determinant of colonization of widely microbial taxa and number of hypothetical relationships between plant performance and microbial diversity and composition have been proposed [624]. Salinity resistant Pseudomonas fluorescens, P. aeruginosa, and P. stutzeri ameliorated sodium chloride stress in tomato plants, and an increase in roots and length were observed. In the past decade, bacteria belonging to different genera including Rhizobium, Bacillus, Pseudomonas, Pantoea, Paenibacillus, Burkholderia, Achromobacter, Azospirillum, Microbacterium, Enterobacter and Methylobacterium have been reported to endow host plants under different abiotic stress environments [235]. P. fluorescens produces 2,4-diacetyl phloroglucinol, which inhibits the growth of phytopathogenic fungi [293].
Soil Microbiome Engineering
Global food supply must grow sustainably within the context of the ever-increasing competition for natural resources, particularly land and water, and competition for food and biofuel, and by the need to operate in a carbon- constrained economy [537,596]. Increased anthropogenic activities have major effect on environment [358] as a whole and sustainable agriculture in particular [176]. Increasing the productivity of agricultural land in order to produce more food in an environment friendly ways in the era of changing climate, concept of ‘sustainable intensification’ [118] requires microbes to have a myriad of functions, and they play an imperative role in sustainability and biogeochemical cycling [132].
Soil microorganisms including bacteria, archaea and fungi play a diverse and often decisive role towards the functioning of ecosystem such as driving the cycling of major elements (e.g. N, C and P). This cycling of elements besides shaping the structure and function of ecosystems also enriches the soil with the abilities that can provide varied services to the people [15]. Soil microbiome and allied functions determines the productivity of agro ecosystems [625] and this paper accentuates the role of biofertilizer in soil microbiome engineering.
Climate-changing parameters are well known to affect both the micro as well as macro-organisms (plants) and is a major global problem affecting the life on the planet [121,537] and believed to impart both direct and indirect effects on plant–soil–microbe interactions [4,79,609], by altering the community structure, relative abundance and function, as the soil community taxa vary greatly in their physiology, growth rates and temperature sensitivity [40,248]. Soil is the most diverse and complex habitat that consists of millions of fungi, billions of bacteria and other macro organisms [55]. Microorganisms present in soil play important roles in nutrient cycling and shielding plant from harmful effects of abiotic and biotic stresses [11,12,13,247,310,317]. Plant growth promoting rhizobacteria, a group of diverse rhizospheric microbe, produce a variety of bioactive chemical substances that besides promoting the growth of plants, protect the plants from pathogens [157]. Plants rely on the propensity of their roots to communicate with variety of microbes. The first step in root colonization is production of chemotactic response towards variety of root exudates released by roots of plants. The different types of exudates released by roots include amino acids and organic compounds [708]. The root microbiome is the main determinant for the plant growth and health and does so by assisting the host plant in nutrient uptake, protection against pathogen attack and by supporting abiotic stress tolerance [69,90,157,527].
Various soil microbes in different biofertilizer formulations in agriculture interact with each other as well as with plants in a myriad of different ways that help in maintaining and shaping different components of ecosystem [70,249,477]. The direct impact of climatic change on function and composition of microbial communities have been extensively reviewed by different researchers [1,107,109,111,250,363] but unfortunately the indirect effects via shifting soil microbe–microbe and plant–soil microbe interactions receive very less study. Soil health deliver a range of ecosystem and agronomic functions and services in order to maintain environmental health and quality, biological productivity, promote plant and animal health [327]. Microbes enhances soil health, improving water holding capacity, carbon storage, root growth, availability and cycling of essential nutrients, filtering pollutants and also in conservation of biodiversity [294,407] (Figure 2).
In Table 9 functional diversity of symbionts, root microbiome can complement each other each other in obtaining various limiting nutrients and in driving ecosystem functions [626,659]. Chemical fertilizers disruption of Earth’s biogeochemical cycles (soil degradation, eutrophication, and greenhouse gas emissions) reported by Amundson et al., [22], Steffen et al., [578]. Understanding potential of soils to sequester carbon by microbes are of fundamental importance [22]. Sustainable agricultural practices capable of generating higher crop yields via multidisciplinary coordination among ecology, agronomy, soil science, genetics, economics and social sciences and also without the full engagement of farmers was reported by scholars [110,318]. Soil microbes are the key components of ecosystem and are responsible for crop yield [157], nutrient cycling and carbon sequestration [67], and environmental restoration [359]. There is a wealth of unexplored knowledge about the role of these microbes in ecosystem functioning and climate change. Scientists [254,610,697] suggested that microbes possess natural ability to capture and sequester CO2 via different metabolic pathways. Similarly, researchers reported that [237,414,686] in their studies discussed the significance of microbes for mitigating the harmful effect of greenhouse gases. Application of metagenomic approaches to study soil microbiomes could greatly help in understanding and restoring ecosystem functioning, which at present are under severe pressure, as these approaches seem to be instrumental in providing useful knowledge about taxonomic, genetic, and functional aspects of soil microbial taxa [188,557,617] (Tables 1&2).
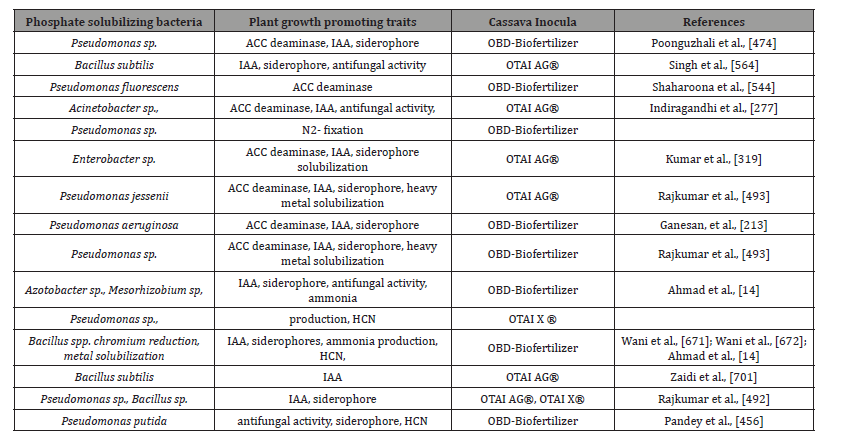
Table 1: Inoculants in biofertilizers applied for cassava cultivation potential for soil microbiome engineering [459].
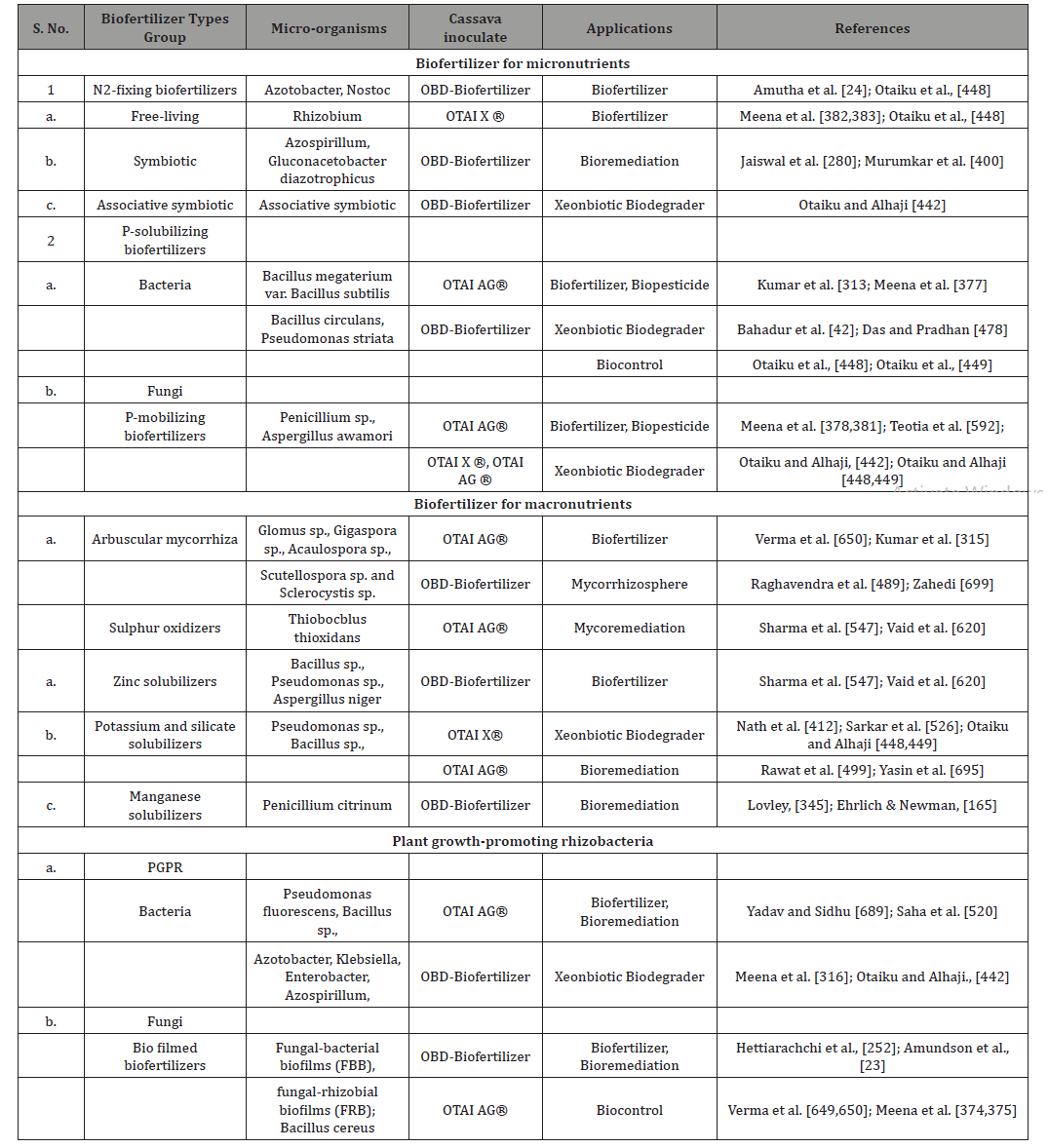
Table 2: Various organic or inorganic substances produced by plant growth promoting rhizobacteria facilitating resource acquisition to stimu plant growth.
Results and Discussion
Sustainable cassava agro-systems
The largest number of cassava diseases is found in Latin America and the Caribbean, the plant’s centre of origin and sub- Saharan Africa and Asia according to FAO [181]. Many diseases are caused by pathogens, whose damage symptoms appear on the leaves, stems and storage roots [390]. The common diseases of cassava are cassava mosaic disease, cassava bacterial blight, cassava anthracnose disease, cassava bud necrosis and root rot. Some of these diseases attack the leaves and stems of cassava plants while others attack the storage roots, [438]. Cassava mosaic disease is caused by the African cassava mosaic virus which occurs inside the leaves and stems and causes yield reductions of up to 90 percent [271]. The leaves become discoloured with patches of normal green colour mixed with light green, yellow and white area (chlorosis). When cassava mosaic attack is severe, the leaves become very small and distorted and the plants are stunted [323]. The symptoms are more pronounced on younger plants, usually under six months, than older plants. The disease is spread through infected cuttings and by whiteflies Bemisia tabaci [21]. China is world leading importer of cassava products, importing an estimated 9.5 million tons of flour and starch and also accounted for 63% world share of cassava import value in 2017 [182,430]. China ranked number one, exporting cassava worth of $1.37billion while Canada’s cassava export was $57.8 million [430] (Table 3).
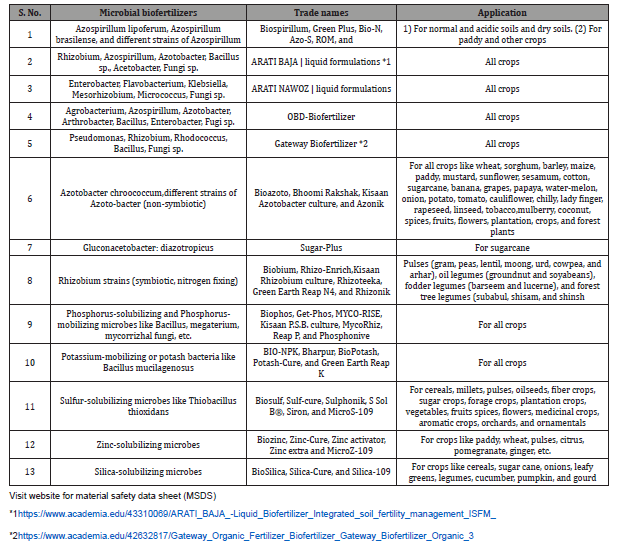
Table 3: Different microbial biofertilizers available in market and their application.
Biofertilizer inoculant: Quorum sensing
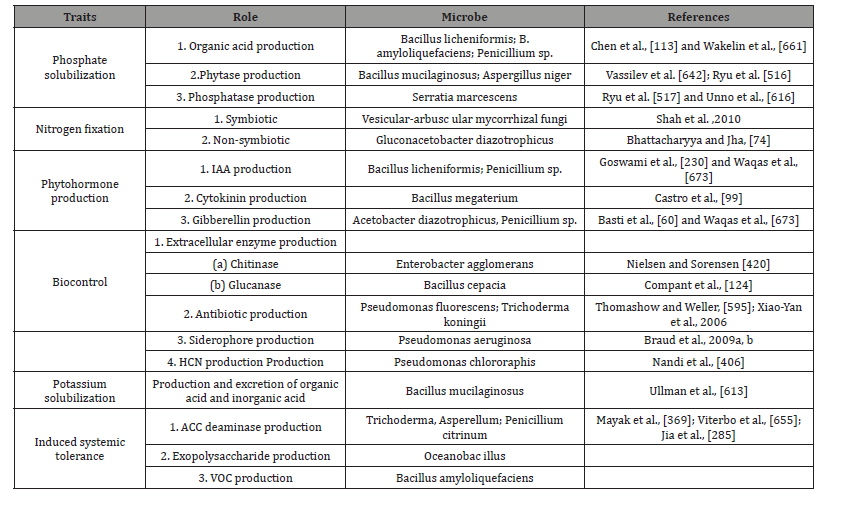
The nitrogen-fixing efficiency of rhizobia bacteria, an important group of biofertilizers that contains organisms like Rhizobium, Bradyrhizobium, Sinorhizobium, Azorhizobium, Mesorhizobium and Allorhizobium, can vary till 450 kg N / ha among different strains and host legume species, in which root nodules are formed [232,574,575,614,615,631]. The rhizobial biofertilizers can be in powder, liquid, and granular formulations, with different sterilized carriers like peat, perlite, mineral soil, and charcoal [581]. In rhizobia, a nitrogen-fixing actinomycete, can form root nodules in several woody plants [68,139,151,266,607,663]. Other ecologically microbes include phosphorus-solubilizing bacteria (PSB) like Bacillus and Pseudomonas can increase phosphorus availability to plants by mobilizing it from the unavailable forms in the soil [503] called phosphorus-mobilizing biofertilizers or phosphate absorbers.
The mycorrhizal fungi form obligates or facultative functional mutualistic symbioses with more than 80% of all land plants, in which the fungus is dependent on host for photosynthates and energy and in return provides a plethora of benefits to its host [567,593]. The mycelium of the fungus extends from host plant root surfaces into soil, thereby increasing the surface area for more efficient nutrient access and acquisition for the plant, especially from insoluble phosphorus sources and others like calcium, copper, zinc, etc. [563]. Mycorrhizal fungi enhance soil quality, soil aeration, water dynamics, and heavy metal and drought tolerance of plants and protects root pathogens or herbivores [505,593]. The inoculum source [75,301] determine the impacts on the results in amended and homogeneous crop growth.
Biofertilizer compost is produced from a wide variety of materials like straw, leaves, cattle-shed bedding, fruit and vegetable wastes, biogas plant slurry, agriculture waste, etc reported. Biofertilizer compost is biodegraded in anaerobic biodigester inoculated with broad spectrum decomposing microorganisms like Trichoderma viridae, Aspergillus niger, A. terreus, Bacillus spp., several Gram-negative bacteria (Pseudomonas, Serratia, Klebsiella, and Enterobacter), etc. that have plant cell wall-degrading cellulolytic or lignolytic and other activities as narrated in Paper 1 [448]. Biofertilizer has proteolytic activity and antibiosis (by production of antibiotics) that suppresses other parasitic or pathogenic microorganisms [83].
The challenges of biofertilizer are lower shelf-life, temperature sensitivity, being contamination prone, and becoming less effective by low cell counts. Consequently, liquid formulations have been developed for Rhizobium, Azospirillum, Azotobacter, and Acetobacter which although costlier, have the advantages of having easier production, higher cell counts, longer shelf-life, no contamination, storage up to 45 °C, and greater competence in soil [419]. The application practices of microbial biofertilizers include seed treatment (plant growth regulator, PGR), seedling root dipping, and soil application. There are several microbial biofertilizers available as dried or liquid cultures under different trade names in the market, which are used for a variety of purposes including enhancement of plant growth and soil fertility. For instance, the rhizobia biofertilizers can fix 50-300 kg N ha that increases yield by 10–35%, maintains soil fertility, and leaves residual nitrogen for succeeding crops [113,138]. The Azotobacter biofertilizer used for almost all crops can fix 20–40 mg N/g of carbon source that causes up to 15% increase in yield; maintains soil fertility; produces growth-promoting substances such as vitamin B complexes, indole acetic acid, and giberellic acid; and is further helpful in biocontrol of plant diseases by suppressing some of the plant pathogens [2,321]. The phosphorus-solubilizing bacterial biofertilizers, which are nonspecific and suitable for all crops, produce enzymes which mineralize the insoluble organic phosphorus into a soluble form, thereby increasing crop yield by 10–30% [548,549,550].
Plant–microbe metagenomic
Biofertilizers mineralization in soil is an integral function of the microbe’s inoculants with core mechanism for mineral phosphate solubilization is the production of organic acids and acid phosphatases. The mechanism of mineral phosphate solubilization is the action of organic acids synthesized by soil microorganisms [379,551,556,644]. The rhizosphere makes a source of gene pool with a huge potential, particularly for agricultural applications with the aim to improve crop productivity and quality of agricultural products and shield crops from pests executed the genes responsible for PGPR activities and application of the recombinant molecules to soil.
An activity that improves the plant fitness and, hence, improves crop production is apparently ACC deaminase activity. Interestingly Nikolic et al., [426] also analyzed ACC deaminase genes (acdS) of bacterial endophytes colonizing field-grown potato plants and discovered the presence of two unique types of acdS genes, the dominant one showing high homology to an acdS gene derived from P. fluorescens through PCR analysis. The fundamental study on siderophores is mainly fascinating due to its triple function application, nutritional, systemic resistance inductor (ISR) and biocontrol reported by Bakker et al., [50]. The sequencing of whole genomes from a number of species permits to delineate their organization and provides the basis for understanding their functionality [392], as a consequence favoring metagenomic– agricultural practices.
Genomics contribution to agriculture spans the identification and manipulation of genes linked to specific phenotypic traits [706]. In addition to molecular breeding by marker-assisted selection of variants [344]. In the future, agricultural metagenomics without doubt aims to reveal several innovative solutions through the study of crops or livestock genomes, achieving information for protection and sustainable productivity for food industry reported by Bulgarelli et al., [89] and impacts on metagenomics study of the soybean rhizosphere [454]. Currently, the great majority of bacterial species are still unidentified [497]. Metagenomics application in agriculture also proved to be suitable for depicting the multifarious patterns of interactions occurring among microorganisms in soil and in plant rhizosphere [454]; the environmental changes [454]; agricultural management [572] and can help decipher the role of soil microbes in plant nutrition [470] or in the cycle of elements [580].
Plant, soil, and microbiome also play a crucial role in agriculture provided that it determines plant fitness [244] and soil biogeochemical properties and affects both yield and quality traits [149]. Bio-nanotechnology applications which employ nanoparticles made of inorganic or organic materials could also provide new avenues for the development of carrier-based microbial inocula [360] like the cassava inoculants can be in biomaterials or nanomaterials called value added materials (VAS) will become carrier-based microbial inocula in the future climatesmart agriculture was reported by Otaiku et al., [448]; Otaiku et al., [449] posit that characterisation of advanced materials, and particularly of materials with necessarily complex structures such as bio and functional ones, requires analytical tools for observation and monitoring all relevant length scales (nano, micro, meso and macro).
Rhizosphere biocommunication
Rhizosphere microflora coordinated the expression of the specific target genes within the Rhizosphere and other eukaryotic cell types interactions of organisms to occupy particular habitat adapting to environmental conditions and against several adverse conditions. to share symbiotic and a symbiotic relationship with plants benefiting its growth directly or indirectly. These coordinated responses are generally induced by a group of chemicals signaling and are referred to as quorum sensing (QS) reported by Fuqua & Greenberg [201]. QS signals produced by rhizosphere microflora are significantly higher compared to other organisms isolated from bulk soil. Scholars reports on QS signals with distinct chemical structures produced by Gram-negative rhizosphere bacteria and their potential to regulate a wide array of genes in the population [42,43,245,282,284,312,314,366,372].
Roots are reported to secrete exudates with a wide array of chemicals involved in regulation of both beneficial and pathogenic microorganisms in the rhizosphere. The variation in these chemicals helps in stabilizing the equilibrium of beneficial microbes in terms of its number and by which evading the continuous attack by soil borne pathogenic bacteria [47,662]. The cross talk between the plant roots and beneficial microbes is playing a vital role in growth and development of the entire plant kingdom in general and agricultural crops in particular [295,361,545]. The release of chemicals involved in trans-specific communication from roots can occur passively upon decay and may export signals actively to the extracellular environment. Ten to 40% of the photosynthetic carbon assimilation is released by the plants as root exudates, and it is a mixture of sugars, amino acids, organic acids, sugar alcohols, and secondary metabolites [46] microbial activity through nutrient bioavailability and regulating phytotoxic elements is vital [416,558]. The rhizosphere is a hotspot of several communications involving a wide range of microorganisms with diverse physiological importance [313,412,650]. Among these communications, quorum sensing and quorum quenching are attracting scientific community for its beneficial exploitation in plant growth regulations ultimately leading to yield enhancement. Gram-negative bacteria use homoserine lactones (LuxR/LuxI) as communication signals [532,587], whereas Gram-positive bacteria use oligopeptides in quorum sensing. During quorum sensing, it is important for the organisms to differentiate between speciesspecific signaling and signaling associated with interspecies behavior modulations [59,185,675].
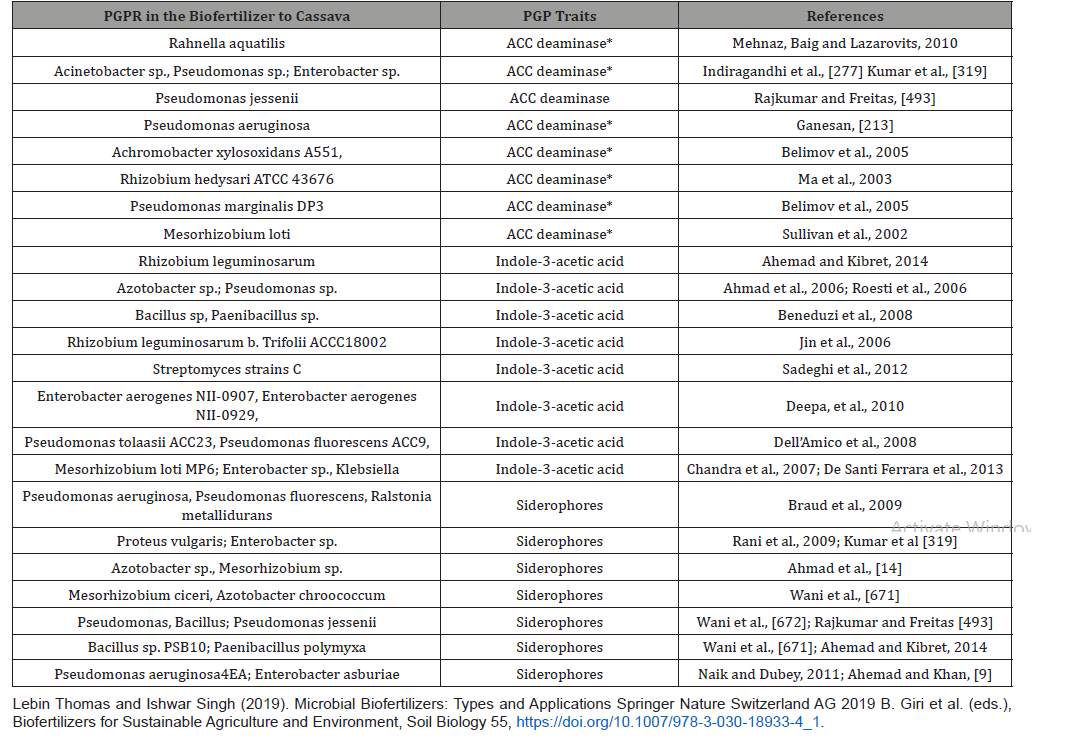
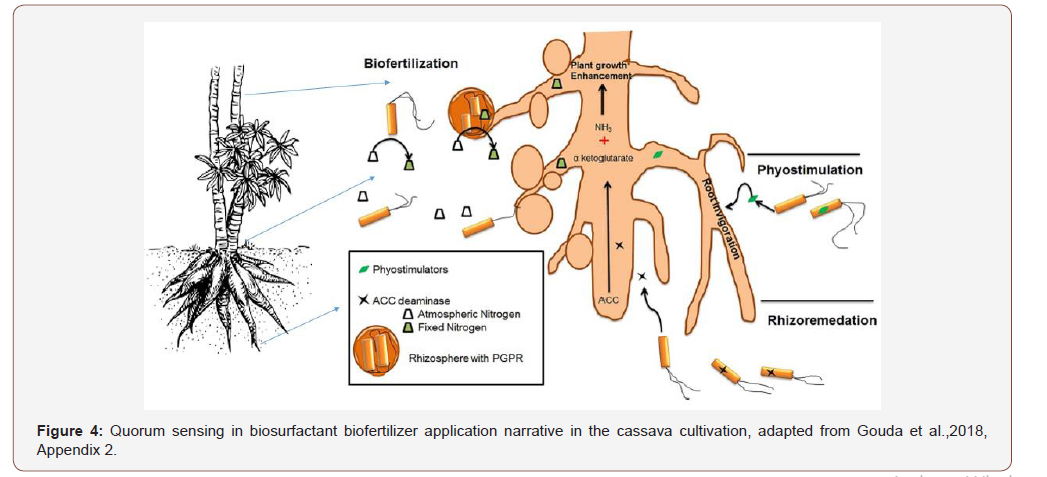
Mycorrhizal fungi support the growth of bacteria by releasing few nutrients, and in turn soil bacteria with its wide array of enzymes degrade the complex soil organic nutrients and make it easily available for the fungi [80,81]; supports the plant growth by extending its hyphae to the areas where plant roots are not able to reach; due to this extension, the plant gets sufficient nutrients supplied by both roots and fungi compared to uninfected roots. The law of limiting factor supported by adaptive radiation of the species is favored by coexistence of bacterial life with suitable interactions to scavenge the limiting factor required to colonize the specific habitat and suitably favored in competition with other groups of organisms [526,650]. Generally, quorum sensing in bacteria falls into three classes: the first is, as mentioned earlier, AHL-dependent LuxI/LuxR-type QS observed in Gram-negative bacteria, the second is the small peptide-mediated QS observed in Gram-positive bacteria, and the third observed in both these bacteria is luxS-encoded autoinducer 2 (AI-2) QS. These signal molecules are operating with precise sensing and regulatory network [159,185,388,457,532,675]. Two types of quorum sensing systems are reported in Gram-positive bacteria in contrast to Gramnegative bacteria [159,191,194,427]. The first comprises of a threecomponent signaling peptide referred to as autoinducing peptide (AIP) and the other is a two-component signal transduction system which specifically responds to an AIP [159,388,532], Appendix 2 (Figure 3).
Biofertilizer impacts: Quorum sensing
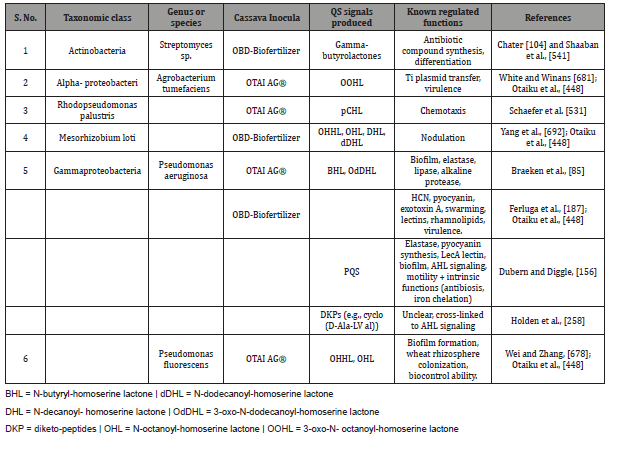
Soil microorganisms in general and rhizosphere microflora in particular are considered as treasure houses of the soil defining its fertility and plant growth promotion. Table 4 represents QS signals and its regulated functions. Cell-to-cell signaling regulates the expression of the rhlAB operon responsible for production of biosurfactants [432,433,463,466]. RhlI, N-butyryl homoserine lactone autoinducer synthase gene, and transcriptional activator encoding rhlR that are the major QS system and rhamnosy transferase encoded by rhlC which is coordinately regulated along with rhlAB are responsible for biosurfactant production in microorganisms [491]. These systems are under the influence of nutritional factor and QS signals [236]. They also came out with interesting observation that the nutritional conditions supersede cell-to-cell communication and hence correlate more positively with upregulation of quorum sensing-controlled genes such as rhlAB. Bacteria through social traits get several benefits such as coordinated population behavior (Vibrio fischeri, Ps. aeruginosa, and Staph. aureus), biofilm formation to get protection from adverse environmental conditions, nutrient and niche protection in nocules (Rhizobium sp.), enhanced colonization and growth in specialized niches (siderophores production for iron acquisition in bacteria), autolysis to provide nutrients and DNA for biofilm development (Ps. aeruginosa), coordinated movement toward nutrient source (Yersinia sp., Myxococcus xanthus, Ps. aeruginosa), antibiotic resistance through production of extracellular enzymes to break down antimicrobials (E. coli and Klebsiella spp.), and also immune modulation to facilitate survival within the host (Ps. aeruginosa, Porphyromonas gingivalis, Helicobacter pylori) [150]. Microorganisms develop protective adaptations easily at high density than at low density to adverse environmental conditions such as acidic condition, alkalinity, pressure, etc. [131,195,196,336]. Usually, microorganisms develop protective adaptations easily at high density than at low density to adverse environmental conditions such as acidic condition, alkalinity, pressure, etc. [131,195,196,336]. Signaling pathways are well documented and described [339,418,425,473]. The link between soil signaling and nitrogen cycling is also investigated by De Angelis et al. [145]. They reported that many alpha-proteobacteria were newly found with QS-controlled extracellular enzyme activity, and even cell division, symbiotic plasmid transfer, gene expression in the rhizosphere, symbiosome development and nitrogen fixation, and nodule number in Rhizobium bacteria are regulated by QS. QS is also reported to play an important role in expression of genes associated with virulence factors [41,374,384,518,560,689] (Figure 4) (Table 4).
Table 4: Activities of soil borne bacterial functions regulated by Quorum Sensing (QS) signals [490].
The plant microbiome has the ability to buffer plant hosts against abiotic and biotic stress, facilitate nutrient uptake and nutrient use efficiency, and promote growth [356,405,525]. Endophytic bacteria can be used to improve plant productivity and stress tolerance in the absence of pesticides and inorganic fertilizers, and to facilitate phytoremediation heavy metals and hydrocarbons, but more research is needed on how to best inoculate plants in field settings [93].
Diseases and pests of cassava
Cassava anthracnose disease is caused by a fungus which occurs on the surface of cassava stems and leaves [21] and appears as cankers (sores) on the stems and bases of leaf petioles. Cankers weaken the petioles so that the leaf droops downwards and wilts [694]. The wilted leaves die and fall causing defoliation and shoot tip die-back or complete death of the shoot. Soft parts of cassava stems become twisted under severe attack by the disease. The disease usually starts at the beginning of the rains and worsens as the wet season progresses [323]. Cassava bacterial blight, Leaf spot diseases, Cassava brown streak disease, Cassava root rot diseases, cassava mealybug. The cassava green mite Table 4. The treatment of the cassava disease and pests by disease suppression bio-control broad spectrum microbial PGPR inoculants in the biofertilizer (Tables 5a,5b & 6) (Plates 1A & 1B).
Table 5: Impacts of Biofertilizer on Diseases and Pests of Cassava cultivation.
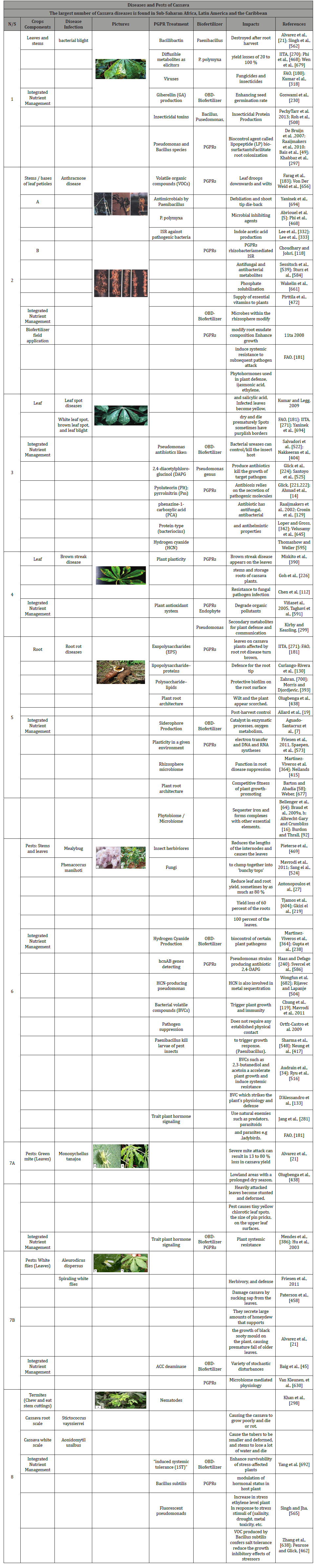
Table 6: Annotation of the pictures of Bio-control impacts Biofertilizer Cassava crops.
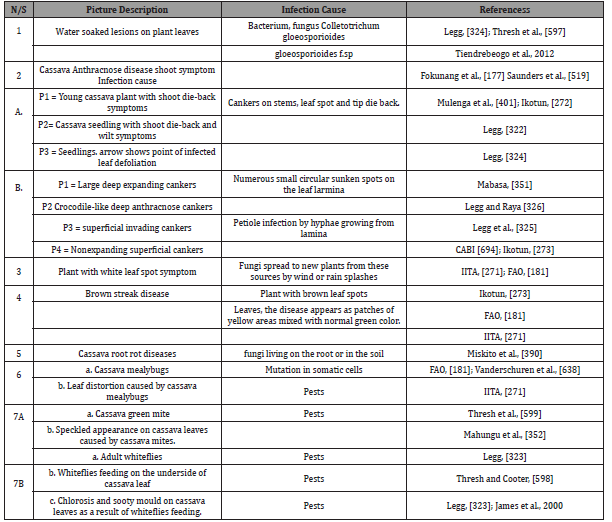
Table 7: List of some beneficial plant growth-promoting traits in the OBD-Biofertilizer.
Microorganisms affecting stress tolerance
Bacteria with the potential to act as bio stimulants have been isolated from a number of ecosystems with saline, alkaline, acidic, and arid soils. These bacteria belong to several genera such as Rhizobium, Bradyrhizobium, Azotobacter, Azospirillum, Pseudomonas, and Bacillus. Members of these genera have developed strategies to adapt and thrive under adverse conditions [40,389]. Amongst these adaptations, alterations to the composition of the cell wall and the ability to accumulate high concentrations of soluble solutes are common. These allow for enhanced water retention and increased tolerance to osmotic and ionic stress. Cell wall composition is altered through enrichment for exopolysaccharides (EPS) and lipopolysaccharide–proteins and polysaccharide - lipids which form a protective biofilm on the root surface [235,700]. Plant growth-promoting rhizobacteria (PGPR) inoculated soils can ameliorate plant abiotic stress responses (Tables 7&8).
Table 8: Progress and current status of cassava genetic transformation.
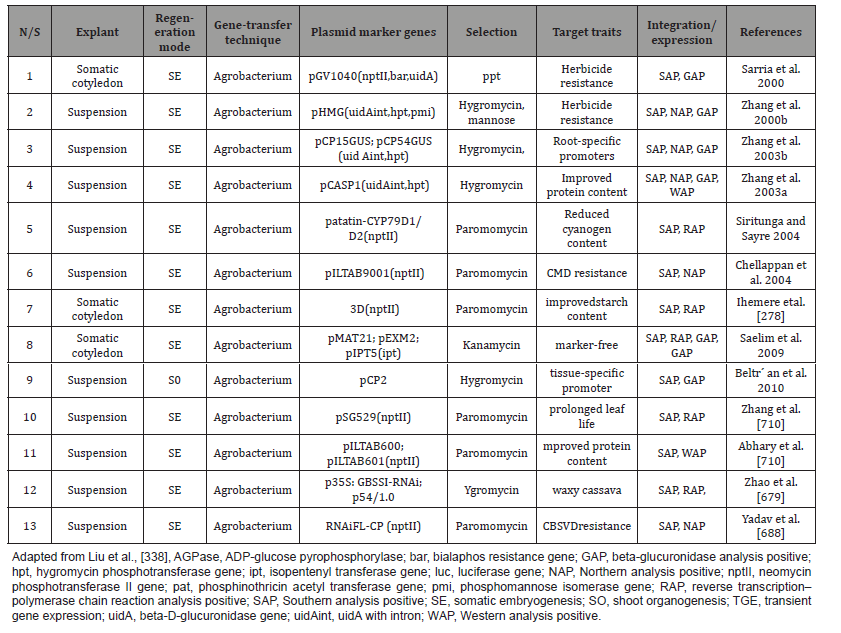
Table 9: Microbial Biofertilizers: Market Types and Application.
Endophytes are microorganisms that live within the plants’ tissues without causing any damage to the host. Entophytes could be classified as fungi, bacteria or algae [535]. Endophytes primarily assist in promoting the growth of plants that they inhabited as shown Figure 2 and Table 1. Facultative endophytes grow outside its host plant, obligate endophytes are dependent on their host plant for their growth and survival. Endophytic bacteria are correlated with the enhanced plant growth by the production of hormones that increase accessibility of nutrients, such as nitrogen, potassium and phosphorus reported by Glick, 2012, Table 8 While induced disease resistance activities are allied with the abilities to produce secondary metabolites, such as antibiotics or chitinase enzyme, which can inhibit growth of plant pathogens and act as biocontrol agents [128,670].
Endophytic bacteria have the capacity to cope with phytopathogenic fungi with induced systemic resistance (ISR) [469]. Due to their beneficial function such as plant growth promotion and disease control, endophytes can be used in the form of bio-formulations (seed treatment, soil application and seedling dip) in agriculture. Endophytic bacteria can also induce seedling emergence and stimulate plant growth under stress conditions. The genera of Bacillus and Pseudomonas are identified as frequently occurring bacteria in agricultural crops. It has been reported that most of Gram-negative endophytes act as agents of biological control [302], while among the Gram-positive bacteria, the dominant endophytic species are Bacillus species. The root exudates contain that colonize different bacterial genera and they differ normally according to plant species. The apical root zone having thin-walled surface of root cells includes cell elongation and the root hair zone (zone of active penetration), and the basal root zone with small cracks are the preferable sites of bacterial attachment and subsequent entry caused by the emergence of lateral roots (zone of passive penetration)of lateral roots (penetration). Root colonization or rhizospheric beneficial microorganisms are familiar biocontrol agents and plant growth promoters. Innumerable compounds such as hydrocyanic acids (HCN), DAPG, phenazines, pyrrolnitrin, enzymes and phytohormones to protect plant from toxic effect of fungal pathogens are considered as the significant products to help endophytes to be colonized in rhizosphere, Figure 4. Bacteria are able to trigger signaling pathways to produce extracellular metabolites with higher toxicity for other microorganism lead to destruction of higher pathogen, called induced systemic resistance (ISR).
Myriad of bacteria has been documented for beneficial effects, alleviation of several abiotic and biotic stresses. Pseudomonas and Bacillus sp. have been studied as potential candidate to provide ISR to plants. On an average, most of mineral nutrients in soil are present in millimolar amounts but P is present in micromolar or even lesser quantities. However, plants are well adapted to uptake of P from low concentration soil solution. Therefore, it is presumed that the supply and availability of P to the root surface is influenced by the root and microbial processes. Schematic illustration of important mechanisms known for plant growth promotion by PGPR. Different mechanisms can be broadly studied under (1) Biofertilization, and (2) Biocontrol of pathogens. Biofertilization encompasses: (a) N2Fixation, (b) Siderophore production, (c) P inorganic solubilization by rhizobacteria. Biocontrol involves: (a) Antibiosis, (b) Secretion of lytic enzymes, and (c) Induction of Systemic Resistance (ISR) of host plant by PGPR.
Growth, Yield and Root Quality: Biofertilizer
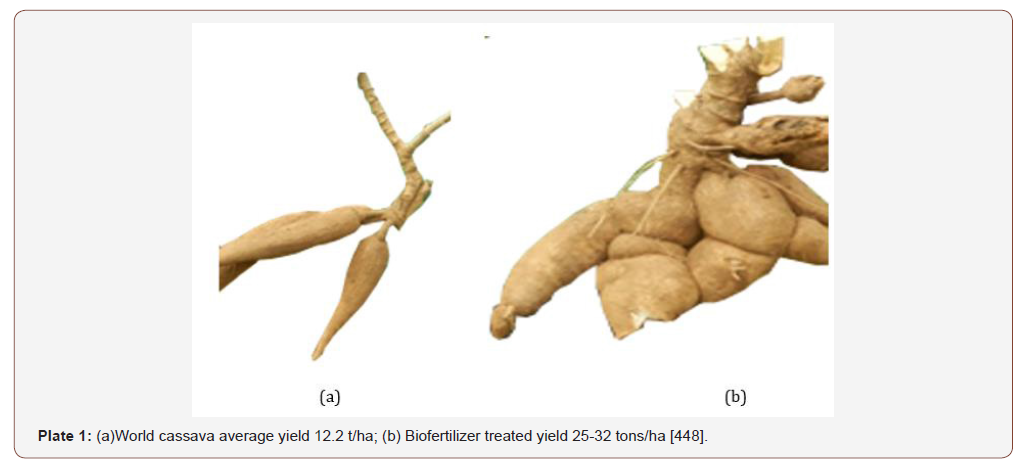
Biofertilizer facilitate the below-ground biological activity of earthworms, bacteria and fungi, and supply a wide range of nutrients, including secondary and micro-nutrients Adoa reported highest plant height with the application of poultry manure on Nkabom and IFAD cassava varieties. Adjei-Nsiah & Issaka (2013) observed that average fresh tuber yield increase from 13.7 t/ha without amendment to 23.7 t/ha with application of 4 t/ha poultry manure and compared with where biofertilizer application at 5t/ ha yield ; 16 t/ha and control yield 12 t/ha [448], Plates 1 and 2 Organic fertilizer promote the growth of stems and leaves of cassava, increase the chlorophyll content and the photosynthesis of leaves and improve the physiological metabolism of cassava. The period of maximum rate of dry matter partitioning depends on genotype-byenvironmental interaction. Canopy spread in cassava ensures large surface solar interception for photosynthesis. Nutrient supplied by poultry manure enhances increase in plant height due to increase in cell elongation of plant tissues as a result of steady release and mineralization of nutrients.
Amanullah et al., (2006), Parkes et al., (2012) observed that the number of roots per plant was significantly influenced by organic fertilizer treatment steady availability of nutrients throughout the crop growth period favourable changes in soil, such as loose and friable soil conditions, enabling better root formation, Plate 1. An increase in the number of storage roots per plant in response to organic fertilizer application has been reported by Kasele (1980) and Pellet & El- Sharkawy (1997). Leo & Kabambe (2014), observed a significant increase in number of roots per plant, and tuber diameter having a positive correlation with fertilizer treatment [448]. Manure application has resulted in higher root yields of cassava. Manure application enhances the cooking quality (mealiness) of cassava. Various observations have been made of a positive correlation between dry matter content and cooking quality of cassava.
Findings
Biofertilizer biodegradation
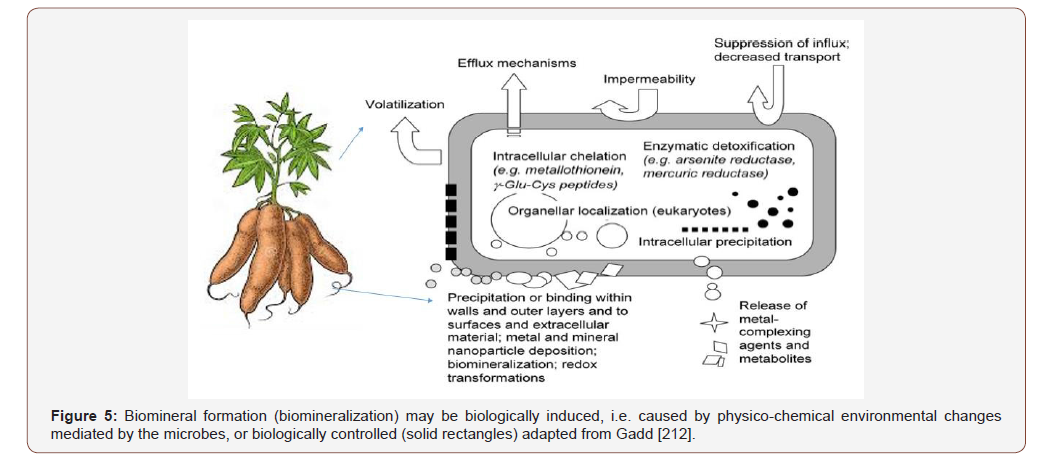
Metals are directly and/or indirectly involved in all aspects of microbial growth, metabolism and differentiation [205]. Bacterial resistance mechanisms generally involve efflux or enzymic detoxification, which can also result in release from cells, e.g. Hg (II) reduction to Hg (0) [421-424,450,510,553,554]. Bacterial plasmids have resistance genes to many toxic metals and metalloids, e.g. Ag+, AsO3, Cd2+, CrO2, Cu2+, Hg2+, Ni2+, Sb3+, TeO2 and Zn2+. Related systems are also frequently located on bacterial chromosomes, e.g. Hg2+ resistance in Bacillus, Cd2+ efflux. Bacillus and arsenic efflux in Escherichia coli [443,445,510,553]. Microbes are intimately associated with the biogeochemical cycling of metals, and associated elements, where their activities can result in mobilization and immobilization depending on the mechanism involved and the microenvironment where the organism (s) are located [165,210,211,654].
Despite apparent toxicity, many microbes grow and even flourish in apparently metal-polluted locations, and a variety of mechanisms, both active and incidental, contribute to resistance [35,193,203,259,397] Biofetilizer can be used for biodegradation of hydrocarbon pollution soil that impacts Niger Delta cassava cultivation (Gure, Yorla and Kpean) during hydrocarbon exploration reported by Otaiku,2019 where total N for the impacted soils range (0.58 to 0.179%).The concentration range for N% was <4.5 for deficient reported by Howeler [263]. The available P of the impacted soils ranges from 0.011 to 0.019%. The critical concentration for deficiency is P < 0.2% for cassava growth [263]. The polluted soil can be remediated and fortified with biofertilizer OBD-Biofertilizer inoculated with OTAI AG ® microbes to support cassava growth reported by Otaiku et al., [448].
Microbial resistance to toxic metals is widespread, with frequencies ranging from a few per cent in pristine environments to nearly 100 % in heavily polluted environments [554]. Chemical and biological methylation BY microbes playing significant roles in the latter process [208,594]. All microbial materials can be effective bio sorbents for metals except for mobile alkali metal cations like Na+ and K+, and this can be an important passive process in living and dead organisms [207,209,582,669]. Microbial biodegradation of organometallic (and organometalloid) compounds, still widely used in agriculture and industry, can result from direct enzymic action, or by microbial facilitation of abiotic degradation, e.g. by alteration of pH and excretion of metabolites [208,209] other xenobiotic that may be anthropogenically produced like TNT, RDX and other heavy metals [165,345,442-445]. The insoluble glycoprotein glomalin, produced in copious amounts on hyphae of arbuscular mycorrhizal fungi, can sequester such metals, and could be considered a useful stabilization agent in remediation of polluted soils [229]. Phytostabilization strategies may be suitable to reduce the dispersion of uranium (U) and the environmental risks of U-contaminated soils. Biomineralization is itself an important interdisciplinary research area, and one that overlaps with geomicrobiology [51,52,153,304]. There is growing awareness of the geochemical significance of microbes among researchers in geology, mineralogy, geochemistry and related disciplines [2 3,51,82,212,220,304,354,618,674]. Xenobiotic chemicals which may be carcinogens [444], drugs, food additives, hydrocarbons, pesticides, and many other forms of environmental pollutants. The chemical reactions in soil necessarily should facilitate conversion of xenobiotic to simpler compounds (mineralization) or sometimes alternatively xenobiotic undergo activation (conversion into toxic molecule). almost all the organic compound can be mineralized under this process [362]. Munition xenobiotic can be biodegraded by bacteria [443,445] and Fungi [442].
The Rhizosphere engineering: rhizomicrobiome for better plant health
In a rhizosphere microbiome, not all of the microbes are needed to fulfill the ecological services to plants because functional redundancy in microbial communities across diverse environments is common [152,572] (Figure 5).
The plants and the associated microbes are not seen individually as a unit of inheritance and evolution, rather as a halobiont or superorganism. The approach involves microbial population engineering rather than single strain engineering. The rhizosphere engineering holds great promise for future plant breeding programs and biotechnological application like in cassava crop. Microbiome assembly can be very sensitive to host genetic and environmental parameters and can vary even between different plant tissues. Rhizosphere microbiome diversity and their inheritance had been projected to be equally important as that of plant genome, since number of genes in plant microbiome is more than number of genes in a host [371]. The rhizosphere management methods should primarily focus on the hypothesis of increase in yield by altering the dynamics of host genotype-x-environment-x-microbe interactions [93] like in cassava crop and the ability to manage and manipulate microbiome is limited. There are three main approaches in building a productive microbiome - the first one relies on construction of a high yielding microbial consortium, second and third approaches involve manipulating the plant or the superorganism respectively.
Rhizosphere environment variations are induced by altering the physical and chemical environment in the rhizosphere through plant- affected characters which change the spectrum of the fitness and interactions among microbes and evolution of new microbes better suited to the rhizosphere environment [329]. These changes in microbiome structure and function are usually attributed to differences in root exudate chemistry [46,48,498], root architecture and in plant nutrient uptake rates [62] which makes it possible to engineer these traits into crops through gene editing tools. The most direct way to alter the microbiome is through inoculation with several strains or mixed cultures of bacteria, fungi rhizobia, endophytes etc. designated as biofertilizers. The concept of synthetic microbial consortium (SMC) is different from co-cultures, mixed cultures, microbial consortia and other similar concepts in a way that it includes, not only living together but also labor division [141,234,511,623].
There are two ways for designing and constructing SMCs [288].
The Meta-organism or superorganism approach is based on the fact that both microbiome and the plants are highly dependent on each other as the microbiome contributes a significant portion of the secondary genome of the host plant. The heritability of the meta organisms is not solely dependent on the genetics of microbes but the genetics of host plant as well. No general-purpose framework for the reconstruction of SMCs used to promote plant health is yet available [93]. The existence of functional redundancy in microbial communities across diverse environments is common [152,572]. Based on relative occurrence of microbes in microbiomes can be classified as core or minimal microbiome. A core microbiome (CM) is comprised of the members common to two or more microbial assemblages associated with a habitat [243,612].
There are various ways to define the CM within a habitat using bioinformatics-based approaches. Shade and Handelsman [542,543] suggested five parameters, including membership, composition, phylogeny, persistence, and connectivity, to discover the core microbiota based on a Venn diagram analysis. The concept of minimal microbiome (MM) implied the smallest but functionally indispensable subset of the total microbiome [482]. The ultimate goal of identifying such CMs or MMs is to exploit them in reconstruction of synthetic microbial consortium (SMC) with desirable member microbes [241]. SMCs are composed of multiple species with well-defined genetic background and help in accomplishing specific function through interactions among microorganisms. Plants release 10-20% of their photosynthates as exudates, which alter the physical and chemical properties of soil that in turn provides suitable niches for microbial proliferation [161,698]. Root exudates include a wide range of compounds, like carbohydrates, amino acids, organic acids, fatty acids, nucleotides, flavones, vitamins, and enzymes [54].
Cassava inoculants: Biofertilizer
Plant growth-promoting bacteria (PGPB) are generally obtained from soils [123,128]. Bacillus species including Lysinibacillus sphaericus, B. amyloliquefaciens B. cereus, B. mycoides, B. subtilis, B. pasteurii, B. pumilus, and B. thuringiensis may reduce the incidence or severity of plant diseases through the elicitation. of induced systemic resistance against pathogens of plants; hence, these bacteria can indirectly promote the plant growth [117,465,487,668]. Bacillus thuringiensis (Bt) is a unique soil bacterium that is gram-positive aerobic or facultative spore-forming and is included in the genus Bacillus. Bt-related studies are mostly focused on its insecticidal activity due to its entomopathogenic properties. Bt regarding its ability to interact with plants [128]. It was also reported that Bt can successfully colonize cabbage, cotton, soybean, and rice as an endophyte [31,475,480], Appendix 1.
The insecticidal toxins (Cry toxins) are usually expressed as δ-endotoxin and specifically act on some pest insect species [37,38,291]. Genes encoding these toxins are termed cry genes [127,533]. One significant common feature of the cry genes is that they are expressed during the stationary growth phase. These proteins start to appear during the 3rd phase of sporulation and persist until end of the 7th phase [91,268]. Some Bt strains produce non-parasporal insecticidal proteins during vegetative growth termed VIP (Vegetative Insecticidal Proteins). Generally, bacterial strains that have useful effects on plant growth are considered PGPB [289,389]. PGPB are beneficial microorganisms that help plant development [e.g., by producing indole-3-acetic acid (IAA), 1-aminocyclopropane-1-carboxylate-deaminase (ACC-deaminase), phosphate - solubilizing enzyme (PSE), and siderophores (SD)], exhibit antimicrobial activity against plant pathogens (e.g., by producing bacteriocin, zwittermicin, fengycin, chitinase, and cell wall-degrading enzyme reported by scholars Sharma & Saharan [546], Jouzani et al., [289], Raddadi et al., [486].
Phytohormones have important functions in plant growth and development as regulators and signals. They are produced by bacteria that colonize plant roots and play key roles in plant growth, plant pathology, and plant–microorganism interactions [225,486,538]. IAA is a phytohormone of the auxin class and is the most physiologically, biochemically, and genetically studied plant growth hormone [146,486]. Some Bt strains colonize plant roots and have plant growth-promoting properties [32,44,227,289,389,546].
Praça et al., [475] emphasized that effective colonization of Bt on the surface of seedling roots can affect physiology of host plants and that this bacterium may act as a growth. Biological fertilizer can be defined as a substance that increases a plant’s mineral nutrient intake and transportation when applied to seed and contain viable microorganisms that can be found on the plant surface or in the soil, rhizosphere colonies, or plant interior [486,651].
Although phosphate (P) is present in high amounts in many types of soils, it is an important limiting factor of plant growth. Phosphate solubilization can be improved through various mechanisms, such as hydrolysis or processes involving enzymes like phosphatases and phytases Matos et al., [365]. Bacillus spp. are known as one of the most significant phosphate-solubilizing bacteria (PSB) Behera et al., [61], Abdallah et al., [3] PSB convert the nonsoluble phosphate to the soluble form by enzymatic activity reported by Fitriatin et al., 2014 (Table 9) (Figure 6).
Table 10: Cassava Inoculants: Biofertilizer and Biostimulator phosphate solubilizing bacteria [701].
Cassava cultivar phytoremediation and Re-generative agriculture
Alves (2002) stated that cassava was a subsistence crop, grown by resource poor, small-holder farmers for land optimization techniques for crop failure in the tropics and low inputs crops. The future of cassava is to improve cultivar development increase crop yield, improves value chain development; income; soil health and yield and improve cassava cultivated on marginal soils [694]. Hillocks [18], suggested that the observed increase in acreage is related to declining soil fertility levels in Africa for chemical fertilizer application. According to FAO (2006), average cassava yields in Africa have gradually increased from 6 to 10 t/ha over the past five decades.
At present, the average African farmer harvests approximately 20% less cassava per hectare than the world average 12.2 t/ha due to no or low fertilizer inputs instead of addition of soil inputs that can yield 25-32 tons/ha [448] and there is the need to apply supplementary nutrients for sustainable crop production.
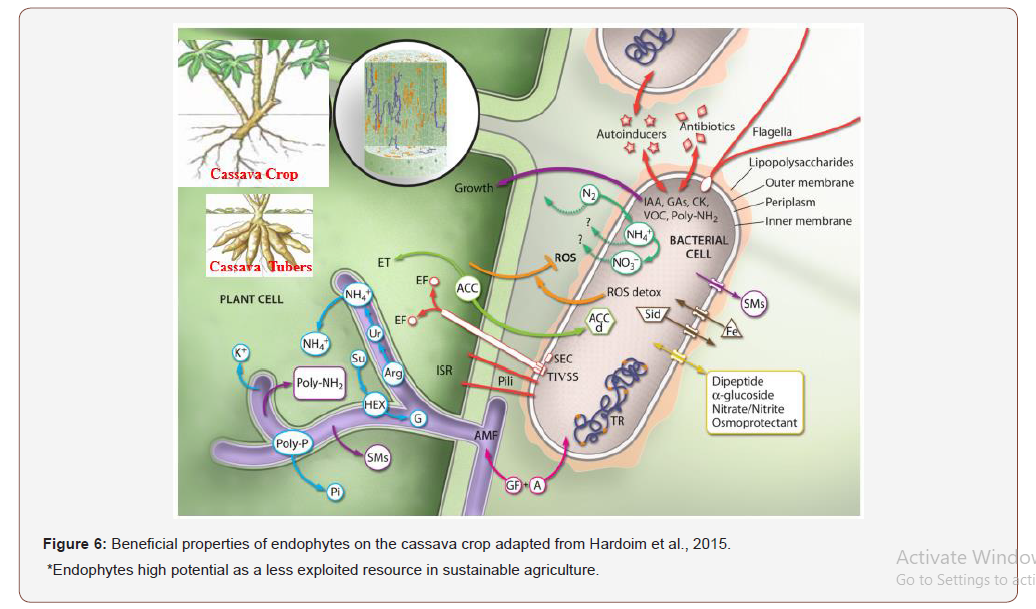
Howler (1990) earlier stated that large bulk of foliage are created by the action of nitrogen and consequently an extensive assimilating area, a pre-requisite for the good development of the roots. Roots development per plant is attributed to metabolites promotes the photosynthetic organs in the plant to produce and make available more assimilates to the root and increase the yield of cassava [711]. Biofertilizer application to crop cultivation and the role of PGPR is narrated in Figure 6 and adapted from Hardoim et al., 2015.
The cassava crop plants inoculated beneficial microorganisms significantly improve plantgrowth based on microorganisms in the biofertilizer inoculated to elaborate mechanisms of action in Tables 2 and 8. In Figure 6, bacteria (orange) and fungi (purple), can colonize the internal tissues of the plant (middle panel). Once inside the plant, the endophytic bacteria and fungi interact intimately with the plant cells and with surrounding microorganisms (large panel). Endophytic fungi, represented here as arbuscular mycorrhizal fungi (AMF) (lilac), might form specialized structures, called arbuscules, where plant- derived carbon sources, mainly sucrose (Su), are exchanged for fungus-provided phosphate (Pi), nitrogen (NH4+), and potassium (K+) elements (blue). Plant cytoplasmic sucrose is transported to the peri arbuscular space, where it is converted to hexose (HEX) to be assimilated by the fungus. Hexose is finally converted to glycogen (G) for long-distance transport reported by Hardoim et al., (2015).
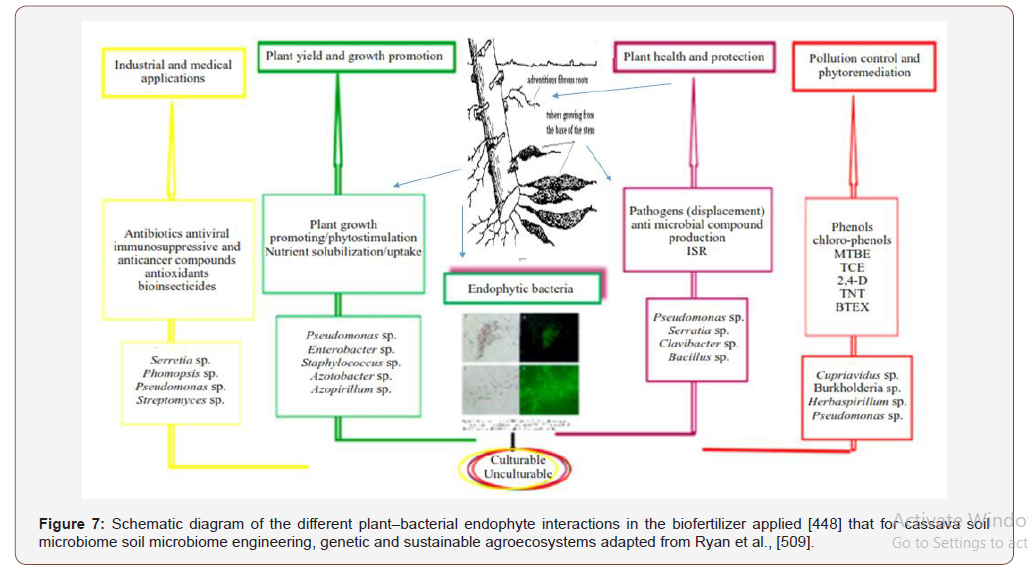
The production of secondary metabolites is undoubtedly the major mode of action amongst endophytes. Endophytes are regarded as a micro-organism that lives in plant tissues partly or in all of their lifecycle, classified as beneficial, neutral and or detrimental depending on the kind of interaction with their host plant and example, mycorrhizal fungi and rhizobia are regarded as the beneficial microbes. Endophytes possess the ability to control the pathogens of plant, insects and nematodes [311,509] xenobiotic degradation [442,444,445]. The emerging use of endophytesbased nanoparticles as value added materials [446,227] has showed promising results for future drug development. In the near future, the application of endophytes may revolutionize drug formulations like the pharmaceutical starch from cassava. Host plants can be induced to produce required metabolites of interest such as those used in drugs for treating cancer. Endophyte(s)-based bioformulations applied on seeds or aerial parts will be far more effective because once the microbe is inside the plant tissue, it will not face the competition of other soil microbes, which is common in the case of rhizosphere microbes (Table 9).
Endophyte-based bioformulations for remediation of contaminated soils, pollutants and biodegradation (Peng et al., 2013 and Muponda, 2014) that exhibit their symbiotic responsibilities to its host producing metabolites (Kumar et al.,2014 ; Gao et al., 2015) and support root development and access to nutrients (Tan et al., 2001) protect the plant from desiccation and from insects as well as parasitic fungi (Taghavi et al., 2011); root-knot entomopathogenic microbes (nematodes) treatment Elmi et al., 2000 ; Sikora et al., 2008 ; Hirose and Murakami (2011) illuminated in Figure 7.
Biofertilizer: Remediation-to-Biofuel sustainable development
Germain et al.,2006 studied the degradation of herbicides with bacterial endophytes (Pseudomonas sp.) and reported that there was no sign of accumulation of the herbicide in the plant tissue and no sign of phytotoxicity, unlike the uninoculated plant. The use of endophytes capable of degrading environmental contaminants in addition to the specific plants could offer an efficient, economic and sustainable remediation technology. Cassava crop new development cultivars could be used for phytoremediation of polluted soils (hydrocarbon) base on the report by Otaiku [446], that cassava cultivation in hydrocarbon producing communities of Niger -Delta have food security challenges because of impacted soils nutrients. Applied biofertilizer to polluted soils will open a new research future development for roots crops re-generative agriculture in Niger Delta called remediation-to-biofuel (cassava crop harvested converted to bioethanol) sustainable development. This paradigm shifts for polluted degraded soils called pollution construct, remediation, restoration and re-use (PC3R Technology) where microbial inocula in Tables 1 and 9 are applied for the xenobiotic biodegradation [442,444]. PC3R technology encapsulate genetics, bioremediation, phytoremediation, re-generative agriculture other techniques (Phyto stabilization, phytovolatilization, rhizofiltration, etc.). PC3R genetic studies using endophytes and omics techniques improved cassava cultivar gene carrier affirmed by scholars [535,539]. The genetically engineer cassava cultivar and microbial endophytes which will serve as protection for the host like cassava crop for remediation-to-biofuel development and convert remediated polluted soils in the tropics the bio-ethanol economy (waste-to-wealth project). The PC3R technology application includes are xenobiotics (munitions waste) biodegradation [442- 445], heavy metals, POPs and high molecular weight pesticides with their recalcitrant, bioaccumulation and bioconcentration properties, that generally defy conventional remediation practices and techniques [353,680]. The limitation of phytoremediation is often as a result of the toxicity of these chemicals or their toxic end products in plants [370,622] can be ameliorated through endophyte-assisted phytoremediation [267,576] and endophytic microbes activity [30,535] by incorporating into plant endophyte those natural microbe ability to conjugate with each other by means of movable DNA elements (vectors) between microbial populations [29] Case study report the introducing bacterial genes pTOM-Bu61 involved in the metabolism of toluene and TCE biodegradation [576].
Crude oil had variable effects on the microbial biomass [163] weakens soil microbe’s activity influences plant root development [164], soil water absorption by plants [33], biotoxicity [33], soil structure, water stress and nutrients deficiencies [434] and decline in crop performance [200]. as elevated accumulation has direct consequences to man and ecosystem [8]. The low pH of the soil could explain the presence of cyanogenic glycosides in the cassava effluent contaminated soil. Low clay content was reported as soil conditions that increase cyanide mobility. The biodegradability of cassava effluent impacts on the physicochemical characteristics on soil dynamics and structure was reported by scholars the microbial contents isolated from the studies areas of Niger Delta, Nigeria was similar to the biofertilizer microbial inocula [36,167,279,303,436].
Biofertilizer biosafety
Populations of microorganisms applied to the environment commonly decline to a density naturally sustainable within that environment, often to undetectable levels. Plant-associated microorganisms introduced as biocontrol agents into the rhizosphere or phyllo sphere, the population of the microbial biocontrol agent declines to background levels when the supporting plant dies, and it must be applied again with the next planting of that crop [125,595], this promotes re-generative agriculture for modern agriculture. Evidence shows that the effects are short-term and subtle and that non-target populations stabilize relatively quickly after application is discontinued [93]. Studies have confirmed that plant-associated microorganisms introduced into soil remain virtually in the row where introduced and decline to undetectable populations soon after and sometimes before the supporting plant completes its life cycle [195] and see the narrative in Figure 6 and the challenge can be corrected deploying PC3R technology.
Impacts of Genomics on Cassava Development
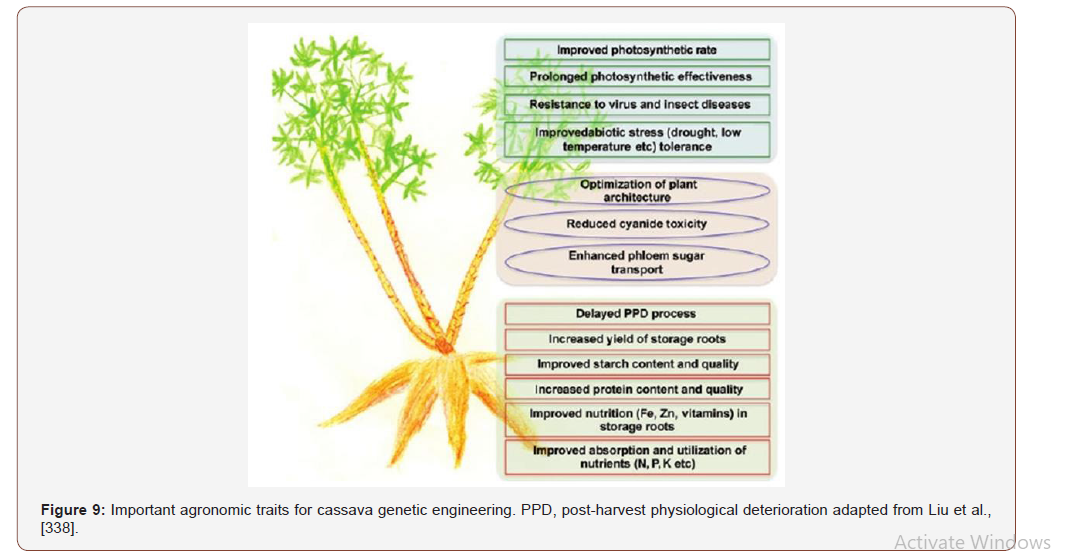
Cassava is vegetative propagated through stem cuttings, and its growth cycle is longer than 10 months. Cassava breeding is hampered due to the high degree of genetic heterozygosity, genetic overloading, serious separation of progeny, few flowers, low pollen fertility, self-incompatibility, and low fruit set rate [101]. Genetic engineering shows great potential in cassava genetic improvement and can compensate for the limitations of conventional breeding for cassava. Programs, such as HarvestPlus and BioCassava Plus, have made remarkable achievements by transforming traditional breeding into molecular breeding [529]. Schopke et al., (1996) reported milestone for cassava molecular breeding.
The commonly used methods for the genetic transformation of cassava include Agrobacterium-mediated gene delivery and particle bombardment. Agrobacterium is one of the microbes in the biofertilizer reported by Otaiku et al., [448] (Paper 1). The explants used for transformation include somatic cotyledons and FEC. Usually cassava transformation is carried out using FEC and/or embryogenic suspensions by Agrobacterium tumefaciens or particle bombardment. Gonzalez et al., [229] successfully transformed FEC of the West African cultivar 60444 (also known as TMS60444) with the Agrobacterium tumefaciens strain ABI. The most prominent advantage of the Agrobacterium-mediated transformation system is the availability of a large number of transgenic plants; thus, it is the most widely used method for cassava genetic engineering. Climate change vulnerabilities necessitated the breeding of new cassava varieties with increased nutrition, high stress resistance and starch content [101] using the genetic engineering in germplasm innovation by improving specific traits without changes in other important traits using genome editing technology‘-omics’ tools have led to intensive cassava starchy storage root development, starch accumulation, health-promoting components (e.g. betacarotene), and stress response and regulation [529,693].
Genetic development using Agrobacterium-mediated transformation protocols for TMS60444 friable embryogenic callus (FEC) by Liu et al., [338]. In Table 7 and Figure 7 several genetic transformation systems of farmer-preferred cassava cultivars have been successfully established using African, Asian and South American cultivars they still need to be optimized due to cultivar dependence. The first successful Agrobacterium- mediated cassava genetic transformation that was reported was from the Potrykus laboratory at ETH Zurich in 1996 using Agrobacterium strains harboring different binary vectors (e.g. LBA4404 (pTOK233) and others to transform cassava somatic cotyledons. Sarria et al. (2000) successfully transformed an herbicide (phosphinotricin, ppt)-resistance gene into the cotyledons of cassava MPer183 by an Agrobacterium-mediated method and obtained stable transgenic plants resistant to Basta spray (at concentrations of 200 mg/L).
Also, Siritunga & Sayre (2003) developed transgenic cassava with a lower cyanide content using MCol2215 cotyledon explants). Analysis of the transgenic plants revealed the integration of the target gene into the genome and its expression at the transcript level. Field experiments showed that the transgenic plants had significantly delayed leaf senescence, drought tolerance, and altered cytokinin content in their leaves. Transformation efficiency was enhanced with low Agrobacterium density during co-cultivation and co-centrifugation of FEC with Agrobacterium, possibly facilitating plant regeneration [105,428]. Application of TALEN and CRISPR/ Cas9 systems to mutant target genes is now in demand in cassava [683] and the utilization of this tool in cassava is still in its infancy and no published reports involving genome editing of cassava to date, although several laboratories are working on different genes and traits. Based on draft cassava genome sequences [476,670]. Cassava breeders are at the turning point of trait improvements in important root crops.

The candidate gene identification study is still dependent on traditional methods, such as Cdna library screening under stress [521] or transcriptome analysis under different treatments [619] or development stages [693]. Researchers are seeking specific ways to study massive microarray data [619,693], RNA-seq [340,350] and even genome sequencing [670]. Agrobacterium sp is microbe used in the biofertilizer formation as inocula reported by Otaiku et al., [448]. Cassava mosaic disease (CMD), one of the major viraldiseases in Africa, is responsible for yield reductions of 20–95% in certain areas [322] and cassava brown streak disease (CBSD) result in great loss of cassava production in sub-Saharan Africa and the Indian subcontinent [323]. In the model cassava cultivar TMS60444, CMD-resistant transgenic cassava has been developed using both antisense and dsRNA technology [633- 635,688,707]. To develop CMD-resistant cassava, African breeders used resistant germplasms of wild cassava relatives (Manihot glaziovii) to obtain new cultivars of cassava resistant to CMD, which have been widely adopted in the major epidemic regions, reduced, and cassava cultivation has gradually been recovered (Figures 8 & 9).
However, this variety comes from West Africa and is not resistant to African cassava mosaic disease (CMD) or cassava brown streak virus disease (CBSD). It is necessary to develop FEC-based transformation systems in other cultivars that are preferred by farmers and the industry. In this regard, several laboratories have made unremitting efforts by investigating various genotypes for FEC production, and success has been achieved in several cultivars, such as TME7, Ebwanatereka, TME1, TMS91/02327, Rosinha, and Buja Preta [268]. Transgenic technology, as a powerful tool, also plays an important role in obtaining virus-resistant cultivars [633]. Chellappan et al., (2004) used pILTAB9001 and pILTAB9002 harboring the wild-type and mutant AC1 genes of ACMV-Kenya, which were regulated by the cassava vein mosaic virus promoter and the pea Rubisco terminator for the production of transgenic TMS60444 lines with increased resistance to mosaic disease. Insecticide proteins, such as Bt Cry proteins, protease inhibitors, α-amylase inhibitor, and plant lectins, could pave the way for insecticides, as a high expression of these products in transgenic cassava might be useful for increased insect resistance.
Transcriptomic studies revealed a rapid change in cassava genes after infection by this disease [343]. Using the leaf senescenceinduced promoter, SAG12, to express the ipt gene, transgenic cassava showed not only prolonged leaf life, but also improved resistance to drought stress [705]. Transgenic plants also showed altered composition of amino acids and reduced cyanide content. Therefore, a transgenic approach to cassava protein enhancement is practical and is a useful way to reduce protein deficiency in poverty-stricken regions [638]. Biofortification of cassava by BioCassava Plus and of Harvest Plus advanced breeding and the nutrition issues of zinc, iron, and vitamins A and β-carotene-rich cassava [269,529]. Cassava accumulates a large amount of starch in its storage roots and its native starch has many shortfalls such as low solubility and retrogradation. To overcome these limitations, native starch is often modified chemically, physically or biotechnologically [292]. The challenge in most countries are not yet equipped to approve transgenic waxy cassava through the regulatory process. Storage roots are the cassava plant’s main commercial product, and therefore understanding storage root development is key to improving starch accumulation and root production. Jørgensen et al. (2005) conducted a similar experiment by RNAi and found that the cyanogenic glucoside contents of cassava storage roots were reduced by 92%. White et al. [599] reported that at transcript level, the hydroxy nitrile lyase content in roots is only 6% of that in leaves. The overexpression of hydroxy nitrile lyase can reduce the acetone cyanohydrin content of roots, thus accelerating the detoxification process. To achieve this goal, the cDNA of the gene encoding hydroxy nitrile lyase was cloned between the CaMV 35S promoter and the pea ribulose bisphosphate carboxylase terminal sequence, and transformed into MCol2215, Appendix 1.
Comparative proteomes also show that root formation may be influenced by metabolic and regulatory processes in cassava leaves [391]. Through AFLP-based transcript profiling, Sojikul et al. [569] found that four genes in the MeKD family exhibit critical expression in initiation and early stage development of the cassava storage root. An estimated 26% of total cassava production is lost owing to post-harvest physiological deterioration (PPD)reported by Sayre et al., [529]. Genetic improvement can potentially delay or inhibit PPD in cassava and PPD process differs among cassava genotypes and PPD-susceptible HMC-1 variety and PPD-tolerant experimental hybrid AM206-5 were significantly different in PPD level and in secondary metabolic synthesis [523] and engineering of cassava storage roots with prolonged shelf life. Among the significantly impacted pathways, glycolysis / gluconeogenesis was the most vital one [693], suggesting the importance of carbon mobilization during storage root development. Recent studies based on cDNA microarray-based transcriptomes [502] and iTRAQ proteomics [453] showed that the onset of PPD is related to signal transduction pathways involving multiple enzymes, cellular processes including reactive oxygen turnover, signal transduction, stress response, metabolism and biosynthesis.
Reactive oxygen species (ROS) production and turnover in the root is closely associated with PPD. O2− and H2O2 quickly accumulate in the root within 15 minutes after harvest, with peak levels at 4hrs and 24hrs, respectively [502]. Accumulation of ROS in the storage root can be observed directly by fluorescent probes (singlet oxygen sensor green, dihydroergotamine 123), which indicates oxidative bursts during the PPD process [686]. Zidenga et al., [712] reported that PPD is cyanide-dependent, probably because of a cyanidedependent inhibition of respiration. Post-harvest physiological deterioration has a close relationship with reactive oxygen species (ROS). Using the cDNA-AFLP technique, Beaching’s laboratory has analysed the proteins and enzymes affecting PPD, most of which are involved in signal transduction, ROS, cell wall repair, programmed cell death, metabolite transport, signal transduction, and a series of biological processes [502]. The upregulation or downregulation of key enzymes or factors in the PPD pathway by the overexpression or RNAi might effectively slow or reduce the occurrence of PPD (Figures 10 & 11).
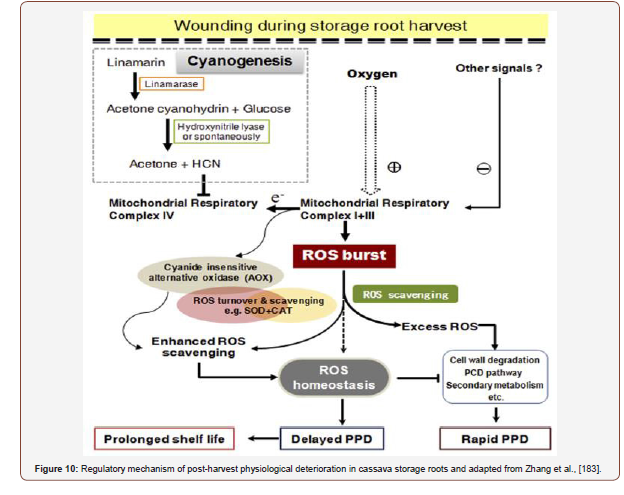
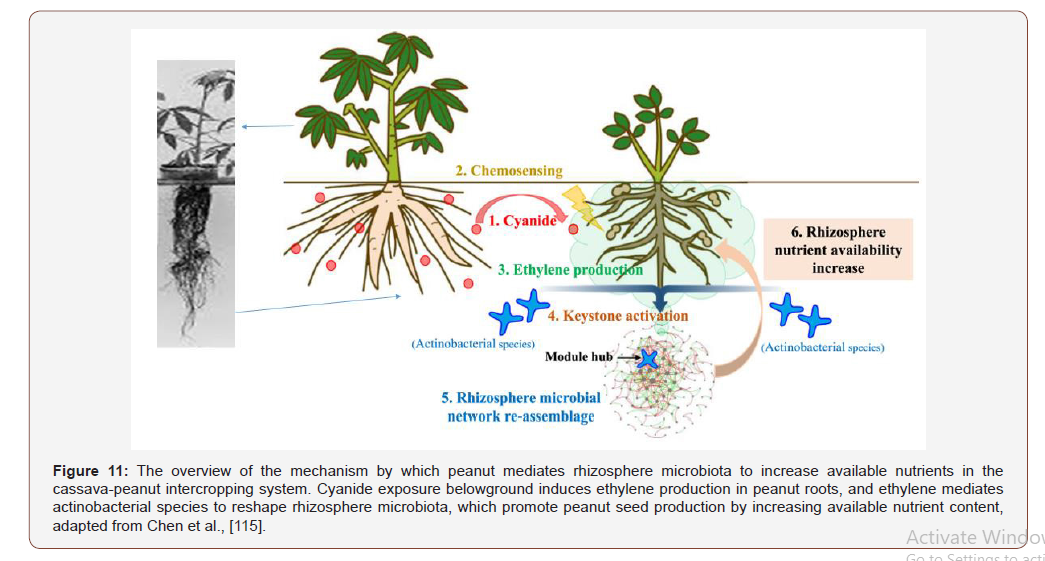
The production of reactive oxygen species (ROS) is an inevitable consequence of an aerobic lifestyle. ROS participate in signaling pathways in plants, animals, and fungi [189,536,606] and even in interspecies communication [606]; and it has also been proposed that they play a role in the development of multicellularity [328]. ROS is in fact beneficial to longevity through the adaptive mechanism called hormesis [455,506]. During hormesis, low doses of stress or toxin induce mechanisms that protect the organism against this stressor and evoke cross adaptation to other stresses. Lethal attacks from bacterial and viral species also result in ROS production in target cells. As a model prey or target organism, Escherichia coli can be killed by the T6SS activities of a number of bacteria including Pseudomonas aeruginosa and Acinetobacter baylyi ADP1.
Cassava Sustainable and Ecological Intensification
The growing concern for ecologically grown foods due food safety and security, environmental protection, biodiversity and human health [144,178,530]. The distribution of cassava is related to climatic conditions and most of the cassava producing regions are between Tropic of Cancer and Tropic of Capricorn and elevations up to 2300m above mean sea level [18]. Cassava, intermediate photosynthetic pathway between the C3 and C4 [172] can be adaptive “efficient” C4 pathway of higher plants [169]. Cassava can yield 80t/ha of fresh tuber (experimental farms) and 40t/ha of fresh tuber improved cultivars (commercial fields); productivity in seasonally dry and semi-arid environments without fertilization is much less [173,481]. Average yield of fresh tuber of cassava varies from 1.13 to 32.68 t/ha among 104 cassava growing countries with a global average yield of 11.80 t/ha, which is far below the potential yield of 80–100t/ha [94]. A large yield gap exists between yield potential and farmers yields due to lack of adoption of improved varieties, better agronomy and soil fertility management [170,171,186]. Because of its inherent tolerance to prolonged drought and infertile soils [172], cassava production is expanding into more marginal lands and drier environments for subsistence [186,515].
Secondary nutrients influence cassava cultivation, namely, calcium, magnesium and sulphur reported by [306,478,394,395]. Liming increased the tuber yield and starch content and decreased the HCN content [405]. Mohankumar & Nair, [395], Howeler [264] reported that Magnesium deficiency was observed in cassava cultivated in Oxisols, Ultisols, Inceptisols and Entisols (Application of Sulphur at 50kg/ha resulted in a significantly higher tuber yield and starch content and a lower HCN content, total protein and methionine contents. Cerilles [100] reported significance of organic cassava mitigating the effects of climate change in the Philippines. Zhongyong et al., [711] reported that cassava bio-organic fertilizer treatment promoted the leaf and stem growth, increased the chlorophyll content, photosynthesis of leaves, improved the physiological metabolism of cassava, transfer of photosynthates to storage roots and increased yield and starch content in the storage roots of cassava. Organic nutrient management also resulted in greater N and K uptake over chemical system, Also, similar impacts was reported for compost and inoculated with mixed biofertilizers [552] which affirmed the report by Otaiku et al., [448]. Radhakrishnan [494,495] 2019 reported ultimately reflected in tuber yield under organic management (27.26 t/ha). Organic and inorganic nutrient sources had significant effect on quality of cassava tubers [451]. Cyanogenic glucoside levels decreased with the application of organic fertilizers while inorganic fertilizer increased the level of cyanogenic glucoside and decreased the phytochemical contents in both the leaves and tubers. Nutritional quality was improved by organic fertilization with significant effects on antioxidant activity and phenolic metabolites in cassava reported by [451]. Omar et al. [452] also observed that application of organic fertilizers like vermicompost was favorable for enhancement of antioxidants and total phenolic acids in cassava leaves that contributed to nutritional value of leaves used as vegetable.
Conclusion
The ability of endophytes to colonize every plant tissue has led to the opportunity of using the microorganism in agriculture and environmental biotechnology for sustainable development like the biofertilizer technology. The different constraints in biofertilizer technology can be mitigated [611,655] using nanobiotechnology and value-added materials [446], Table 13 as an alternative to chemical fertilizer. Cassava adaptation exhibited by plants growing in an extreme environment and expressing genes for adaptation can be improved using genetic engineering to become phytoremediation xenobiotic cultivar for pollution control and remediation-to-biofuel regenerative agriculture because of the rhizosphere microbe’s potentials like Bacillus Pseudomonas, and Agrobacterium species with their outstanding root-colonizing ability, and Horizontal Gene Transfer (HGT), catabolic flexibility and ability to produce a wide range of metabolites for ecological services and agrosystems management to improves the cassava crop sustainable development goals.
Acknowledgement
None.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest [Appendix 1 (Figure 12)] [Appendix 2 (Tables 10-13)].
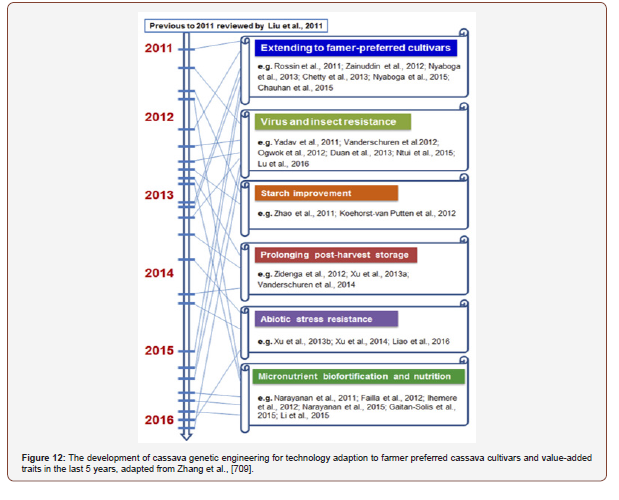
Table 11: Advantage and disadvantage of using biofertilizer.
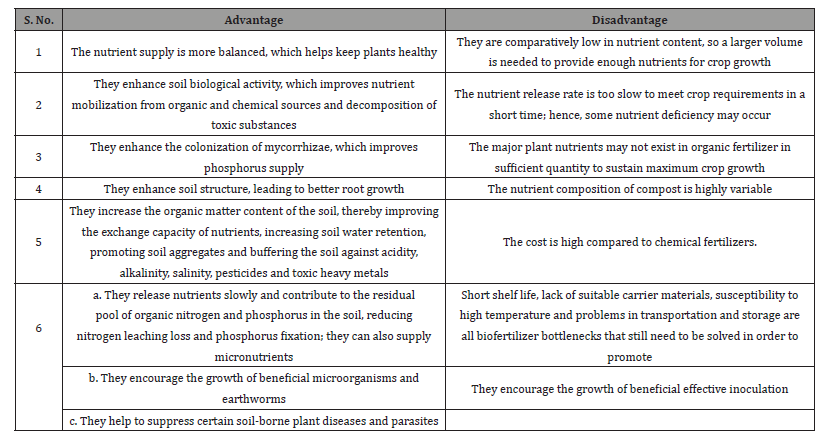
Table 12: Horizontal Gene Transfer in Soil and the Rhizosphere: Impact on Ecological.
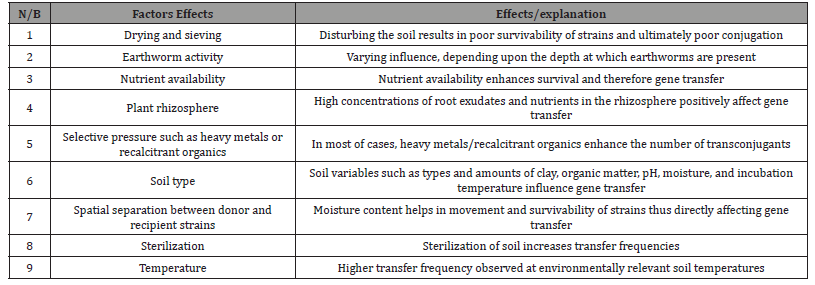
Table 13: Advantage and disadvantage of chemical fertilizer.
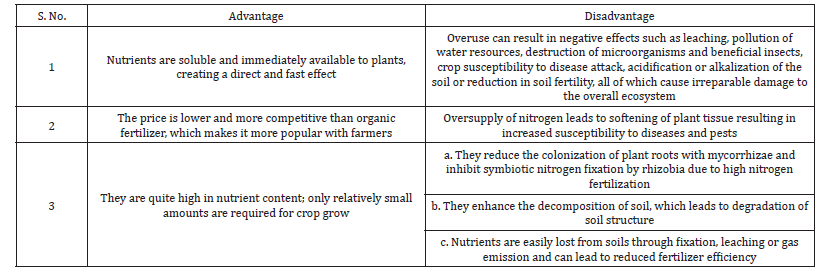
References
- A Bear, Jones TH, Boddy L (2014) Potential impacts of climate change on interactions among saprotrophic cord-forming fungal mycelia and grazing soil invertebrates. Fungal Ecol 10: 34-43.
- Abd, El-Lattief EA (2016) Use of Azospirillum and Azobacter bacteria as biofertilizers in cereal 434 crops: a review. IJREAS 6: 36-44.
- Abdallah DB, Frikha-Gargouri O, Tounsi S (2018) Rizhospheric competence, plant growth a promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol Control 124: 61-67.
- Abhilash, PC, Dubey RK, Tripathi V, Pankaj Srivastava, Jay Prakash Verma, et al. (2013) Remediation and management of POPs- contaminated soils in a warming climate: challenges and perspectives. Environ Sci Pollut Res 20: 5879-5885.
- Abriouel H, Franz CMAP, Omar NB, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35: 201-232.
- Adler PB, Dalgleish HJ, Ellner SP (2012) Forecasting plant community impacts of climate variability and change: when do competitive interactions matter? J Ecol 100: 478-487.
- Aguado-Santacruz, Get al (2012) Impact of the microbial siderophores and phytosiderophores on the iron assimilation by plants: a synthesis. Rev Fitotec Mex 35(1): 9-21.
- Agbozu, IE, Ekweozor IKE, Opuene K (2007) Survey of heavy metals in the catfish Synodontic claries. International Journal of Environmental Science Technology 4 (1): 93-7.
- Ahemad M, Khan MS, (2012) Evaluation of plant-growth promoting activities of rhizobacterium Pseudomonas putida under herbicide stress.Ann. Microbiol 62: 1531-1540.
- Ahkami, A, White RA, Handakumbura PP, Jansson C (2017) Rhizosphere engineering: enhancing sustainable plant ecosystem productivity. Rhizosphere 3: 233-243.
- Ahmad, P, Hameed A, Abd-Allah E.F, Sheikh SA, Wani M R, et al. (2013) Biochemical and molecular approaches for drought tolerance in plants. In: Ahmad P, Wani MR (eds.), Physiological mechanisms and adaptation strategies in plants under changing environment. Springer, USA, pp: 1-29.
- Ahmad P, Kumar A, Ashraf M, Akram NA (2012) Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr J Biotechnol 11(11): 2694-2703.
- Ahmad R, Lim CJ, Kwon S-Y (2013) Glycine betaine a versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol Rep 7: 49-57.
- Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163(2): 173-181.
- Aislabie J, Deslippe JR, Dymond JR (2013) Soil microbes and their contribution to soil services. In: Dymond J.R (ed) Ecosystem services in New Zealand: conditions and trends. Manaaki Whenua Press, Lincoln 135: 143-161.
- Albrecht-Gary AM, Crumbliss AL (1998) Coordination chemistry of siderophores: thermo dynamics and kinetics of iron chelation and release. In Metal Ions In: Biological Systems 129: 239-327.
- Alexander I, Lee S (2005) Mycorrhizas and Ecosystem Processes in Tropical Rain Forest: Implications for Diversity. Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity. Cambridge University Press: New York pp. 165-203.
- Allem AC, (2002) The origins and taxonomy of cassava. In: Hillocks RJ, Thresh JM., Bellotti A (Eds.), Cassava: Biology, Production and Utilization. CABI, Wallingford, UK, pp. 1-16.
- Allard S, Enurah A, Strain E, Millner P, Rideout SL, Brown EW, et al. (2014) In situ evaluation of Paenibacillus alvei in reducing carriage of Salmonella enterica serovar Newport on whole tomato plants. Appl Environ Microbiol 80: 3842-3849.
- Allstadt, A J, Vavrus SJ, Heglund PJ, Pidgeon AM, Thogmartin WE, et al. (2015) Spring plant phenology and false springs in the conterminous US during the 21st century. Environmental Research Letters 10(10):104008.
- Alvarez, E., Llano, G.A. and Mejia, J.F. (2012). Cassava diseases in Latin America, Africa and Asia. In R.H. Howeler, (ed.), Thecassava handbook - A reference manual based on the Asian regional cassava training course, held in Thailand. Cali, Colombia, CIAT. pp. 258-304.
- Amundson, R, Berhe AA, Hopmans JW Carolyn Olson, A Ester Sztein, et al. (2015) Soil and human security in the 21st century. Science 348(6235).
- Amundson R, Richter DD, Humphreys GS, Jobbagy EG ,Gaillardet J (2007) Coupling between biota and Earth materials in the critical zone. Elements 3: 327-332.
- Amutha, R, Karunakaran S, Dhanasekaran S, Hemalatha K, Monika R, Shanmugapriya P, et al. (2014) Isolation and mass production of biofertilizer (Azotobacter and phospho- bacter). Int J. Lat Res Tech 3(1): 79-81.
- An D, Yang J, Zhang P (2012) Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics 13: 64.
- Annual Review of Plant Biology (2013) 64. Palo Alto: Annual Reviews, pp. 807-38.
- Antonopoulos DF, Tjamos SE, Antoniou PP, Rafeletos P, Tjamos EC (2008) Effect of Paenibacillus alvei, strain K165, on the germination of Verticillium dahliae microsclerotia in planta. Biol Control 46: 166-170.
- Antonova ES, Hammer BK (2011) Quorum-sensing autoinducer molecules produced by members of a multispecies biofilm promote horizontal gene transfer to Vibrio cholera. FEMS MicrobiolLett 322(1): 68-76.
- Andreolli MS, Lampis M, Poli, G Gullner, B Biro, G Vallini (2013) Endophytic Burkholderia fungorum DBT1 can improve phytoremediation efficiency of polycyclic aromatic hydrocarbons. Chemosphere 92: 688-694.
- Anyasi RO, HI Atagana (2016) Endophytes: An indicator for improved phytoremediation of industrial waste. Proceedings of the 23rd WasteCon Conference, Emperors Palace, Johannesburg, South Africa Institute of Waste Management of Southern Africa pp. 140-150.
- Argôlo-Filho R, Loguercio L (2014) Bacillus thuringiensis is an environmental pathogen and host-specificity has developed as an adaptation to human-generated ecological niches. Insects 5(1): 62-91.
- Armada E, Probanza A, Roldán A, Azcón R (2016) Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J Plant Physiol 192: 1-12.
- Atuanya EI (1987) Effect of oil pollution on physical and chemical properties of soil: a case study of waste oil contaminated Delta soil in Bendel State. Journal of Applied Sciences 5: 23-8.
- Audrain B, MA Farag, CM Ryu, J. M. Ghigo (2015) Role of bacterial volatile 24 compounds in bacterial biology. FEMS Microbiol Rev 39 (2): 222-233.
- Avery S V (2001) Metal toxicity in yeast and the role of oxidative stress. Adv Appl Microbiol 49: 111-142.
- Aiyegoro OA, Akinpelu DA, Igbinosa EO, Ogunmwonyi HI (2007) Effect of cassava effluent on the microbial population dynamic and physicochemical characteristic on soil community. Sci Focus 12: 98-101.
- Azizoglu, U, Yilmaz S, Ayvaz A, Karabörklü S, Akbulut M (2011) Characterization of local Bacillus thuringiensis isolates and their toxicity to Ephestia kuehniella (Zeller) and Plodia interpunctella (Hübner) larvae. Egypt Biol Pest Control 21: 143-150.
- Azizoglu, U, Yilmaz S, Ayvaz A, Karabörklü S, Atcıyurt ZB (2017) Mosquitocidal potential of native Bacillus thuringiensis strain SY49-1 against disease vector, Culex pipiens (Diptera: culicidae). Trop Biomed 34: 256-269.
- Baetz U, Martinoia E (2014) Root exudates: The hidden part of plant defense. Trends in Plant Science 19 (2): 90-98.
- Bagri DS, Upadhyaya DC, Kumar A, Upadhyaya CP (2018) Overexpression of PDX-II gene in potato (Solanum tuberosum L.) leads to the enhanced accumulation of vitamin B6 in tuber tissues and tolerance to abiotic stresses. Plant Sci 272: 267-275.
- Bahadur I, Maurya BR, Kumar A, Meena VS, Raghuwanshi R (2016a) Towards the soil sustainability and potassium-solubilizing microorganisms. In: Meena V.S, Maurya B.R, Verma J.P, Meena R.S (eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, India, pp. 225-266.
- Bahadur I, Maurya BR, Meena VS, Saha M, Kumar A, et al. (2016b) Mineral release dynamics of tricalcium phosphate and waste muscovite by mineral-solubilizing rhizobacteria isolated from indo-gangetic plain of India. Geomicrobiol J, pp. 454-466.
- Bahadur I, Meena VS, Kumar S (2014) Importance and application of potassic biofertilizer in Indian agriculture. Int Res J Biol Sci 3: 80-85.
- Bai Y, Zhou X, Smith DL (2003) Enhanced soybean plant growth resulting from co-inoculation of strains with Bradyrhizobium japonicum. Crop Sci 43: 1774-1781.
- Baig KS, Arshad M, Shaharoona B, Khalid A, Ahmed I (2012) Comparative effectiveness of Bacillus spp. Possessing either dual or single growth-promoting traits for improving phosphorus uptake, growth and yield of wheat (Triticum aestivum L.). Annals of Microbiology 62: 1109-1119.
- Bais H, Weir T, Perry L, Gilroy S, Vivanco J (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57: 233-266
- Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2003) How plants communicate using the underground information superhighway. Trends Plant Sci 9: 26-32.
- Bais HP, Weir TL, Perry LG, Gilory S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57: 233-266.
- Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus Subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and Surfactin production. Plant Physiol 134: 307-319.
- Bakker PAH, Corné M, Pieterse J, Van Loon LC (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 97(2): 239-243.
- Banfield JF and Nealson KH (editors) (1997) Geomicrobiology: Interactions between Microbes and Minerals, Reviews in Mineralogy and Geochemistry, Washington, DC: Mineralogical Society of America 35.
- Banfield JF, Cervini-Silva J Nealson K H (editors) (2005) Molecular Geomicrobiology, Reviews in Mineralogy and Geochemistry, Washington, DC: Mineralogical Society of America 59.
- Bano A, Fatima M (2009) Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biology and Fertility of Soils 45(4): 405-413.
- Bansal M, Mukerji KG (1996) Root exudates in rhizosphere biology. In: Mukerji K.G, Singh V.P, Dwivedi S (eds) Concepts in applied microbiology and biotechnology. Aditya books, New Delhi 98-120.
- Bardgett RD, Van Der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515: 505-511.
- Barkal LJ, Theberge AB, Guo CJ, Spraker J, Rappert L, et al. (2016) Microbial metabolomics in open microscale platforms. Nature Communications 7:10610
- Bar On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proc Natl Acad Sci USA.
- Barton LL, Abadia J (2006) Iron nutrition in plants and Rhizospheric microorganisms. Springer, Dordrecht.
- Bassler BL (1999) How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2(6): 582-587.
- Bastian F, Cohen A, Piccoli P, Luna V, Baraldi R, et al. (1998) Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically defined culture media Plant Growth Regulation Kluwer Academic Publishers. Printed in the Netherlands 24: 7-11.
- Behera BC, Singdevsachan SK, Mishra RR, Dutta SK, Thatoi HN (2014) Diversity, mechanism, and biotechnology of phosphate solubilizing microorganism in mangrove-a review. Biocatal Agric Biotechnol 3(2): 97-110.
- Bell CW, Asao S, Calderon F (2015) Plant nitrogen uptake drives rhizosphere bacterial community assemblyduring plant growth. Soil Biol Biochem 85: 170-182.
- Bell TH, Hurteau BC, Al Otaibi F, Turmel MC, Yergeau E, et al. (2015) Early rhizosphere microbiome composition is related to the growth and Zn uptake of willows introduced to a former landfill. J Environ Biol 17(8): 3025-3038.
- Bellenger JP, Wichard T, Kustka AB, Kraepiel AML (2008) Uptake of molybdenum and vanadium by a nitrogen-fixingsoil bacterium using siderophores. Nature Geosci 1: 243-246.
- Bellotti A, Campo, BVH, Hyman G (2012) Cassava production and pest management: present and potential threats in a changing environment. Tropical Plant Biology 5(1): 39-72.
- Bellotti AC, Herrera CJ, Hernadez MP, Arias B, Guerrero JM, et al. (2012b) Chapter 10: Cassava Pests in Latin America, Africa, and Asia. In: Howeler, RH (Ed.) The cassava handbook: A reference manual based on the Asian regional cassava training course, held in Thailand. CIAT & The Nippon Foundation. Accessed 5: 19-13.
- Bender SF, Wagg C, van der Heijden MGA (2016) An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol Evol 31: 441-452.
- Benson DR, Silvester WB (1993) Biology of Frankia strains, actinomycete symbionts of 461 actinorhizal plants. Microbiol Rev 57: 293-319.
- Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17: 478-486.
- Berg M P, Toby Kiers E, Driessen G,Marcel VDH, Bob w et al (2010). Adapt or disperse: understanding species persistence in a changing world. Glob Change Biol 16: 587-598.
- Bever JD, Westover KM, Antonovics J (1997) Incorporating the soil community into plant population dynamics: The utility of the feedback approach. Journal of Ecology 85(5): 561-573.
- Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, et al. (2010) Rooting theories of plant community ecology in microbial interactions. Trends in Ecology & Evolution 25 (8): 468-478.
- Bever JD (2003) Soil community feedback and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytologist 157(3): 465-473.
- Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR ): emergence in agriculture. World J. Microbiol Biotechnol 28(4): 1327-1350.
- Biermann, B, Linderman RG (1983) Mycorrhizal roots, intraradical vesicles and extraradical vesicles as Inoculum. New Phytol 95: 97-120.
- Biocyclopedia (2018) Commercial Producers of Biofertilizers.
- Biotech International Limited (2018).
- Black T, Chen W, Barr A, Arain M, Chen Z, et al. (2000) Increased carbon sequestration by a boreal deciduous forest in years with a warm spring. Geophysical Research Letters 27(9): 1271-1274.
- Bojko O, Kabala C (2017) Organic carbon pools in mountain soils - sources of variability and predicted changes in relation to climate and land use changes. CATENA 149: 209-220.
- Bonfante P (2003) Plants, mycorrhizal fungi and endobacteria: a dialog among cells and genomes. Biol Bull 204: 215-220.
- Bonfante, P, Anca IA (2009) Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol 63: 363-383.
- Bottjer DJ (2005) Geobiology and the fossil record: eukaryotic, microbes, and their interactions. Palaeogeogr Palaeoclimatol Palaeoecol 219: 5-21.
- Boulter JI, Trevors JT, Boland GJ (2002) Microbial studies of compost: bacterial identification, and their potential for turfgrass pathogen suppression. World J Microbiol Biotechnol 18: 661.
- Boyer JS (1982) Plant productivity and environment. Science. 218(4571): 443-448.
- Braeken K, Daniels R, Ndayizeye M, Vanderleyden J, Michiels J (2008) Quorum sensing in bacteria- plant interactions. In: Nautiyal CS, Dion P (eds) Molecular mechanisms of plant and microbe coexistence, Soil biology, Vol 15. Springer, Berlin, pp.265-290.
- Braun V (2001) Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol 291: 67-79.
- Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. Biochemistry and Molecular Biology of Plants 1158: e1203.
- Brüssow H, Canchaya C, Hardt W (2004) Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev 68(3): 560-602
- Bulgarelli, D, Garrido-Oter R, Munch PC, Weiman A, Droge J, et al. (2015) Structure and function of the bacterial root microbiota in wild and domesticated barley.Cell Host Microbe 17(3): 392-403.
- Bulgarelli, D, Schlaeppi K, Spaepen S, Emiel VLT,Paul SL et al. (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64: 807-838.
- Bulla LA, Bechtel DB, Kramer KJ, Shethna YI, Aronson AI, et al. (1980) Ultrastructure, physiology, and biochemistry of Bacillus thuringiensis. Crit Rev Microbiol 8: 147-204.
- Burdon JJ, and Thrall PH (2009) Coevolution of plants and their pathogens in natural habitats. Science 324: 755-756.
- Busby PE, Soman C, Wagner MR, Friesen ML, Kremer J, et al. (2017) Research priorities for harnessing plant microbiomes in sustainable agriculture. PLOS Biology 15: 1-14.
- Byju G, Nedunchezhiyan M, Ravindran CS, SanthoshMithra VS, Ravi V, (2012) Modeling the response of cassava to fertilizers: a site specific nutrient managementapproach for higher tuberous root yield. Commun. Soil Sci. Plant Anal 43: 1-14.
- CABI, Crop Protection Compendium (2008) Manihot esculenta datasheet.
- Callaway E (2016) Devastating wheat fungus appears in Asia for first time. Nature 532: 421-422.
- Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383: 3-41.
- Campbell R, Greaves M (1990) Anatomy and community structure of the rhizosphere. The Rhizosphere 11-34 Carbonetto, B, Rascovan N, Álvarez R, Mentaberry A, Vázquez MP (2014) Structure, compositionand metagenomic profile of soil microbiomes associated to agricultural land use and tillage systems in argentine pampas.
- Castro RO, Cantero EV, Bucio JL (2008) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3(4): 263-265.
- Cerilles AWE (2015) Gahung-Gahung organic cassava farming system: a climate change adaptive and poverty-alleviating farming strategy. J. Agric. Technol 11(8): 1669-1675.
- Ceballos H, Kulakow P, Hershey C (2012) Cassava breeding: current status, bottlenecks and the potential of biotechnology tools. Tropical Plant Biology 5(1): 73-87.
- Chaabouni I, Guesmi A, Cherif A (2012) Secondary metabolites of Bacillus: potentials in biotechnology. In: Sansinenea E (ed) Bacillus thuringiensis biotechnology chapter 18. Springer, Dordrecht 347-366.
- Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. ISME J 8: 790-803.
- Chater KF (1993) Genetics of differentiation in Streptomyces. Annu Rev Microbiol 47: 685-713.
- Chauhan RD, Beyene G, Kalyaeva M, Fauquet CM, Taylor N (2015) Improvements in Agrobacterium-mediated transformation of cassava (Manihot esculenta Crantz) for large-scale production of transgenic plants. Plant Cell, Tissue & Organ Culture 121: 591-603.
- Chetty CC, Rossin CB, Gruissem W, Vanderschuren H, Rey MEC (2013) Empowering biotechnology in southern Africa: establishment of a robust transformation platform for the production of transgenic industry-preferred cassava. N Biotechnol 30(2): 136-143.
- Chen, D, Cheng J, Chu P, Shuijin Hu, Yichun Xie, et al. (2015a) Regional-scale patterns of soil microbes and nematodes across grasslands on the Mongolian plateau: relationships with climate, soil, and plants. Ecography 38(6): 622-631.
- Chen, Alexandra Koumoutsi, Romy Scholz, Andreas Eisenreich, Kathrin Schneider, et al. (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42 Nat Biotechnol 25(9): 1007-1014.
- Chen S, Zou J, Hu Z, Haishan Chend, Yanyu Lu (2014) Global annual soil respiration in relation to climate, soil properties and vegetation characteristics: summary of available data. Agric for Meteorol 198: 335-346.
- Chen X-P, Cui Z L, Vitousek PM, Kenneth G Cassman, Pamela A Matson, et al. (2011) Integrated soil-crop system management for food security. Proc Natl Acad Sci USA 108(16): 6399-6404.
- Chen Z, Yu G, Ge J, Qiufeng Wang, Xianjin Zhu, et al. (2015b) Roles of climate, vegetation and soil in regulating the spatial variations in ecosystem carbon dioxide fluxes in the Northern Hemisphere. PLoS One 10(4): e0125265.
- Chen AH, Chen LJ, Wu ZJ (2012) Relationships among persistence of Bacillus thuringiensis and cowpea trypsin inhibitor proteins, microbial properties and enzymatic activities in the rhizosphere soil after repeated cultivation with transgenic cotton. Applied Soil Ecology 53: 23-30.
- Chen JH (2006) The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. In: International workshop on sustained management of the soil- rhizosphere system for efficient crop production and fertilizer use. Land Development Department, 16 p. 20, Bangkok, Thailand.
- Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, et al. (2006) Phosphate solubilizing Bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34(1): 33-41.
- Chen Yan, Michael Bonkowski, Yi Shen, Bryan S, Griffiths, et al. (2020) Root ethylene mediates rhizosphere microbial community reconstruction when chemically detecting cyanide produced by neighbouring plants. Microbiome 8: 4.
- Chaudhary A, Gustafson D, Mathys A (2018) Multi-indicator sustainability assessment of global food systems. Nat Commun 9(1): 848.
- Choudhary DK, Johri BN (2009) Interactions of Bacillusspp and plants with special reference to induced systemic resistance (ISR). Microbiol Res 164(5): 493-513.
- Chowdhary K, Kumar A, Sharma S, Pathak R, Jangir M (2018) Ocimum sp.: source of biorational pesticides. Ind Crops Prod 122: 686-701.
- Chung JH, Song GC, Ryu CM (2016) Sweet scents from good bacteria: case studies on bacterial volatile compounds for plant growth and immunity. Plant Mol Biol 90(6): 677-687.
- Classen AT, Sundqvist MK, Henning JA, Gregory S Newman, Jessica AM Moore, et al. (2015) Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere 6(8): 1-21.
- Compant S, Clement C, Sessitsch A (2010) Plant growth-probacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42: 669-678.
- Compant S (2007) Interaction between grapevine, Vitis vinifera L and the endophytic bacterium Burkholderia phytofirmans strain PsJN: colonization, induced defense responses and systemic resistance towards Botrytis cinerea. URCA, Remis, pp. 220.
- Compant S, Van Der Heijden MGA, Sessitsch A (2010) Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol Ecol 73(2): 197-214.
- Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action and future prospects. Appl Environ Microbiol 71(9): 4951-4959.
- Cook LC, Dunny GM (2014) The influence of biofilms in the biology of plasmids. Microbiol Spectr 2(5): 0012.
- Crickmore, N, Baum J, Bravo A, Lereclus D, Narva K, et al. (2016) Bacillus thuringiensis toxin nomenclature.
- Crickmore, N, Zeigler DR, Feitelson J, Schnepf E, Van Rie J, et al. (1998) Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev 62(3): 807-813.
- Cristian Vidal Quist J, Hilary JR, Mahenthiralingam E, Berry C (2013) Bacillus thuringiensis colonizes plant roots in a phylogeny dependent manner. FEMS Microbiol Ecol 86(3): 474-489.
- Cronin D, Y Moenne Loccoz, A Fenton, C Dunne, D Dowling, et al. (1997) Role of 2, 4-diacetyl phloroglucinol in the interaction of the biocontrol of Pseudomonas strain F113 with the potato cyst nematode Globodera rostochiensis. Appl Environ Microbiol 63(4): 1357-1361.
- Curlango Rivera G, Pew T, Vanetten HD, Zhongguo X, Yu N, et al. (2013) Measuring root disease suppression in response to a compost water extract. Phytopathology CSIR-Agra, Projects SHP (2012) Soil Health Projects (SHP-014). 103(3): 255-260.
- Cui S, Meng J, Bhagwat A (2001) Availability of glutamate and arginine during acid challenge determines cell density-dependent survival phenotype of Escherichia coli strains. Appl Environ Microbiol 67(10): 4914-4918.
- Curtis TP, Sloan WT (2005) Exploring microbial diversity -a vast below. Science 309: 1331-1333.
- D Alessandro M, Erb M, Ton J, Brandenburg A, Karlen D, et al. (2014) Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ 37(4): 813-826.
- D Alvise PW, Sjoholm OR, Yankelevich T, Jin Y, Wuertz S, et al. (2010) TOL plasmid carriage enhances biofilm formation and increases extracellular DNA content in Pseudomonas putida KT2440. FEMS Microbiol Lett 312(1): 84-92.
- Dahlberg C, Chao L (2003) Amelioration of the cost of conjugative plasmid carriage in Escherichia coli K12. Genetics 165(4): 1641-1649.
- Meena RS, Verma JP, Meena VS (2016) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, India. pp. 281-291.
- Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol R 74(3): 417-433.
- Davis RD (1996) The impact of EU and UK environmental pressures on the future of sludge treatment and disposal. Water Environ J 10: 65-69.
- Dawson JO (1986) Actinorhizal plants: their use in forestry and agriculture. Outlook Agr 15(4): 202-208.
- De Bruijn I, de Kock MJD, Yang M, de Waard P, Van Beek TA, et al. (2007) Genome-based discovery, structure prediction and functional analysis of cyclic lipopeptide antibiotics in Pseudomonas species. Mol Microbiol 63(2): 417-428.
- De Roy K, Marzorati M, Van den Abbeele P, Van de Wiele T, Boon N (2014) Synthetic microbial ecosystems: an exciting tool to understand and apply microbial communities. Environ Microbiology 16(6):1472-1481.
- De Souza EM, Granada CE, Sperotto RA (2016) Plant pathogens affecting the establishment of plant-symbiont interaction. Frontiers in Plant Science 7: 15.
- De Vries FT, Wallenstein MD (2017) Below-ground connections underlying above-ground food production: a framework for optimizing ecological connections in the rhizosphere. J Ecol 105(4): 913-920.
- De Schutter O (2011) Agroecology and the right to food.
- De Angelis KM, Lindow SE, Firestone MK (2008) Bacterial quorum sensing and nitrogen cycling in rhizosphere soil. FEMS Microbiol Ecol 66(2): 197-207.
- Del Pozo JC, Lopez Matas MA, Ramirez Parra E, Gutierrez C (2005) Hormonal control of the plant cell cycle. Physiol Plant 123(2): 173-183.
- Denison RF, Kiers ET (2018) Life histories of symbiotic rhizobia and mycorrhizal fungi. Current Biology 21(18): 775-785.
- Dessaux, Y, Grandclement C, Faure D (2016) Engineering the rhizosphere. Trends Plant Sci 21(3): 266-278.
- Deusch S, Tilocca B, Camarinha Silva A, Seifert J (2015) News in livestock research-use of omics-technologies to study the microbiota in the gastrointestinal tract of farm animals. Comput Struct Biotechnol J 13: 55-63.
- Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, et al. (2007) The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14(1): 87-96.
- Dommergues YR (1995) Nitrogen fixation by trees in relation to soil nitrogen economy. Fertil Res 42: 215-230.
- Dopheide A, Lear G, He Z, Zhou J, Lewis GD (2015) Functional gene composition, diversity and redundancy in microbial stream biofilm communities. PLoS ONE 10(4): 1-21.
- Dove PM, De Yoreo JJ, Weiner S (2003) Biomineralization. Reviews in Mineralogy and Geochemistry 54. Washington DC: Mineralogical Society of America.
- Dragoni D, Schmid HP, Wayson CA, Potter H, Grimmond CSB, et al. (2011) Evidence of increased net ecosystem productivity associated with a longer vegetated season in a deciduous forest in south-central Indiana, USA. Global Change Biology 17(2): 886-897.
- Duan X, Xu J, Ling E, Zhang P (2013) Expression of Cry1Aa in cassava improves its insect resistance against Helicoverpa armigera. Plant Molecular Biology 83(1): 131-141.
- Dubern JF, Diggle SP (2008) Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Bio Syst 4(9): 882-888.
- Dubey, A, Kumar A, Abd_Allah EF, Hashem A, Khan ML (2018) Growing more with less: breeding and developing drought resilient soybean to improve food security. Ecol Indic 105: 425-437.
- Dubey Anamika, Muneer Ahmad Malla, Farhat Khan, Kanika Chowdhary, Shweta Yadav, et al. (2019) Soil microbiome: a key player for conservation of soil health under changing climate. Biodiversity and Conservation 28: 2405-2429.
- Dunny GM, Leonard BA (1997) Cell-cell communication in gram-positive bacteria. Annu Rev Microbiol 51 :527-564.
- Dutta C, Sarkar M (2015) Horizontal gene transfers and bacterial diversity. In: Encyclopedia of Springer, New York, pp. 251-257.
- Edwards J, Johnson C, Santos-Medellin C, Lurie E, Podishetty NK, et al. (2015) Structure, variation and assembly of the root-associated microbiomes of rice. PNAS 112: E91-E20.
- Egamberdieva, D, Jabborova D, Hashem A (2015) Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi Journal of Biological Sciences 22(6): 773-779.
- Ekpo MA, Nwankpa II (2006) The effect of crude oil on microorganisms and growth of ginger (Zingiber officinale) in the tropics. Journal of Sustainable Tropical Agricultural Research 16: 67-71.
- Ekpo MA (2002) Microbial degradation of petroleum drilling and activities and plant root development. World Journal of Biotechnology 3: 377-386.
- Ehrlich HL, Newman DK (2009) Geomicrobiology 5th Edn, Boca Raton, FL: CRC Press/Taylor & Francis.
- Ehrlich HL (1996) How microbes influence mineral growth and dissolution. Chem Geol 132(1-4): 5-9.
- Ehiagbonare JE Enabulele SA, Babatunde BB, Adjarhore R (2009) Effect of cassava effluent on Okada denizens. Sci Res Essay 4(4): 310-313.
- Elmi AA, CP West, RT Robbins, TL Kirkpatrick (2000) Endophyte effects on reproduction of a root knot nematode (Meloidogyne marylandi) and osmotic adjustment in tall fescue. Grass for Sci 55(2): 166-172.
- El-Sharkawy MA, Cock JH (1987) Response of cassava to water stress. Plant and Soil 100 (1-3): 345-360.
- El-Sharkawy MA, Cock JH, Lynam JK, del Pilar Hernandez A, Cadavid LF (1990) Relationships between biomass, root-yield and single-leaf photosynthesis in field- grown cassava. Field Crop Res 25(3-4): 183-201.
- El-Sharkawy MA (1993) Drought-tolerant cassava for Africa, Asia, and Latin America. Bioscience 43(7): 441-451
- El-Sharkawy MA (2006) International research on cassava photosynthesis, productivity, eco-physiology, and responses to environmental stresses in the tropics. Photosynthetica 44(4): 481-512.
- El-Sharkawy MA (2010) Cassava: physiological mechanisms and plant traits underlying tolerance to prolonged drought and their application for breeding cultivars in the seasonally dry and semiarid tropics. In: da Matta, FM (Ed), Ecophysiology of Tropical Tree Crops. Nova Science Publishers, Hauppauge, New York, USA, pp. 71-110.
- Erisman JW, Galloway JN, Seitzinger S, Albert Bleeker, Nancy B Dise, et al. (2013) Consequences of human modification of the global nitrogen cycle. Philos Trans R Soc B Biol Sci 368(1621): 20130116.
- Failla ML, Chitchumroonchokchai C, Siritunga D, De Moura FF, Fregene M, et al. (2012) Retention during processing and bioaccessibility of b-carotene in high b-carotene transgenic cassava root. Journal of Agricultural Food Chemistry 60(15): 3861-3866.
- Fang J, Yu G, Liu L, Hu S, Chapin FS (2018) Climate change, human impacts, and carbon sequestration in China. Proc Natl Acad Sci USA 115(16): 4015-4020.
- Fokunang CN, Dixon AG, Ikotun T, Tembe EA, Akem CN, et al. (2001) Anthracnose: An Economic Disease of Cassava in Africa. Pakistan Journal of Biological Sciences 4(7): 920-925.
- Asian Network for Scientific Information. FAO (2010) Final document. In: International Scientific Symposium Biodiversity and Sustainable Diets United Against Hunger, 3-5 November 2010. FAO headquarters, Rome.
- Farm Guide (2018) Farm Information Bureau. Government of Kerala, India.
- FAO (2012) Save and Grow: A policy maker’s guide to the sustainable intensification of smallholder crop production. Rome. pp. 98-150.
- FAO (2013) Save and Grow: Cassava A guide to sustainable production intensification. Food and Agriculture Organization of the United Nations. Rome. pp. 25-40.
- FAO (2018) Food Outlook: Biennial Report on Global Food Markets 2018 Rome.
- Farag, MA, Zhang H, Ryu CM (2013) Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J Chem Ecol 39(7): 1007-1018.
- Faure D, Vereecke D, Leveau JH (2009) Molecular communication in the rhizosphere. Plant and Soil 321(1-2): 279-303.
- Federle MJ, Bassler BL (2003) Interspecies communication in bacteria. J Clin Invest 112(9): 1291-1299.
- Fermont AM (2009) Cassava and Soil Fertility in Intensifying Small Holder Farming Systems of East Africa. Ph.D. Thesis. Wageningen Agricultural University, Wageningen, The Netherlands.
- Ferluga S, Staindler L, Ventiru V (2008) N-acyl homoserine lactone quorum sensing in gram negative rhizobacteria. In: Karlovsky P (ed) Secondary metabolites in soil ecology. Soil biology 14: 69-92.
- Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, et al. (2012a) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6(5): 1007-1017.
- Finkel T (2011) Signal transduction by reactive oxygen species. Journal of Cell Biology 194(1): 7-15.
- Fitriatin BN, Yuniarti A, Turmuktini T, Ruswandi FK (2014) The effect of phosphate solubilizing microbe producing growth regulators on soil phosphate, growth and yield of maize and fertilizer efficiency on Ultisol. Eurasian J Soil Sci 3: 101-107.
- Fleuchot B, Gitton C, Guillot A, Vidic J, Nicolas P, et al. (2011) Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in Streptococci. Mol Microbiol 80(4): 1102-1119.
- Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, et al. (2011) Solutions for a cultivated planet. Nature 478(7369): 337-342.
- Fomina M, Burford EP, Gadd GM (2005c) Toxic metals and fungal communities. In The Fungal Community. Its Organization and Role in the Ecosystem, pp. 733-758. Edited by J Dighton, JF White & P Oudemans. Boca Raton, FL: CRC Press.
- Fontaine L, Boutry C, de Frahan MH, Delplace B, Fremaux C, et al. (2010) Novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophiles and Streptococcus salivarius. J Bacteriol 192(5): 1444-1454.
- Foster J (1995) Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit Rev Microbiol 21(4): 215-237.
- Foster J, Hall H (1991) Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol 173(16): 5129-5135.
- Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, et al. (2011) Microbially mediated plant functional traits. Annual Reviews of Ecology. Evolution and Systematics 42: 23-46.
- Frost LS, Leplae R, Summers AO, Toussaint A (2005) Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 3(9): 722-732.
- Fuentes-Ramirez LE, Caballero-Mellado J (2005) Bacterial biofertilizers. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, The Netherland, pp. 143-172.
- Gaskin A, Kio-Jack FS, Isirimah NO (2000) Remediation of crude polluted soils using municipal waste compost for soy-beans production in the Niger Delta. Proceedings of the 26th Annual Conference of Soil Science Society of Nigeria held at Nigeria Ibadan.
- Fuqua C, Greenberg EP (2002) Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol 3(9): 685-695.
- Gaitán-Solís E, Taylor NJ, Siritunga D, Stevens W, Schachtman DP (2015) Overexpression of the transporters AtZIP1 and AtMTP1 in cassava changes zinc accumulation and partitioning. Frontiers in Plant Science 6: 492.
- Gadd GM, Griffiths AJ (1978) Microorganisms and heavy metal toxicity. Microb Ecol 4(4): 303-317.
- Gadd GM (1986) The uptake of heavy metals by fungi and yeasts: the chemistry and physiology of the process and applications for biotechnology. In Immobilisation of Ions by Bio-sorption, pp. 135-147. Edited by H. Eccles & S. Hunt. Chichester: Ellis Horwood.
- Gadd GM (1992a) Metals and microorganisms: a problem of definition. FEMS Microbiol Lett 100(1-3): 197-204.
- Gadd GM (1992b) Microbial control of heavy metal pollution. In Microbial Control of Pollution, pp. 59-88. Edited by JC Fry, GM Gadd, RA Herbert, CW Jones, I Watson-Craik. Cambridge: Cambridge University Press.
- Gadd GM (1993a) Interactions of fungi with toxic metals. New Phytol 124(69): 25-60.
- Gadd GM (1993b) Microbial formation and transformation of organometallic and organo-metalloid compounds. FEMS Microbiol Rev 11(4): 297-316.
- Gadd GM (2000b) Microbial interactions with tributyltin compounds: detoxification, accumulation, and environmental fate. Sci Total Environ 258(1-2): 119-127.
- Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122(2-4): 109-119.
- Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res 111(Pt 1): 3-49.
- Gadd GM (2008a) Bacterial and fungal geomicrobiology: a problem with communities? Geobiology 6(3): 278-284.
- Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56(4): 403-407.
- Garbeva, P, Hordijk C, Gerards S, de Boer W (2014a) Volatile-mediated interactions between phylogenetically different soil bacteria. Frontiers in Microbiology 5: 285-290.
- Garbeva, P, Hordijk C, Gerards S, De Boer W (2014b) Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiology Ecology 87(3): 639-649.
- Garbeva P, Hordijk C, Gerards S, de Boer W (2014) Volatile-mediated interactions between phylogenetically different soil bacteria. Frontiers in Microbiology 5: 285-290.
- Ghigo JM (2001) Natural conjugative plasmids induce bacterial biofilm development. Nature 412(6845): 442-445.
- Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18(1): 1-22.
- Gkizi D, Lehmann S, L’Haridon F, Serrano M, Paplomatas EJ, et al. (2016) The innate immune signaling system as a regulator of disease resistance and induced systemic resistance activity against Verticillium dahliae. Mol Plant-Microbe Interact. 29(4): 313-323.
- Gleeson D, McDermott F, Clipson N (2007) Understanding microbial active biogeochemical environments. Adv Appl Microbiol 62: 81-104.
- Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41: 109-117.
- Glick BR (1995) The enhancement of plant growth by free-living bacteria. Canadian Journal of Microbiology 41(2): 109-117
- Glick BR, Patten CL, Holguin G, Penrose D (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London, UK.
- Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase containing soil bacteria. Eur J Plant Pathol 119: 329-339.
- Glickmann E, Gardan L, Jacquet S, Hussain S, Elasri M, et al. (1998) Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol Plant Microbe Interact 11(2): 156-162.
- Goh C-H, Veliz Vallejos DF, Nicotra AB, Mathesius U (2013) The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J Chem Ecol 39(7): 826-839.
- Gomes AMA, Mariano RLR, Silveira EB, Mesquita JCP (2003) Isolation, selection of bacteria, and effect of Bacillus spp. in the production of organic lettuce seedlings. Hort Brasileira 21(4): 701-705.
- Gao Y, Q Liu, P Zang, X Li, Q Ji, et al. (2015) An endophytic bacterium isolated from Panax ginseng C.A Meyer enhances growth, reduces morbidity and stimulates ginsenoside biosynthesis. Phytochem Lett 11: 132-138.
- Gonzalez-Chavez MC, Carrillo-Gonzalez R, Wright SF, Nichols KA (2004) The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130(3): 317-323.
- Goswami, D, Thakker JN, Dhandhukia PC (2016) Portraying mechanics of Plant Growth Promoting Rhizobacteria (PGPR). Cogent Food Agric 2(1): 1-19.
- Goudaa Sushanto, Rout George Kerryb, Gitishree Dasc, Spiros Paramithiotisd, Han-Seung Shine, et al. (2018) Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol Res 206: 131-140.
- Graham PH, Vance CP (2000) Nitrogen fixation in perspective: an overview of research and extension needs. Field Crops Res 65(2-3): 93-106.
- Groenhagen U, Baumgartner R, Bailly A, Gardiner A, Eberl L, et al. (2013) Production of bioactive volatiles by different Burkholderia ambifaria strains. J Chem Ecol 39(7) :892-906.
- Großkopf T, Soyer OS (2014) Synthetic microbial community. Curr Opin Microbiol 18: 72-77
- Grover M, Ali SZ, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World Journal of Microbiology and Biotechnology 27(5): 1231-1240.
- Guerra-Santos LH, Käppeli O, Fiechter A (1986) Dependence of Pseudomonas aeruginosa continuous culture biosurfactant production on nutritional and environmental factors. Appl Microbiol Biotechnol 24: 443-448.
- Gupta C, Prakash DG (2014) Role of microbes in combating global warming. Int J Pharm Sci Lett 4: 359-363.
- Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant Growth Promoting Rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7(2): 96-102.
- Gururaj B, Hamsa KR, Ramesh Mahadevaiah GS (2017) Doubling of small and marginal farmers income through rural non-farm and farm sector in Karnataka. Econ Aff 62(4): 581-587.
- Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3(4): 307-319.
- Hacquard S (2016) Disentangling the factors shaping microbiota composition across the plant holobiont. New Phytol 209(2): 454-457.
- Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, et al. (2008) Plant host habitat and root exudates shape soil bacterial community structure. The ISME Journal 2(12): 1221-1230.
- Hamady M, Knight R (2009) Microbial community profiling for human microbiome projects: tools, techniques and challenges. Genome Res 19(7): 1141-1152.
- Haney CH, Samuel BS, Bush J, Ausubel FM (2015) Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat Plant 1(6).
- Hartmann A, Rothballer M, Hense BA, Schroder P (2014) Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front Plant Sci 5: 131.
- Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant and Soil 312(1-2): 7-14.
- Hashem, A, Abd_Allah EF, Alqarawi AA, Radhakrishnan R, Kumar A (2017) Plant defense approach of bacillus subtilis (Bera 71) against Macrophomina phaseolina (tassi) Goid in mung bean. J Plant Interact 12(1): 390-401.
- Hashem A, Kumar A, Al-Dbass AM, Alqarawi AA, Al-Arjani ABF, et al. (2018) Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J Biol Sci 26(3): 614-624.
- Hassani MA, Durán P, Hacquard S (2018) Microbial Interactions within the plant holobiont. Microbiome 6(1): 58.
- Henry HAL (2013) Reprint of Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 56: 53 -59.
- Herrera, Paredes S, Lebeis SL (2016) Giving back to the community microbial mechanisms of plant-soil interactions. Functional Ecology 30(7): 1043-1052.
- Hettiarachchi RP, Dharmakeerthi RS, Jayakody AN, Seneviratne G, de Silva, et al. (2014) Effectiveness of fungal bacterial interactions as biofilmed biofertilizers on enhancement of root growth of Hevea seedlings. J Environ Prof Sri Lanka 3(2): 25-40
- Heuer H, Smalla K (2012) Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol Rev 36(6): 1083-1104.
- Hicks N, Vik U, Taylor P, Ladoukakis E, Park J, et al. (2017) Using prokaryotes for carbon capture storage. Trends Biotechnol 35(1): 22-32.
- Hill KE, Top EM (1998) Gene transfer in soil systems using microcosms. FEMS Microbiol Ecol 25(4): 319-329.
- Hirose E, A Murakami (2011) Microscopic anatomy and pigment characterization of coral-encrusting black sponge with cyanobacterial symbiont, Terpios hoshinota. Zool Sci 28(3): 199-205.
- Hiruma K, Gerlach N, Sacristán S, Nakano RT, Hacquard S, et al (2016) Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 165(2): 464-474.
- Holden MT, Ram Chhabra S, de Nys R, Stead P, Bainton NJ, et al. (1999) Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol 33(6): 1254-1266.
- Holden JF, Adams MWW (2003) Microbe-metal interactions in marine hydrothermal vents. Curr Opin Chem Biol 7(2): 160-165.
- Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agricultural and Biological Chemistry 42(10): 1825-1831.
- Hoppert M, Flies C, Pohl W, Gunzl B, Schneider J (2004) Colonization strategies of lithobiontic microorganisms on carbonate rocks. Environ Geol 46: 421-428.
- Horton MW, Bodenhausen N, Beilsmith K, Meng D, Muegge BD, et al. (2014) Genome-wide association study of Arabidopsis thaliana leaf microbial community. Natur Commun 5: 5320.
- Howeler RH (1978) The mineral nutrition and fertilization of cassava. In: Cassava Production Course. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia, pp. 247-292.
- Howeler RH (2014) Sustainable Soil and Crop Management of Cassava in Asia. CIAT, Cali, Colombia.
- Ho YN, Chiang HM, Chao CP, Su CC, Hsu HF, et al. (2003) In planta biocontrol of soilborne Fusarium wilt of banana through a plant endophytic bacterium, Burkholderia cenocepacia 869T2. Plant and Soil 387(1-2): 295-306.
- Huss-Danell K (1997) Actinorhizal symbioses and their N2 New Phytol 136(3): 375-405.
- Hur M, Y Kim, HR Song, JM Kim, YI Choi, et al. (2011) Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Applied Environ Microbiol 77(21): 7611-7619.
- Ibrahim AM, Griko N, Junker M, Bulla LA (2010) Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng Bugs 1(1): 31-50.
- IFPRI (2014) Biofortification Progress Briefs. Harvest Plus, pp. 1-82.
- IITA (2004) IITA Brief: Biological control. Ibadan. Nigeria, pp 68-83.
- IITA (2008) Starting a Cassava Farm - IPM Field Guide for Extensions Agents. Technical Leaflet No 6 In: New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research. Pp, 329-339.
- Ikotun T (1977) Survival of Xanthomonas manihotis in cassava tissues. Nigerian Journal of Plant Protection 3: 31-36.
- Ikotun T (1978) Effect of Xanthomonas manihotis on cassava tissues. In: Cassava Bacterial Blight in Africa. Past, Present and Future. Report of an interdisciplinary workshop 26-30 June 1978. IITA, Ibadan, Nigeria 1979. Editors: Terry ER, Persley GJ, Cook SA. pp. 29-32
- İnceoğlu Ö, van Overbeek LS, Salles JF, van Elsas JD (2013) Normal operating range of bacterial communities in soil used for potato cropping. Applied and Environmental Microbiology 79(4): 1160-1170.
- India mart (2018) Green Earth Liquid Biofertlilizers Reap p Phosphate Solubilizing Bacteria, For Soil Application, Packaging Type: Bottle.
- India mart (2018) Green Earth Liquid Biofertlilizers Reap p Phosphate Solubilizing Bacteria, For Soil Application, Packaging Type: Bottle.
- Indiragandhi P, Anandham R, Madhaiyan M, Sa TM (2008) Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Curr Microbiol 56(4): 327-333.
- Ihemere UE, Narayanan NN, Sayre RT (2012) Iron biofortification and homeostasis in transgenic cassava roots expressing the algal iron assimilatory gene, FEA1. Front Plant Sci 3(3): 171.
- Igbinosa EO, Ozede NI (2015) The Impact of Cassava Effluent on the Microbial and Physicochemical Characteristics on Soil Dynamics and Structure. Jordan Journal of Biological Sciences 8(2): 107-112.
- Jaiswal DK, Verma JP, Prakash S, Meena VS, Meena RS (2016) Potassium as an important plant nutrient in sustainable agriculture: a state of the art. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 21-29.
- Jang CS, Tang H, Bowers JE, Lemke C, Paterson AH (2008) Evolutionary fate of rhizome specific genes in a nonrhizomatous Sorghum genotype. Heredity 102(3): 266-273.
- Jat LK, Singh YV, Meena SK, Parihar M, Jatav HS, (2015) Does integrated nutrient management enhance agricultural productivity? J Pure Appl Microbiol 9(2): 1211-1221.
- Jeong SJ, Ho CH, Gim HJ, Brown ME (2011) Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982-2008. Global Change Biology 17(7): 2385-2399.
- Jha Y, Subramanian RB (2015) Reduced apoptosis like cell death and improved cell membrane integrity in paddy under salinity by root associate bacteria. Theor Exp Plant Physiol 27: 227-235.
- Jia Y, Huang H, Zhong M, Wang FH, Zhang LM, et al. (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47(7): 3141-3148.
- Jia YJ, Ito H, Matsui H, Honma M (2006) 1-aminocyclopropane-1-carboxylate (ACC) deaminase induced by ACC synthesized and accumulated in Penicillium citrinum intracellular spaces. Biosci Biotechnol Biochem 64(2): 299-305.
- Jiang C, Sheng, X, Qian M, Wang Q (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72(2): 157-164.
- Jiao X, Lyu Y, Wu X, Li H, Cheng L, et al. (2016) Grain production versus resource and environmental costs: Towards increasing sustainability of nutrient use in China. J Exp Bot 67: 4935-4949.
- Jouzani GS, Valijanian E, Sharafi R (2017) Bacillus thuringiensis: a successful insecticide with new environmental features and tidings. Appl Microbiol Biotechnol 101(7): 2691-2711.
- Kai M, Haustein M, Molina F, Petri A, Scholz B, et al. (2009) Bacterial volatiles and their action potential. Applied Microbiology and Biotechnologz 81(6): 1001-1012.
- Karabörklü S, Azizoglu U, Azizoglu ZB (2018) Recombinant entomopathogenic agents: a review of biotechnological approaches to pest insect control. World J Microbiol Biotechnol 34(1): 1-12.
- Kaur B, Ariffin F, Bhat R, Karim AA (2012) Progress in starch modification in the last decade. Food Hydrocolloids 26(2): 398-404.
- Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, et al. (1992) Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2, 4-diacetylphloroglucinol. Molecular Plant-Microbe Interactions 5(1): 4-13.
- Keesstra SD, Geissen V, Mosse K, Piiranen S, Scudiero E, et al (2012) Soil as a filter for groundwater quality. Curr Opin Environ Sustain 4(5): 507-516.
- Kent AD, Triplett EW (2002) Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu Rev Microbiol 56: 211-236.
- Khan Z, S Doty (2011) Endophyte-assisted phytoremediation. Plant Biol 12: 97-105.
- Khabbaz SE, L Zhang, LA Cáceres, M Sumarah, A Wang, et al. (2015) Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot diseases. Ann Appl Biol 166(3): 456-471.
- Khan Z, Son S, Akhtar J, Gautam N, Kim Y (2012) Plant growth-promoting rhizobacterium (Paenibacillus polymyxa) induced systemic resistance in tomato (Lycopersicon esculentum) against root-knot nematode (Meloidogyne incognita). Indian J Agric Sci 82: 603-607.
- Kirby J, Keasling D (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60: 335-355.
- Kirk JL, Klironomos JN, Lee H, Trevors JT (2005) The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environ Pollut 133(3): 455-465.
- Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12: 181-184.
- Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, et al. (2005) Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot 56(415): 1305-1316.
- Knowles CJ (1988) Cyanide utilization and degradation by microorganisms. Ciba Found Symp 140: 3-15.
- Konhauser K (2007) Introduction to Geomicrobiology. Oxford: Blackwell, UK.
- Koehorst-Van Putten H, Sudarmonowati E, Herman M, Pereira-Bertram I, Wolters A, et al. (2012) Field testing and exploitation of genetically modified cassava with low-amylose or amylose-free starch in Indonesia. Transgenic Res 21(1): 39-50.
- Korah PA, Usha PB, Sudharmayi Devi CR (1989) Effect of sulphur on the yield and quality of cassava (Manihot utilisima) in the laterite soils of Kerala. Madras Agric J 76(1): 57-60.
- Korde VV, Dhas SS, Gurave NA (2016) Hairy root culture: a promising approach in biotransformation. Asian J Plant Sci 6: 6-11.
- Kristiansson E, Hugenholtz P, Dalevi D (2009) Shotgun functionalize R: An R-package for functional comparison of metagenomes. Bioinformatics 25: 2737-2738.
- Kroer N, Barkay T, Sørensen S, Weber D (1998) Effect of root exudates and bacterial metabolic activity on conjugal gene transfer in the rhizosphere of a marsh plant. FEMS Microbiol Ecol 25 (4): 375-384.
- Kumar A, Gupta A, Azooz MM, Sharma S, Ahmad P, Dames J (2013) Genetic approaches to improve salinity tolerance in plants. In: Ahmad P, Azooz MM, Prasad MNV (eds) Salt stress in plants. Springer, New York.
- Kumar U, C Aggarwal, S Paul, K Annapurna (2014) Endophytes as biocontrol agents of plant pathogens and insects. Kavaka 41: 92-95.
- Kumar A, Bahadur I, Maurya BR, Raghuwanshi R, Meena VS, et al. (2015) Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J Pure Appl Microbiol 9: 715-724.
- Kumar A, Maurya BR, Raghuwanshi R, Meena VS, Islam MT (2017a) Co-inoculation with Enterobacter and Rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under indo-gangetic plain of India. J Plant Growth Regul.
- Kumar A, Meena R, Meena VS, Bisht JK, Pattanayak A (2016a) Towards the stress management and environmental sustainability. J Clean Prod 137: 821-822.
- Kumar A, Meena, Maurya BR, Raghuwanshi R, Bisht JKA (2017b) Towards the biological nitrogen fixation and nitrogen management in legume under sustainable agriculture. Appl Soil Ecol.
- Kumar A, Patel JS, Bahadur I, Meena VS (2016b) The molecular mechanisms of KSMs forenhancement of crop production under organic farming. In: Meena V.S, Maurya B.R, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 61-75.
- Kumar A, Sharma S, Mishra S (2010) Influence of arbuscular mycorrhizal (AM) fungi and salinity on seedling growth, solute accumulation, and mycorrhizal dependency of Jatropha curcas L. J Plant Growth Regul 29: 297-306.
- Kumar A, Sharma S, Mishra S (2016) Evaluating effect of arbuscular mycorrhizal fungal consortia and Azotobacter chroococcum in improving biomass yield of Jatropha curcas. Plant Biosyst 150: 1056-1064.
- Kumar KV, Singh N, Behl HM, Srivastava S (2008) Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 72: 678-683.
- Kumar S, Chauhan PS, Agrawal L, Raj R, Srivastava A, Gupta S (2016) Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of cucumber mosaic virus. PLoS ONE 11: e0149980.
- Kurrey DK, Lahre MK, Pagire GS (2018) Effect of Azotobacter on growth and yield of onion (Allium cepa L). J Pharmacogn Phytochem 7: 1171-1175.
- Legg JP (1999) Emergence spread and strategies for controlling the pandemic of cassava mosaic virus disease in east and central Africa. Crop Protection 18(10): 627-637.
- Legg JP (2010) Epidemiology of a whitefly-transmitted cassava mosaic geminivirus pandemic in Africa. In: Bemisia: Bionomics and Management of a Global Pest, [ed. by Stansly PA, Naranjo SE]. Springer Science + Business Media BV, pp. 233-255.
- Legg JP (1996) Host-associated strains within Ugandan populations of the whitefly Bemisia tabaci (Genn.), (Hom., Aleyrodidae). Journal of Applied Entomology 120(9): 523-527.
- Legg JP, Owor B, Sseruwagi P, Ndunguru J (2006) Cassava mosaic virus disease in east and central Africa: epidemiology and management of a regional pandemic. Advances in Virus Research 67: 355-418.
- Legg JP, Raya D (1998) Survey of cassava virus diseases in Tanzania. International Journal of Pest Management 44: 17-23.
- Lal R (2016) Soil health and carbon management. Food Energy Secur 5: 212-222.
- Lalucque H, P Silar (2003) “NADPH oxidase: an enzyme for multicellularity?” Trends in Microbiology 11(1): 9-12.
- Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321: 83-115.
- Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root micro- biomes. Plant Molecular Biology. 90 (6): 575-587.
- Lee JY, Passalacqua KD, Hanna PC, Sherman DH (2011) Regulation of petrobactin and bacillibactin biosynthesis in Bacillus anthracis under iron and oxygen variation. PLoS ONE 6(6).
- Lee S, Flores Encarnacion M, Contreras Zentella M, GarciaFlores L, Escamilla JE, et al. (2004) Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome C biogenesis genes J Bacteriol 186: 5384-5391.
- Lee B, Farag MA, Park HB, Kloepper JW, Lee SH, Ryu CM (2012) Induced resistance by a long-chain bacterial volatile: elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS ONE 7: e48744.
- Lebin, Thomas and Ishwar Singh (2019) Microbial Biofertilizers: Types and Applications Springer Nature Switzerland AG 2019 B. Giri et al. (eds.), Biofertilizers for Sustainable Agriculture and Environment, Soil Biology 55.
- Li KT, Moulin M, Mangel N, Albersen M, Verhoeven Duif NM, et al. (2015) Increased bioavailable vitamin B6 in field-grown transgenic cassava for dietary sufficiency. Nature Biotechnology 33(10): 1029-1032.
- Li Y, Hanna M, Svensater G, Ellen R, Cvitkovitch D (2001) cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J Bacteriol 183: 6875-6884.
- Liao WB, Wang G, Li YY, Wang B, Zhang P, Peng M (2016) Reactive oxygen species regulate leaf pulvinus abscission zone cell separation in response to water-deficit stress in cassava. Scientific Reports 6: 21542.
- Liu J, Zheng Q, Ma Q, Gadidasu KK, Zhang P (2011) Cassava genetic transformation and its application in breeding. Journal of Integrative Plant Biology 53: 552-569.
- Loh J, Carlson RW, York WS, Stacey G (2002) Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. Proc Natl Acad Sci U S A 99: 14446-14451.
- Liu J, Yang J, Bi H, Zhang P (2014) Why mosaic? Gene expression profiling of African cassava mosaic virus-infected cassava reveals the effect of chlorophyll degradation on symptom development. Journal of Integrative Plant Biology 56(2): 122-132.
- Lombard N, Prestat E, van Elsas JD, Simonet P (2011) Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol Ecol 78: 31-49.
- Loper JE, Gross H (2007) Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf-5. Eur J Plant Pathol 3: 265-278.
- Lopez Fernandez SP, Sonego M, Moretto M, Pancher K, Engelen I, et al. (2015) Whole-genome comparative analysis of virulence genes unveils similarities and differences between endophytes and other symbiotic bacteria. Front. Microbiol (6).
- Lovene M, Barone A, Frusciante L, Monti L, Carputo D (2004) Selection for aneuploid potato hybrids combining a low wild genome content and resistance traits from Solanum commersonii. Theor Appl Genet 109(6): 1139-1146.
- Lovely DR (2000) Fe (III) and Mn (IV) reduction. In Environmental Microbe-Metal Interactions Edited by DR, Lovley. Washington, DC: American Society for Microbiology, pp. 3-30.
- Lu JX, Burton SD, Xu YS, (2014) The flexible structure of the K24S28 region of Leucine-Rich Amelogenin Protein (LRAP) bound to apatites as a function of surface type, calcium, mutation, and ionic strength. Front Physiol 5: 254
- Lu F, Liang X, Lu H, Li Q, Chen Q, et al. (2016) Overproduction of superoxide dismutase and catalase confers cassava resistance to Tetranychus cinnabarinus 7: 40179.
- Scientific Reports Lugtenberg, B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annual Review of Microbiology. 63: 541-556.
- Ma T, Zhou C (2012) Climate-associated changes in spring plant phenology in China. International Journal of Biometeorology 56(2): 269-275.
- Ma Q, Zhou W, Zhang P (2015) Transition from somatic embryo to friable embryogenic callus in cassava: dynamic changes in cellular structure, physiological status, and gene expression profiles. Frontiers in Plant Science 6: 824.
- Mabasa KG (2007) Epidemiology of cassava mosaic disease and molecular characterization of cassava mosaic viruses and their associated whitefly Bemisia tabaci vector in South Africa. Msc. Johannesburg, South Africa: University of Witwatersrand.
- Mahungu NM, Dixon AGO, Kumbira JM (1994) Breeding cassava for multiple pest resistance in Africa. African Crop Science Journal 2(4): 539-552.
- MacKinnon G, HJ Duncan (2013) Phytotoxicity of branched cyclohexanes found in the volatile fraction of diesel fuel on germination of selected grass species. Chemosphere 90: 952-957.
- Macalady J, Banfield JF (2003) Molecular geomicrobiology: genes and geochemical cycling. Earth Planet Sci Lett 209: 1-17.
- Madsen JS, Burmolle M, Hansen LM, Sorensen SJ (2012) The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol 65: 183-195.
- Maheshwari, Meenu Hussein H. Abulreesh, Mohammad Shavez Khan, Iqbal Ahmad, John Pichtel (2017) Horizontal Gene Transfer in Soil and the Rhizosphere: Impact on Ecological Fitness of Bacteria, In: Agriculturally Important Microbes for Sustainable Agriculture. Volume I: Plant-soil-microbe nexus.
- Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Frontiers in Microbiology 6: 198.
- Malla MA, Dubey A, Yadav S, Hashem A, Abd_Allah EF (2018a) Exploring the human microbiome: the potential future role of next-generation sequencing in disease diagnosis and treatment. Front Immunol 9: 1-23.
- Malla MA, Dubey A, Yadav S, Kumar A, Hashem A, Abd_Allah EF (2018b) Understanding and designing the strategies for the microbe-mediated remediation of environmental contaminants using omics approaches. Front Microbiol 9.
- Malusá E, L. Sas Paszt J, Ciesielska (2012) Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. The Scientific World Journal, pp. 12.
- Manefield M, Turner SL (2002) Quorum sensing in context: out of molecular biology and into microbialecology. Microbiology 148: 3762-3764.
- Mansour M, Scheunert I, Korte F (1992) Fate of persistend organic compounds in soil and water. In: Petruzzelli D, Freidreich G (eds) Migration and fate of pollutants in soils and suboils, Vol 32.
- Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93: 930-938.
- Martinez Viveros O, Jorquera M, Crowley DE, Gajardo G, Mora ML (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria, Journal of Soil Science and Plant Nutrition 10: 293-319.
- Matos ADM, Gomes ICP, Nietsche S, Xavier AA, Gomes WS, et al. (2017) Phosphate solubilization by endophytic bacteria isolated from banana trees. Ann Bra Ac Sc 89 (4): 2945-2954.
- Maurya BR, Meena VS, Meena OP (2014) Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos 27: 181-187.
- Maurya BR, Verma JP, Meena RS (2016) Potassium solubilizing microorganisms for sustainable agriculture. Springer, India, pp. 149-162.
- Mavrodi DV, Mavrodi OV, Parejko JA, Weller DM, Thomashow LS (2011) The role of 2,4-diacetylphloroglucinol- and phenazine-1-carboxylic acid-producing Pseudomonas spp. In DK Maheshwari (Ed.), Natural protection of wheat from soil borne pathogens. Bacteria in agrobiology: Plant nutrient management pp. 60-63.
- Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42: 565-572.
- Mc Guinness M, D Dowling, (2009) Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J Environ Res Public Health 6: 2226-2247.
- Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic and human pathogenic microorganisms. FEMS Microbiol Rev 37: 634-663.
- Meena OP, Maurya BR, Meena VS (2013a) Influence of K-solubilizing bacteria on release of potassium from waste mica. Agric Sust Dev 1: 53-56.
- Meena VS, Maurya BR, Bohra JS, Verma R, Meena MD (2013b) Effect of concentrate manure and nutrient levels on enzymatic activities and microbial population under submerged rice in alluvium soil of Varanasi. Crop Res 45(1-3): 6-12.
- Meena VS, Maurya BR, Verma R, Meena RS, Jatav GK, et al. (2013c) Soil microbial population and selected enzyme activities as influenced by concentrate manure and inorganic fertilizer in alluvium soil of Varanasi. The Bioscan 8(3): 931-935.
- Meena VS, Maurya BR, Bahadur I (2014a) Potassium solubilization by bacterial strain in waste mica. Ban.gladesh J Bot 43: 235-237.
- Meena VS, Maurya BR, Verma JP (2014b) Does a rhizospheric microorganism enhance K+ avail- ability in agricultural soils? Microbiol Res 169: 337-347.
- Meena RS, Meena VS, Meena SK, Verma JP (2015a) The needs of healthy soils for a healthy world. J Clean Prod 102: 560-561.
- Meena RS, Meena VS, Meena SK, Verma JP (2015b) Towards the plant stress mitigate the agricultural productivity: a book review. J Clean Prod 102: 552-553.
- Meena VS, Maurya BR, Meena RS (2015c) Residual impact of well grow formulation and NPK on growth and yield of wheat (Triticum aestivum L.) Bangladesh J Bot 44(1): 143-146.
- Meena VS, Maurya BR, Verma JP, Aeron A, Kumar A, et al. (2015d) Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecol Eng 81: 340-347.
- Meena VS, Meena SK, Verma JP, Meena RS, Ghosh BN (2015e) The needs of nutrient use efficiency for sustainable agriculture. J Clean Prod 102: 562-563.
- Meena VS, Verma JP, Meena SK (2015f) Towards the current scenario of nutrient use efficiency in crop species. J Clean Prod 102: 556-557.
- Meena RS, Bohra JS, Singh SP, Meena VS, Verma JP, et al. (2016b) Towards the prime response of manure to enhance nutrient use efficiency and soil sustainability a current need: a book review. J Clean Prod 112(1): 1258-1260.
- Meena SK, Rakshit A, Meena VS (2016c) Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal Agric Biotechnol 6: 68-75.
- Meena VS, Bahadur I, Maurya BR, Kumar A, Meena RK, et al. (2016d) Potassium-solubilizing microorganism in evergreen agriculture: an overview. In: Meena VS, Maurya BR.
- Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, et al. (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332: 1097-1100.
- Mend ES LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014) Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8(8): 1577-1587.
- Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55: 165-199.
- Mishra PK, Mishra S, Selvakumar G, Bisht JK, Kundu S, Gupta HS (2009) Coinoculation of Bacillus thuringiensis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J Microbiol Biotechnol 25: 753-761.
- Miskito M, Braima J, Nnodu, E., Legg J, Wydra K, Ogbe F (2000) Disease Control in Cassava Farms. Wordsmithes Printers, Lagos. IITA 978 131: 176-182.
- Mitprasa M, Roytrakul S, Jiemsup S, Boonseng O, Yokthongwattana K (2011) Leaf proteomic analysis in cassava (Manihot esculenta Crantz) during plant development, from planting of stem cutting to storage root formation. Planta 233(6): 1209-1221.
- Morrell PL, Buckler ES, Ross Ibarra J (2012) Crop genomics: advances and applications. Nat Rev Genet 13(2): 85-96.
- Morris AC, Djordjevic MA (2006) The rhizobium leguminosarum biovar trifolii ANU794 includes novel developmental responses on the subterranean clover cultivar Woogenellup. Molecular Plant Microbe Interactions 19: 471-479.
- Mohankumar B, Nair PG (1983) Effect of sulphur containing fertilizers on cassava in acid laterite soil J Root Crops 9(1-2): 15-20.
- Mohankumar B, Nair PG, (1985) Lime, Sulphur and Zinc in Cassava Production. Technical Bulletin No. 2, ICAR-CTCRI, Kerala, India.
- Mosier AR, Syers K, Freney JR (2004) SCOPE 65, agriculture and the nitrogen cycle: 575 assessing the impacts of fertilizer use on food production and the environment. Scientific 576Committee on Problems of the Environment Series, Workshop held by the Scientific 577 Committee on Problems of the Environment in Kampala, Uganda. vol 65.
- Mowll J L, Gadd GM (1984) Cadmium uptake by Aureobasidium pullulans. J Gen Microbiol 130: 279-284.
- Mueller UG, Sachs JL (2015) Engineering microbiomes to improve plant and animal health. Trends Microbiol 2: 606-617.
- Muponda L (2014) An investigation into the anthelmintic properties of seed extracts from endophyte containing pasture grasses. Master’s Thesis, Lincoln University, New Zealand.
- Murumkar DR, Nalawade SV, Indi DV, Pawar SM (2016) Response of sugarcane seed plot to microbial inoculation by Gluconacetobacter diazotrophicus and phosphate-solubilizing bacteria. Sugar Tech 6: 1-7.
- Mulenga RM, Legg JP, Ndunguru J, Miano DW, Mutitu EW, et al. (2016) Survey, molecular detection, and characterization of geminiviruses associated with cassava mosaic disease in Zambia. Plant Disease. 100(7): 1379-1387.
- Nagabhyru PRD, Dinkins CL, Wood CW Bacon, CL Schardl (2013) Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biol 13(1): 127.
- Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiology 132(1): 146-153.
- Nakkeeran S, Fernando WGD, Siddiqui ZA (2006) Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. In: Siddiqui, ZA (ed) PGPR: Biocontrol and biofertilization. Springer, Dordrecht, pp. 257-296.
- Nair PG, Varghese T (1970) Effect of liming on the yield and quality of cassava on laterite soil. Agric Res J Kerala 8: 14-16.
- Nandi M, Selin C, Brassinga AKC, Belmonte MF, Fernando WGD, et al. (2015) Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23exhibits nematicidal and repellent activity against Caenorhabditis elegans. PloS One Journal.
- Nannipieri P, Ascher J, Ceccherini MT (2017) Microbial diversity and soil functions. Eur J Soil Sci.
- Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiology132(1): 146-153.
- Narayanan N, Beyene G, Chauhan RD, Gaitán Solis E, Grusak MA, et al. (2015) Overexpression of Arabidopsis VIT1 increases accumulation of iron in cassava roots and stems. Plant Science 240: 170-181.
- Narayanan NN, Uzoma I, Claire E, Sayre RT (2011) Overexpression of hydroxynitrile lyase in cassava roots elevates protein and free amino acids while reducing residual cyanogen levels. PLoS ONE 6(7): e21996.
- Nash PR, Motavalli PP, Nelson KA (2012) Nitrous oxide emissions from claypan soils due to 579 Nitrogen fertilizer source and tillage/fertilizer placement practices. Soil Sci Soc Am J 580 76: 983-993.
- Nath D, Maurya BR, Meena VS (2017) Documentation of five potassium- and phosphorus- solubilizing bacteria for their K and P-solubilization ability from various minerals. Biocatal Agric Biotechnol 10: 174-181.
- Navarre WW (2016) The impact of gene silencing on horizontal gene transfers and bacterial evolution. Adv Microb Physiol 69: 157-186.
- Nazaries L, Murrell JC, Millard P (2013) Methane, microbes and models: fundamental understanding of the soil methane cycle for future predictions. Environ Microbiol 15: 2395-2417.
- Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chern 270(45): 26723-26726.
- Neumann G (2007) Root exudates and nutrient cycling. In: Marschner P, Rengel Z (eds) Nutrient cycling in terrestrial ecosystems. Springer, Berlin, pp. 123-157.
- Neung S, Nguyen XH, Naing KW, Lee YS, Kim KY (2014) Insecticidal potential of Paenibacillus elgii HOA73 and its combination with organic sulfur pesticide on diamondback moth, Plutella xylostella. J. Kor Soc Appl Biol Chem. 57: 181-186.
- Newton JA, Fray RG (2004) Integration of environmental and host derived signals with quorum sensing during plant-microbe interactions. Cell Microbiol 6: 213-224.
- Ngampimol H, Kunathigan V (2008) The study of shelf life for liquid biofertilizer from vegetable waste. Au J T 11 584: 204-208.
- Nielsen MN, Sorensen J (1999) Chitinolytic activity of Pseudomonas fluorescens isolates from barley and sugar beet rhizosphere. FEMS Microbiol Ecol 30(3): 217-227.
- Nies DH, Silver S (1995) Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol 14: 186-199.
- Nies DH (1992a) Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid 27: 17-28.
- Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51: 730-750.
- Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27: 313-339.
- Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M (2012) Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol 79: 176-191.
- Nikolic B, Schwab H, Sessitsch A (2011) Metagenomic analysis of the 1-aminocyclopropane-1- carboxylate deaminase gene (acdS) operon of an uncultured bacterial endophyte colonizing Solanum tuberosum. L Arch Microbiol 193: 665-676.
- Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48: 1429-1449.
- Nyaboga EN, Njiru JM, Tripathi L (2015) Factors influencing somatic embryogenesis, regeneration, and Agrobacterium-mediated transformation of cassava (Manihot esculenta Crantz) cultivar Frontiers in Plant Science 6: 411.
- Nyaboga E, Njiru J, Nguu E, Gruissem W, Vanderschuren H, et al. (2013) Unlocking the potential of tropical root crop biotechnology in east Africa by establishing a genetic transformation platform for local farmer-preferred cassava cultivars. Frontiers in Plant Science 4: 526.
- OEC (2019) The Observatory of Economic Complexity.
- Ochman H, Lawrence JG, Groisman EA (2000) Lateral gene transfer and the nature of bacterial Nature 405 (6784): 299-304.
- Ochsner UA, Koch AK, Fiechter A, Reiser J (1994) Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 176: 2044-2054.
- Ochsner UA, Reiser J (1995) Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92: 6424-6428.
- Odjegba VJ, Sadiq AO (2002) Effects of spent engine oil on the growth parameters, chlorophyll and protein levels of Amaranthus hybridus L. The Environmentalists 22: 23-28.
- Okon Y, Labandera Gonzalez CA (1994) Agronomic applications of Azospirillum: an evaluation of 20 years’ worldwide field inoculation. Soil Biol Biochem 26: 1591-1601.
- Okoh AI, Badejo MA, Nathaniel IT, Tian G (1999) Studies on the bacteria, fungi and springtails (collembola) of an agroforestry arboretum in Nigeria. Pedobiologia 43: 18-27.
- Oldroyd, GE (2013) Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nature Reviews Microbiology 11(4): 252-263.
- Olugbenga O, AdeOluwa OfosoBudu, Brian Ssebunya (2011) Research Institute of Organic Agriculture, Switzerland.
- Ogwok, E, Odipio J, Halsey M, Gaitán Solís E, Bua A, et al. (2012) Transgenic RNA interference (RNAi)-derived field resistance to cassava brown streak disease. Molecular Plant Pathology 13: 1019-1031.
- Ono NN, Tian L (2011) The multiplicity of hairy root cultures: prolific possibilities. Plant Sci 180: 439-446.
- Orsenigo S, Abeli T, Rossi G, Bonasoni P, Pasquaretta C, Gandini M, et al. (2015) Effects of autumn and spring heat waves on seed germination of high mountain plants. PLoS One 10(7): e0133626.
- Otaiku AA, Alhaji IA (2019) Kachia Military Shooting Range In Situ Fungi Species Biodegradation of Explosives, Kaduna, Nigeria. J Adv Res Biotech 4(2): 1-26.
- Otaiku AA, Alhaji A1 (2020a) Military Shooting Range Xenobiotic Bacteria Consortia In Situ Biodegradation, Kachia, Kaduna, Nigeria. Sci J Biol & Life Sci 1(2).
- Otaiku AA, Alhaji AI (2020b) Characterization of microbial species in the biodegradation of explosives, military shooting range, Kaduna, Nigeria J Appl Biotechnol Bioeng7(3): 128-147.
- Otaiku AA, Alhaji A1 (2020c) Kachia Military Shooting Range Xenobiotics In Situ Biodegradation, Kaduna, Nigeria: Bacteria Species. J Adv Res Biotech 5(1): 1-26.
- Otaiku AA (2019) Effects of oil spillage on soils nutrients of selected communities in Ogoniland, south-eastern Niger Delta, Rivers State, Nigeria. International Journal of Ecology and Ecosolution 6(3): 23-36.
- Otaiku AA (2019) Significance of value-added materials (VAMS) for modernity and development. International Journal of Current Research 10: 7891-7907.
- Otaiku AA, Mmom PC, Ano AO (2019a) Biofertilizer Impacts on Cassava (Manihot esculenta Crantz) Rhizosphere: Crop Yield and Growth Components, Igbariam, Nigeria. World J Agri & Soil Sci 3(5): WJASS.MS.ID.000575.
- Otaiku AA, Mmom PC, Ano AO (2019b) Biofertilizer Impacts on Cassava (Manihot esculenta Crantz) Cultivation: Improved Soil Health and Quality, Igbariam, Nigeria. World J Agri & Soil Sci 4(1).
- Osman D, Cavet JS (2008) Copper homeostasis in bacteria. Adv Appl Microbiol 65: 217-247.
- Omar NF, Hassan SA, Yusoff UK, Abdullah NAP, Wahab PEM, et al. (2012) Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 17: 2378-2387.
- Otieno N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, et al. (2015) Plant growth pro- motion induced by phosphate solubilizing endophytic Pseudomonas isolates. Frontiers in Microbiology 6: 745.
- Owiti J, Grossmann J, Gehrig P, Dessimoz C, Laloi C, et al. (2011) iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration. Plant Journal 67(1): 145-156.
- Pan Y, Cassman N, de Hollander M, Mendes LW, Korevaar H, et al. (2014) Impact of long-term NPK and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiol Ecol 90(1): 195-205.
- Pan Y (20111) “Mitochondria, reactive oxygen species, and chronological aging: a message from yeast,” Experimental Gerontology 46(11): 847-852.
- Pandey A, Trivedi P, Kumar B, Palni LMS (2006) Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a Sub-Alpine location in the Indian Central Himalaya. Curr Microbiol 53: 102-107.
- Papenfort K, Bassler BL (2016) Quorum sensing signal-response systems in gram-negative bacteria. Nat Rev Microbiol 14: 576-588.
- Paterson AH, Schertz KF, Lin YR, Liu SC, Chang YL (1995) The weediness of wild plants: Molecular analysis of genes influencing dispersal and persistence in Johnson grass, Sorghum halepense (L.) Pers Proc Natl Acad Sci USA 92: 6127-6131.
- Parween Talat, Pinki Bhandari, Sumira Jan, Mahmooduzzafar, Tasneem Fatma, et al. (2017) Role of Bioinoculants as Plant Growth-Promoting Microbes for Sustainable Agriculture. In: Meena, Vijay Singh; Pankaj Kumar Mishra; Jaideep Kumar Bisht; Arunava Pattanayak (Eds.), Agriculturally Important Microbes for Sustainable Agriculture Volume I: Plant-soil-microbe nexus. Springer Nature Singapore Pte Ltd.
- Patten CL, Glick BR (2002) Role of Pseudomonas putida indole-acetic acid in development of the host plant root system. Appl Environ Microbiol 68: 3795-3801.
- Peng A, J Liu, Y Gao, Z Chen (2013) Distribution of endophytic bacteria in Alopecurus aequalis Sobol and Oxalis corniculata L. from Soils contaminated by polycyclic aromatic hydrocarbons. PLoS One 8(12): e83054.
- Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Plant 118(1): 10-15.
- Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179: 5756-5767.
- Peñuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, et al. (2014) Biogenic volatile emissions from the soil. Plant Cell Environ 37(8): 1866-1891.
- Pérez García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22: 187-193.
- Pesci EC, Pearson JP, Seed PC, Iglewski BH (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179: 3127-3132.
- Petrova LP, Shelud’ ko AV, Katsy EI (2010) Plasmid rearrangements and alterations in Azospirillum brasilense biofilm formation. Microbiology 79 (1): 121-124.
- Phi QT, Park YM, Seul KJ, Ryu CM, Park SH, et al. (2010) Assessment of root associated Paenibacillus polymyxa groups on growth promotion and induced systemic resistance in pepper. J Microbiol Biotechnol 20: 1605-1613.
- Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC (2014) Induced systemic resistance by beneficial microbes. Ann Rev Phytopathol 52: 347-375.
- Pii Y, Borruso L, Brusetti L, Crecchio C, Cesco S, et al. (2016) The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiology and Biochemistry 99: 39-48.
- Pineda A, Dicke M, Pieterse C, M, Pozo MJ (2013) Beneficial microbes in a changing environment: Are they always helping plants to deal with insects? Functional Ecology 27(3): 574-586.
- Pirttilä AM, Joensuu P, Pospiech H, Jalonen J, Hohtola A (2004) Bud endophytes of Scots pine produce adenine derivatives and other compounds that affect morphology and mitigate browning of callus cultures. Physiol Plant 121: 305-312.
- Podile AR, Vukanti RVNR, Sravani A, Kalam S, Dutta S, et al. (2014) Root colonization and quorum sensing are the driving forces of plant. Proc Indian Natl Sci Acad 80: 407-413.
- Poonguzhali S, Madhaiyan M, Sa T (2008) Isolation and identification of phosphate solubilizing bacteria from chinese cabbage and their effect on growth and phosphorus utilization of plants. J Microbiol Biotechnol 18: 773-777.
- Praça LB, Gomes ACMM, Cabral G, Martins ÉS, Sujii ER, et al. (2012) Endophytic colonization by Brazilian strains of Bacillus thuringiensis on cabbage seedlings grown in vitro. Bt Res 3: 11-19.
- Prochnik S, Reddy MP, Desany B, Rabinowicz PD, Kodira C, et al. (2012)
- Putten W, Bardgett RD, Bever JD, et al. (2013) Plant-soil feedbacks: the past, the present and future challenges. J Ecol 101: 265-276.
- Pushpadas MV, Aiyer RS (1976) Nutritional studies on cassava (Manihot esculenta Crantz) II. Effect of potassium and calcium on yield and quality of tubers. J Root Crops 2: 42-51.
- Qaderi A, Akbari Z, Kalateh jari S, Fatehi F, Tolyat M, et al. (2016) Improving trigonelline production in hairy root culture of fenugreek (Trigonella foenumgraecum). J Med Plants 59: 73-80.
- Qi J, Aiuchi D, Tani M, Asano S, Koike M (2016) Potential of entomopathogenic Bacillus thuringiensis as plant growth promoting rhizobacteria and biological control agents for tomato Fusarium Wilt. Int J Environ Agric Res 2(6): 55-63.
- Ramanujam T (1990) Effect of moisture stress on photosynthesis and productivity of cassava. Photosynthetica 24: 217-224.
- Raaijmakers JM (2015) The minimal rhizosphere microbiome. Principles of Plant-Microbe Interactions 411-417.
- Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81(1-4): 537-547.
- Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34: 1037-1062.
- Raddadi N, Cherif A, Boudabous A, Daffonchio D (2008) Screening of plant growth promoting traits of Bacillus thuringiensis. Ann Microbiol 58(1): 47-52.
- Raddadi N, Cherif A, Ouzari H, Marzorati M, Brusetti L, et al. (2007) Bacillus thuringiensis beyond insect biocontrol: plant growth promotion and biosafety of polyvalent strains. Ann Microbiol 57: 481-494.
- Raddadi N, Crotti E, Rolli E, Marasco R, Fava F, et al. (2012) The most important Bacillus species in biotechnology. In: Sansinenea E (edt.), Bacillus thuringiensis biotechnology chapter 17. Springer, Dordrecht, pp: 329-345.
- Raddadi N, Cherif A, Ouzari H, Marzorati M, Brusetti L, et al. (2007) Bacillus thuringiensis beyond insect biocontrol: plant growth promotion and biosafety of polyvalent strains. Ann Microbiol 57: 481-494
- Raghavendra MP, Nayaka NC, Nuthan BR (2016) Role of rhizosphere microflora in potassium solubilization. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp: 43-59.
- Raghavendra MP (2017) Quorum Sensing in Plant Microbe Interaction In: VS. Meena et al. (eds.), Agriculturally Important Microbes for Sustainable Agriculture, © Springer Nature Singapore Pte Ltd, Singapore.
- Rahim R, Ochsner UA, Olvera C, Graninger M, Messner P, et al. (2001) Cloning and functional characterization of the Pseudomonas aeruginosa rhlC gene that encodes rhamnosyl transferase 2, an enzyme responsible for di-rhamnolipid biosynthesis. Mol Microbiol 40: 708-718.
- Rajkumar M, Nagendran R, Lee KJ, Lee W H, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62: 741-748.
- Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71: 834-842.
- Radhakrishnan S, AR (2017a) Evaluation of Agronomic, Nutritional and Socio- Economic Impacts of Organic Production of Cassava (Manihot esculenta Crantz). PhD Thesis submitted to University of Kerala, Thiruvananthapuram, Kerala, India.
- Radhakrishnan SAR, Suja G, (2017b) How safe is organic cassava? J Root Crops 43(2): 3-9.
- Radhakrishnan SAR, Suja G, (2019) Nutrient release pattern of organic and inorganic resources used in cassava production (Manihot esculenta Crantz). J Plant Nutr. 42: 1301-1315
- Rappé MS, Giovannoni S J (2003) The uncultured microbial majority. Ann Rev Microbiol 57(1): 369-394.
- Rasmann, S, Turlings TCJ (2016) Root signals that mediate mutualistic interactions in the rhizosphere. Curr Opin Plant Biol 32: 62-68.
- Rawat J, Sanwal P, Saxena J (2016) Potassium and its role in sustainable agriculture. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp: 235-253.
- Reinhold-Hurek, B, Hurek T (1998) Life in grasses: diazotrophic endophytes. Trends Microbiol 6: 139-144.
- Reid A (2000) Microbes helping to improve crop productivity. Microbe 6 (10): 435
- Reilly K, Gomez-Vasquez R, Buschmann H, Tohme J, Beeching JR (2003) Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol Biol 53(5): 669-685.
- Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28: 897-906
- Rijavec T, Lapanje A (2016) Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front Microbiol 7: 1785.
- Rillig, MC, Wright SF, Eviner VT (2002) The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: comparing effects of five plant species. Plant Soil 238: 325-333.
- Ristow M, S Schmeisser (2011) Extending life span by increasing oxidative stress. Free Radic Biol Med 51(2): 327-336.
- Robertson P, Hamilton SK, Barham BL (2017) Cellulosic biofuel contributions to a sustainable energy future: choices and outcomes. Science 356: eaal2324
- Roh JY, Choi JY, Li MS, Jin BR, Je YH (2007) Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. Journal of Microbiology and Biotechnology 17: 547-559.
- Ryan PR, K Germaine, A Franks, DJ Ryan, DN Dowling (2008) Bacterial endophytes: Recent developments and applications. FEMS Microbiol Lett 278: 1-9.
- Rosen BP (2002) Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol 133: 689-693.
- Rosier CL, Hoye AT, Rillig MC (2006) Glomalin related soil protein: assessment of current detection and quantification tools. Soil Biology Biochemistry 38: 2205-2211.
- Rout GR, Sahoo S (2015) Role of iron in plant growth and metabolism. Rev Agri Sci 3 :1-24.
- Rossin CB, Rey MEC (2011) Effect of explant source and auxins on somatic embryogenesis of selected cassava (Manihot esculenta Crantz) cultivars. South African Journal of Botany 77: 59-65.
- Rovira AD (1996) Plant root exudates. The Botanical Review 35(1): 35-57.
- Romanoff S, Lynam J (1992) Cassava and African food security: some ethnographic examples. Ecol Food Nutr 27: 29-41.
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, et al. (2003) Bacterial volatiles promote growth in Arabidopsis. Proceedings of the National Academy of Sciences 100 (8): 4927-4932.
- Ryu CM, MA Farag, CH Hu, MS Reddy, HX Wie, et al. (2003) Bacterial volatiles promote growthin Arabidopsis. Proc Natl Acad Sci USA 100: 4927-4932.
- Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A (2016a) Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic Plains of India. Biocatalysis and Agricultural Biotechnology 7: 202-209.
- Saunders K, Nazeera Salim, Mali V R, Malathi V G, Briddon R, et al. (2002) Characterisation of Sri Lankan cassava mosaic virus and Indian cassava mosaic virus: evidence for acquisition of a DNA B component by a monopartite begomovirus. Virology 293(1): 63-74.
- Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A (2016b) Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic Plains of India. Biocatalysis and Agricultural Biotechnology 7: 202-209.
- Sakurai T, Plata G, Rodríguez Zapata F, Seki M, Salcedo A, et al. (2007) Sequencing analysis of 20,000 full length cDNA clones from cassava reveals lineage specific expansions in gene families related to stress response. BMC Plant Biology 7: 66.
- Salvadori JDM, Defferrari MS, Ligabue Braun R, Yamazaki E, Salvadori JR, et al. (2012) Characterization of entomopathogenic nematodes and symbiotic bacteria active against Spodoptera frugiperda (Lepidoptera: Noctuidae) and contribution of bacterial urease to the insecticidal effect. Biololical Control 63: 253-263.
- Sanchez T, Dufour D, Moreno JL, Pizarr M, Aragon IJ, et al. (2013) Changes in extended shelf life of cassava roots during storage in ambient conditions. Postharvest Biology and Technology 86: 520-528.
- Sang K, Kim EN, Han GD, Kwack MS, Jeun YC, et al. (2014) Priming- mediated systemic resistance in cucumber induced by Pseudomonas azotoformans GC-B19 and Paenibacillus elgii MM-B22 against Colletotrichum orbiculare. Phytopathology 104: 834-842.
- Santoyo G, Orozco Mosqueda MDC, Govindappa M (2012) Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Tech 22 (8): 855-872.
- Sarkar, D, Meena VS, Haldar A, Rakshit R (2017) Site-specific nutrient management (SSNM): a unique approach towards maintaining soil health. In: The Adaptive soil management: from theory to practices pp: 69-88.
- Sasse J, Martinoia E, Northen T (2018) Feed your friends: do plant exudates shape the root microbiome? Trends in Plant Science 23(1): 25-41.
- Sastre B, Marquez MJ, García Díaz A, Bienes R (2018) Three years of management with cover crops protecting sloping olive groves soils, carbon and water eects on gypsiferous soil. Catena 171: 115-124.
- Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, et al. (2011) The biocassava plus program: biofortification of cassava for sub-Saharan Africa. Annual Review of Plant Biology 62(1): 251-272.
- SCAR (2011) Sustainable food consumption and production in a resource-constrained world. In: Standing Committee on Agricultural Research of the European Committee: The 3rd SCAR Foresight Exercise. European Commission.
- Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ et al. (2008) A new class of homoserine lactone quorum- sensing signals. Nature 454: 595-599.
- Schauder S, Bassler BL (2001) The languages of bacteria. Genes Dev 15: 1468-1480.
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, et al. (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62: 775-806.
- Schultz RC, Colletti JP, Faltonson RR (1995) Agroforestry opportunities for the United States of 607 America. Agrofor Syst 31: 117-142.
- Schulz B, C Boyle (2006) What are Endophytes? In: Microbial Root Endophytes, Schulz BJE, CJC Boyle, TN Siever (eds.), Springer Verlag Berlin, ISBN: 9783540335252, pp: 1-33.
- Scott B, CJ Eaton (2008) Role of reactive oxygen species in fungal cellular differentiations. Curr Opin Microbiol 11(6): 488-493.
- Sergaki C, Lagunas B, Lidbury I (2018) Challenges and approaches in microbiome research: from fun-damental to applied. Front Plant Sci 9: 1205
- Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215: 229-238.
- Sessitsch A, Howieson JG, Perret X, Antoun H, Martinez Romero E (2002) Advancesin Rhizobium research. Crit Rev Plant Sci 21: 323-378.
- Sessitsch, AM Kuffner, P Kidd, J Vangronsveld, WW Wenzel, et al. (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biology Biochemistry 60: 182-194.
- Shaaban MI, Bar FMA, El-Mahdy AM, Shokralla S (2016) Quorum sensing inhibiting activity of Streptomyces coelicoflavus isolated from soil. Front Microbiol 7: 659-671.
- Shade A, Handelsman J (2012) Beyond the venn diagram: the hunt for a core microbiome. Environ Microbio 14: 4-12.
- Shade A, Handelsman J (2012) Beyond the venn diagram: the hunt for a core microbiome. Environ Microbiol 14: 4-12
- Shaharoona B, Naveed M Arshad M, Zahir ZA: (2008) Fertilizer-dependent efficiency of Pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L) Appl Microbiol Biotechnol 79: 147-155.
- Sharma A, Sahgal M, Johri BN (2003) Microbial communication in the rhizosphere: operation of quorum sensing. Curr Sci 85: 1164-1172.
- Sharma N, Saharan BS (2016) Bacterization effect of culture containing 1-aminocyclopropane-1-carboxylic acid deaminase activity implicated for plant development. BMRJ 16(1): 1-10.
- Sharma P, Kaur N, Gargi K (2012) Revitalizing soil health with biofertilizers. Biofert News Lett 20(2).
- Sharma S, Shahzad A, Sahai A (2013) Hairy root culture: an efficient system for secondary metabolite production. In: Shahid M, Shahzad A, Malik A, Sahai A (eds.), Recent trends in biotechnology and therapeutic applications of medicinal plants. Springer, The Netherlands, pp: 51-78.
- Sharma A, Thakur DR, Kanwar S, Chandla VK (2013) Diversity of entomopatho-genic bacteria associated with the white grub, Brahmina coriacea and lepidopterans. J Pest Sci 86: 261-273.
- Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2: 587
- Shrivastava M, Srivastava PC, D’Souza SF (2016) KSM soil diversity and mineral solubilization, in relation to crop production and molecular mechanism. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp: 221-234.
- Shafeek MR, Omar NM, Mahmad RA, Abd El-Baky MMH (2012) Effect of bioorganic fertilization on growth and yield of cassava plants in newly cultivated land. Middle East J Agric Res 1(1): 40-46.
- Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu RevMicrobiol 50: 753-789.
- Silver S, Phung LT (2009) Heavy metals, bacterial resistance. In Encyclopedia of Microbiology pp: 220-227.
- Simard SW, Durall DM (2004) Mycorrhizal networks: A review of their extent, function, and importance. Canadian Journal of Botany 82(8): 1140-1165.
- Sindhu SS, Parmar P, Phour M, Sehrawat A (2016) Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 171-185.
- Singh BK, Bardgett RD, Smith P, Reay DS (2010) Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8: 779-790.
- Singh G, Mukerji K (2006) Root exudates as determinant of rhizospheric microbial diversity. In: Mukerji K, Manoharachary C, Singh J (eds.), Microbial activity in the rhizosphere. Springer, Berlin, pp: 39-53.
- Singh M, Dotaniya ML, Mishra A, Dotaniya CK, Regar KL, Lata M (2016) Role of biofertilizers in conservation agriculture. In: Bisht JK, Meena VS, Mishra PK, Pattanayak A (eds.), Conservation agriculture: an approach to combat climate change in Indian Himalaya. Springer, Singapore, pp. 113-134.
- Singh NP, Singh RK, Meena VS, Meena RK (2015) Can we use Maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos 28(1): 86-99.
- Singh S, Singh BK, Yadav SM, Gupta AK (2014) Potential of biofertilizers in crop production in 619 Indian agricultures. Am J Plant Nutr Fertil Technol 4: 33-40
- Singh AK, Ghodke I, Chhatpar HS (2009) Pesticide tolerance of Paenibacillus sp. D1 and its chitinase. Journal of Environmental Management 91: 358-362.
- Singh I, Giri B (2017) Arbuscular mycorrhiza mediated control of plant pathogens. In: Mycorrhiza Nutrient uptake, biocontrol, ecorestoration. Springer, Cham, pp: 131-160.
- Singh N, Pandey P, Dubey R, Maheshwari DK (2008) Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (Sarg.) by rhizosphere competent Bacillus subtilis BN1. World J Microbiol Biotechnol.
- Singh RP, Jha PN (2016) The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.) PLoS One 11(6): e0155026.
- Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: Impacts of excess nutrientinputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100(1): 179-196.
- Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, London.
- Soda, N, Wallace S, Karan R (2015) Omics study for abiotic stress responses in plants. Advances in Plants & Agriculture Research 2(1): 00037.
- Sojikul P, Kongsawadworakul P, Viboonjun U, Thaiprasit J, Intawong B, et al. (2010) AFLP-based transcript profiling for cassava genome-wide expression analysis in the onset of storage root formation. Physiol Plant 140: 189-198.
- Sojikul P, Saithong T, Kalapanulak S, Pisuttinusart N, Limsirichaikul S, et al. (2015) Genome-wide analysis reveals phytohormone action during cassava storage root initiation. Plant Mol Biol 88: 531-543.
- Soussi A, Raoudha F, Ramona M, Amel G, Hanene C, et al. (2015) Plant-associated microbiomes in arid lands: diversity, ecology and biotechnological potential. Plant and Soil 405: 357-370.
- Souza RC, Hungria M, Cantão ME, Vasconcelos ATR, Nogueira MA, et al. (2015) Metagenomic analysis reveals microbial functional redundancies and specificities in a soil under different tillage and crop-management regimes. Applied Soil Ecology 86: 106-112.
- Spaepen S, Dobbelaere S, Croonenborghs A, Vanderleyden J (2008) Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant and Soil 312: 15-23.
- Spaink HP, Kondorosi A, Hooykaas PJJ (eds.) (1998) The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, Netherlands.
- Stamford NP, Ortega AD, Temprano F, Santos DR (1997) Effects of phosphorus fertilization and inoculation of Bradyrhizobium and mycorrhizal fungi on growth of Mimosa caesalpiniaefolia in an acid soil. Soil Biology and Biochemistry 29: 959-964.
- Stepniewska Z, A Kuzniar (2013) Endophytic microorganisms-promising applications in bioremediation of greenhouse gases. Applied Microbiol Biotechnol 97: 9589-9596.
- Strobel G, B Daisy (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67: 491-502.
- Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, et al. (2015) Planetary boundaries: guiding human development on a changing planet. Science 347.
- Steinauer K, Tilman D, Wragg PD (2015) Plant diversity effects on soil microbial functions and enzymes are stronger than warming in a grassland experiment. Ecology 96: 99-112.
- Stempfhuber B, Richter-Heitmann T, Regan KM, Kölbl A, Kaul P, et al. (2015) Spatial interaction of archaeal ammonia-oxidizers and nitrite-oxidizing bacteria in an unfertilized grassland soil. Front Microbiol 6: 1567.
- Stephens JHG, Rask HM (2000) Inoculant production and formulation. Field Crops Res 633(65): 249-258.
- Sterflinger K (2000) Fungi as geologic agents. Geomicrobiol J 17: 97-124.
- Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6(3): 199-210.
- Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19: 1-30.
- Sikora RA, A Zum Felde, A Mendoza, R Menjivar, L Pocasangre (2008) In planta suppressiveness to nematodes and longterm root health stability through biological enhancement-do we need a cocktail? Acta Hortic 879: 553-560.
- Svercel M, Duffy B, Defago G (2007) PCR amplification of hydrogen cyanide biosynthetic locus hcn AB in pseudomonas spp. J Microbiol Methods 70(1): 209-213.
- Swift, S, Williams P, Stewart GSAB (1999) N-acylhomoserine lactones and quorum sensing in proteobacteria. In: Dunny GM, Winans S (eds.), Cell-cell signalling in bacteria. Am Soc Microbiol, Washington DC, USA, pp: 291-313.
- Tan RX, WX Zou (2001) Endophytes: A rich source of functional metabolites. Nat Prod Rep 18: 448-459.
- The cassava genome: current progress, future directions. Tropical Plant Biology 5(1): 88-94.
- Tang X, MZ Hashmi, D Long, L Chen, MI Khan, et al. (2014) Influence of heavy metals and PCBs pollution on the enzyme activity and microbial community of paddy soils around an e-waste recycling workshop. Int J Environ Res Public Health 11: 3118-3131.
- Taghavi S, N Weyens J, Vangronsveld, D van der Lelie (2011) Improved Phytoremediation of Organic Contaminants Through Engineering of Bacterial Endophytes of Trees. In: Endophytes of Forest Trees. Forestry Sciences, Pirttila A, A Frank (eds.)., Springer, Dordrecht 80: 205-216.
- Teotia P, Kumar V, Kumar M, Shrivastava N, Varma A (2016) Rhizosphere microbes: potassium solubilization and crop productivity-present and future aspects. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 315-325.
- Thakur P, Singh I (2018) Biocontrol of soilborne root pathogens: an overview. In: Root biology, Soil biology. Springer, pp. 181-220.
- Thayer JS (1989) Methylation: its role in the environmental mobility of heavy elements. Appl Organomet Chem 3: 123-128.
- Thomashow LS, Weller DM (1988) Role of a phenazine antibiotic from Pseudomonas Fluorescens in biological control of Gaeumannomyces graminis var tritici J Bacteriol 170(8): 3499-3508.
- Thornton PK, Herrero M (2010) Potential for reduced methane and carbon dioxide emissions from livestock and pasture management in the tropics. Proc Natl Acad Sci USA 107(46): 19667-19672.
- Thresh JM, Otim Nape GW, Legg JP, Fargette D (1997) African cassava mosaic virus disease: the magnitude of the problem. African Journal of Root and Tuber Crops 2(1/2): 13-19.
- Thresh JM, Cooter RJ (2005) Strategies for controlling cassava mosaic virus disease in Africa. Plant Pathology 54(5): 587-614
- Thresh JM, Otim-Nape GW, Thankappen M, Muniyappa V (1998) The mosaic diseases of cassava in Africa and India caused by whitefly-borne gemini viruses. Review of Plant Pathology 77(9).
- Thwe A, Arasu MV, Li X, Park CH, Kim SJ, et al. (2016) Effect of different agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (Fagopyrum tataricum gaertn). Front Microbiol 7: 318-328.
- Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA.
- Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson A (2017) Perspectives and challenges of 645 microbial application for crop improvement. Front Plant Sci 8: 49.
- Tiendrebeogo F, Lefeuvre P, Hoareau M, Harimalala MA, Bruyn Ade, et al. (2012) Evolution of African cassava mosaic virus by recombination between bipartite and monopartite begomo viruses. Virology Journal 9(67).
- Tjamos SE, Flemetakis E, Paplomatas EJ, Katinakis P (2005) Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Mol Plant-Microbe Interact 18: 555-561.
- Tormo MA, Knecht E, Götz F, Lasa I, Penades JR (2005) Bap-dependent biofilm formation by pathogenic species of Staphylococcus: evidence of horizontal gene transfer?. Microbiology 151(7): 2465-475.
- Torres MA (2010) ROS in biotic interactions. Physiol Plant 138(4): 414-429.
- Torrey JG (1978) Nitrogen fixation by actinomycete-nodulated angiosperms. Bioscience 28(9): 586-592.
- Trevors JT, van Elsas JD, Lee H, van Overbeek LS (1992) Use of alginate and other carriers for encapsulation of microbial cells for use in soil. Microb Releases 1: 61-69.
- Tripathi V, Fraceto LF, Abhilash PC (2015) Sustainable clean-up technologies for soils contaminated with multiple pollutants: plant-microbe-pollutant and climate nexus. Ecol Eng.
- Trivedi P, Anderson IC, Singh BK (2013) Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 21: 641-651.
- Trivedi Pankaj, Matthew D. Wallenstein, Peer M Schenk, Brajesh K Singh (2017) Tiny Microbes, Big Yields: enhancing food crop production with biological solutions. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI (2007) The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 449: 804-810.
- Ullman WJ, David L, Kirchman Susan A, Welch Philippe Vandevivere (1996) laboratory evidence for microbially mediated silicate mineral dissolution in nature. Chern Geol 132: 11-17.
- Unkovich MJ, Pate JS (2000) An appraisal of recent field measurements of symbiotic N2 fixation by 651 annual legumes. Field Crops Res 65: 211-228.
- Unkovich MJ, Pate, Sanford P (1997) Nitrogen fixation by annual legumes in Australian 653 Mediterranean agriculture. Aust J Agric Res 48: 267-293.
- Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M (2005) Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of Lupin analysed by phytate utilization ability. Environ Microbiol 7(3): 396-404.
- Uroz S, Ioannidis P, Lengelle J, et al. (2013) Functional assays and metagenomic analyses reveals differences between the microbial communities inhabiting the soil horizons of a Norway spruce plantation. PLoS ONE.
- Uroz S, Calvaruso C, Turpault MP, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 17(8): 378-387.
- Utsumi Y, Tanaka M, Morosawa T, Kurotani A, Yoshida T, et al. (2012) Transcriptome analysis using a high-density oligomicroarray under drought stress in various genotypes of cassava: an important tropical crop. DNA Research 19(4): 335-45.
- Vaid SK, Kumar B, Sharma A, Shukla AK, Sivastava PC (2014) Effect of zinc solubilizing bacteria on growth promotion and zinc nutrition of rice. J Soil Sci Plant Nutr 13(4): 889-910.
- Valverde A, De Maayer P, Oberholster T, et al. (2016) Specific microbial communities associate with the rhizosphere of Welwitschia mirabilis, a living fossil. PLoS ONE.
- Van Aken B, PA Correa, JL Schnoor (2010) Phytoremediation of polychlorinated biphenyls: New trends and promises. Environ Sci Technol 44: 2767-2776.
- Van der Heijden MGA, de Bruin S, Luckerhoff L, van Logtestijn RSP, Schlaeppi K (2016) A widespread plant‐fungal‐bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J10: 389-399.
- Van der Heijden MG, Hartmann M (2016) Networking in the plant microbiome. PLoS Biology 14(2).
- Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11: 296-310.
- Van der Heijden MGA, De Bruin S, Luckerhoff L, et al. (2015) A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J 10: 389-399.
- Vorholt JA, Vogel C, Carlström CI, Müller DB (2017) Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 22: 142-155.
- Van der Heijden MGA, Van Der Streitwolf-engel R, Riedl R, Siegrist S, Neudecker A, et al. (2004) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure inexperimental grassland. New Phytol 172: 739-752.
- Van Elsas JD, Turner S, Bailey MJ (2003) Horizontal gene transfer in the phytosphere. New Phytol 157(3) :525-537.
- Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13: 235-245.
- Vance CP (1998) Legume symbiotic nitrogen fixation: agronomic aspects. In: Spaink HP (ed) The 655 Rhizobiaceae. Kluwer Academic, Dordrecht, pp. 509-530.
- Vandenkoornhuyse, Achim Quaiser, Marie Duhamel, Amandine Le Van, Alexis Dufresne (2015b) The importance of the microbiome of the plant holobiont. New Phytol 206: 1196-1206.
- Vanderschuren H, Akbergenov R, Pooggin M, Hohn T, Gruissem W et al. (2007b) Transgenic cassava resistance to African cassava mosaic virus is enhanced by viral DNA-A bidirectional promoter-derived siRNAs. Plant Molecular Biology 64: 549-57.
- Vanderschuren H, Alder A, Zhang P, Gruissem W (2009) Dose-dependent RNAi-mediated gem in ivirus resistance in the tropical root crop cassava. Plant Molecular Biology 70: 265-72.
- Vanderschuren H, Moreno I, Anjanapp RB, Zainuddin IM, Gruissem W (2012) Exploiting the combination of natural and genetically engineered resistance to cassava mosaic and cassava brown streak viruses Impacting cassava production in Africa. PLoS ONE 7(9): e45277.
- Vanderschuren H, Nyaboga E, Poon JS, Baerenfaller K, Grossmann J, et al. (2014) Large-scale proteomics of the cassava storage root and identification of a target gene to reduce postharvest deterioration. Plant Cell 26(5): 1913-24.
- Vanderschuren H, Alder A, Zhang Peng, Gruissem W (2009) Dose-dependent RNAi-mediated geminivirus resistance in the tropical root crop Plant Molecular Biology 70(3): 265-272.
- Vanderschuren H, Stupak M, Fütterer J, Gruissem W, Zhang Peng (2007) Engineering resistance to geminiviruses - review and perspectives. Plant Biotechnology Journal 5(2): 207-220.
- Vassilev N, Medina A, Azcon R, Vassilev M (2006a) Microbial solubilisation of rock phosphate as media containing agro industrial wastes and effect of the resulting products on plant growth and phosphorus uptake. Plant Soil 287: 77-84.
- Vassilev N, Vassileva M, Fenice M, Federici F (2001) Immobilized cell technology applied in solubilization of insoluble inorganic (rock) phosphates and P plant acquisition. Bioresour Technol 79: 263-271.
- Vassilev N, Vassileva M, Nikolaeva I (2006b) Simultaneous Psolubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol 71: 137-144.
- Vassilev N, Vassileva M, Bravo V, Fernandez-Serrano M, Nikolaeva I (2007) Simultaneous phytase production and rock phosphate solubilization by Aspergillus niger grown on dry olive wastes. Ind Crops Prod 26: 332-336.
- Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability. A review. Molecules 21(5): 573.
- Velazquez E, Silva LR, Ramírez-Bahena MH, Peix A (2016) Diversity of potassium-solubilizing microorganisms and their interactions with plants. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 99-110.
- Velusamy P, JE Immanuel, SS Gnanamanickam, LS Tomashow (2006) Biological control of rice bacterial blight by plantassociated bacteria producing 2,4-diacetylphloroglucinol. Can J Microbiol 52: 56-65.
- Verma JP, Jaiswa DK, Meena VS, Meena RS (2015a) Current need of organ ic farming for enhancing sustainable agriculture. J Clean Prod 102: 545-547.
- Verma JP, Jaiswal DK, Meena VS, Kumar A, Meena RS (2015b) Issues and challenges about sustainable agriculture production for management of natural resources to sustain soil fertility and health. J Clean Prod 107: 793-794.
- Verma JP, Meena RS (2016) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 1-20.
- Verma R, Maurya BR, Meena VS (2014) Integrated effect of bio-organics with chemical fertilizer on growth, yield and quality of cabbage (Brassica oleracea var capitata). Indian J Agric Sci 8: 914-919.
- Verma R, Maurya BR, Meena VS, Dotaniya ML, Deewan P, et al. (2017b) Enhancing production potential of cabbage and improves soil fertility status of Indo-Gangetic Plain through application of bio-organics and mineral fertilizer. Int J Curr Microbiol App Sci 6(3): 301-309.
- Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255: 571-586.
- Vibha B, Neelam G (2012) Importance of exploration of microbial biodiversity. ISCA J Biol Sci 1(3): 78-83.
- Viñas M, Sabaté J, José M, Solanas AM (2005) Bacterial community dynamics and polycyclic aromatic degradation during bioremediation of heavily creosote contaminated soil. Appl Environ Microbiol 71: 7008-7018.
- Violante A, Huang PM, Gadd GM (2008) Biophysico- chemical Processes of Heavy Metals and Metalloids in Soil Environments. Chichester: Wiley.
- Viterbo A, Horwitz BA (2010) Mycoparasitism. In Cellular and Molecular Biology of Filamentous Fungi. Edited by K. A. Borkovich & D. J. Ebbole. Washington: American Society for Microbiology 42: 676-693
- Von Der Weid I, Artursson V, Seldin L, Jansson JK (2005) Antifungal and root surface colonization properties of GFP-tagged Paenibacillus brasilensis PB177. World J Microbiol Biotechnol 21: 1591-1597.
- Vorholt JA (2012) Microbial life in the phyllosphere. Nature Reviews Microbiology 10(12): 828-840.
- Vorholt, JA, Vogel C, Carlström CI, Müller DB (2017) Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 22: 142-155.
- Vyas P, Kumar D, Dubey A, Kumar A (2018) Screening and characterization of Achromobacter xylosoxidans isolated from rhizosphere of Jatropha curcas L (energy crop) for plant-growth-promoting traits. J Adv Res Biotechnol.
- Wagg C, Boller B, Schneider S, Widmer F, van der Heijden MG (2014) Intraspecific and inter- generational differences in plant-soil feedbacks. Oikos 124(8): 994-1004.
- Wakelin S, Warren R, Harvey P, Ryder M (2004) Phosphate solubilization by Penicillium sp. closely associated with wheat roots. Biol. Fertil. Soils 40: 36-43.
- Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132: 44-51
- Wall LG (2000) The actinorhizal symbiosis.J Plant Growth Regul 19: 167-182.
- Waller, F, Mueller M J, Pedrotti L (2013) Piriformospora indica root colonization triggers local and systemic root responses and inhibits secondary colonization of distal roots. PloS one 8(7).
- Wallenstein MD (2017) Managing and manipulating the rhizosphere microbiome for plant health: a systems approach. Rhizosphere 3: 230-232.
- Wang, Y, Kou S, Jiang Q, Xu B, Liu X, et al. (2014a) Factors affecting transfer of degradative plasmids between bacteria in soils. Appl Soil Ecol 84: 254-261.
- Wang, Y, Jiang Q, Zhou C, Chen B, Zhao W, et al. (2014b) In-situ remediation of contaminated farmland by horizontal transfer of degradative plasmids among rhizosphere bacteria. Soil Use Manag 30(2): 303-309.
- Wang Y, Xiao M, Geng X, Liu J, Chen J (2007) Horizontal transfer of genetic determinants for degradation of phenol between the bacteria living In plant and its rhizosphere. Appl Microbiol Biotechnol 77(3): 733-739.
- Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27: 195-226.
- Wang W, Feng B, Xiao J, Xia Z, Zhou X, et al. (2014) Cassava genome from a wild ancestor to cultivated varieties. Nature Communications 5: 5110.
- Wani PA, Khan MS, Zaidi A (2007a) Chromium reduction, plant growth promoting potentials and metal solubilization by Bacillus sp. isolated from alluvial soil. Curr Microbiol 54: 237-243.
- Wani PA, Khan MS, Zaidi A (2007c) Co-inoculation of nitrogen fixing and phosphate solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron Hung 55: 315-323.
- Waqas, Metal (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17(9): 10754-10773.
- Warren LA, Haack EA (2001) Biogeochemical controls on metal behaviour in freshwater environments. Earth Sci Rev 54: 261-320.
- Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cellcommunication in bacteria. Annu Rev Cell Dev Biol 21: 319-346.
- Waters CM, Bassler BL (2006) The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev 20: 2754-2767.
- Waters, Bassler, Weber RWS (2005) Mycorrhizas: anatomy and cell biology. Mycologist 19(3): 133.
- Wei HL, Zhang LQ (2006) Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 89: 267-280.
- Wen Y, Wu X, Teng Y, Qian C, Zhan Z, Zhao Y, (2011) Identification and analysis of the gene cluster involved in biosynthesis of paenibactin, a catecholate siderophore produced by Paenibacillus elgii B69. Environ Microbiol 13: 2726-2737.
- Weyens N, D Van der Lelie, T Artois K Smeets, S Taghavi et al. (2009) Bioaugmentation with engineered endophytic bacteria improves contaminant fate in phytoremediation. Environ. Sci. Technol 43: 9413-9418.
- White CE, Winans SC (2007) Cell-cell communication in the plant pathogen Agrobacterium tumefaciens. Philos Trans R Soc Lond Ser B Biol Sci 362: 1135-1148.
- Wongfun, Netal (2013) Weathering of granite from theDamma Glacier area: the contribution of cyanogenic bacteria. Geomicrobiol J 31: 93-100.
- Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, et al. (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nature Biotechnology 33(11): 1162-1164.
- Xia J, Wan S (2013) Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Annals of Botany 111(6): 1207-1217.
- Xu, S, Reuter T, Gilroyed BH et al. (2013) Microbial communities and greenhouse gas emissions associated with the biodegradation of specified risk material in compost. Waste Management.
- Xu J, Duan XG, Yang J, Beeching JR, Zhang P (2013a) Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays post-harvest physiological deterioration of cassava storage roots. Plant Physiology 161(3): 1517-1528.
- Xu J, Yang J, Duan X, Jiang Y, Zhang P (2014) Increased expression of native cytosolic Cu/ Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biology 14: 208.
- Yadav J, Ogwok E, Wagaba H, Patil B, Bagewadi B, e al. (2011) RNAi mediated resistance to cassava brown streak Uganda virus in transgenic cassava. Molecular Plant Pathology 12: 677-687.
- Yadav BK, Sidhu AS (2016) Dynamics of potassium and their bioavailability for plant nutrition. In: Meena V. S, Maurya B.R, Verma J.P, Meena R.S (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 187-201.
- Yadav BK, Sidhu AS (2016) Dynamics of potassium and their bioavailability for plant nutrition. In: Meena VS, Maurya BR, Verma J.P, Meena R.S (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, India, pp. 187-201.
- Yang, M, Sun K, Zhou L, Yang R, Zhong Z, Zhu J (2009) Functional analysis of three AHL autoinducersynthase genes in Mesorhizobium loti reveals the important role of quorum sensing in symbiotic nodulation. Can J Microbiol 55: 210-214.
- Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14 (1): 1-4.
- Yang J, An D, Zhang P (2011) Expression profiling of cassava storage roots reveals an active process of glycolysis/gluconeogenesis. Journal of Integrative Plant Biology 53 (3): 193-211.
- Yaninek JBJ, Tumanteh A, Maroya N, Dixon A, Salawu R, et al. (2000) Starting a Cassava Farm. IPM Field Guide for Extension Agents. International Institute for CABI Crop Protection Compendium. Technical Leaflet No.1.
- Yasin M, Munir I, Faisal M (2016) Can Bacillus spp. enhance K+ uptake in crop species. In: Meena VS, Maurya B.R, Verma J.P, Meena R.S (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 163-170.
- Yi HS, Yang JW, Ryu CM (2013) ISR meets SAR outside: Additive action of the endophyte Bacillus pumilus INR7 and the chemical inducer, benzothiadiazole, on induced resistance against bacterial spot in field-grown pepper. Frontiers in Plant Science 4: 122.
- Yuan, H, Ge T, Chen C (2012) Significant role for microbial autotrophy in the sequestration of soil carbon. Appl Environ Microbiol 78: 2328-2336.
- Yuan Z, Druzhinina IS, Labbé J, Redman R, Qin Y, Rodriguez R, et al. (2016) Specialized Microbiome of a Halophyte and Its Role in Helping Non-Host Plants to Withstand Salinity. Scientific Reports 6: 32467.
- Zahedi H (2016) Growth-promoting effect of potassium-solubilizing microorganisms on some crop species. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp. 31-42.
- Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63(4): 968-989.
- Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64: 991-997.
- Zainuddin IM, Schlegel K, Gruissem W, Vanderschuren H (2012) Robust transformation procedure for the production of transgenic farmer-preferred cassava landraces. Plant Methods 8: 24.
- Zavala Gonzalez EA, Rodríguez Cazorla E, Escudero N (2017) Arabidopsis thaliana root colonization by the nematophagous fungus Pochonia chlamydosporia is modulated by jasmonate signaling and leads to accelerated flowering and improved yield. New Phytol.
- Zaidi A, Khan MS, Ahemad M, M Oves (2009) Plant growth promotion by phosphate Solubilizing bacteria. Acta Microbiologica et Immunologica Hungarica 56(3): 263-284.
- Zhang H, Kim MS, Sun Y, Dowd SE, Shi H, Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Molecular Plant- Microbe Interactions 21(6): 737-744.
- Zhang Z, Ober U, Erbe M, Zhang H, Gao N, et al. (2014) Improving the accuracy of whole genome prediction for complex traits using the results of genome wide association studies. PLoS One 9(3): e93017.
- Zhang P, Vanderschuren H, Fütterer J, Gruissem W (2005) Resistance to cassava mosaic disease in transgenic cassava expressing antisense RNAs targeting virus replication genes. Plant Biotechnology Journal 3: 385-397.
- Zheng XY, Sinclair JB (1996) Chemotactic response of Bacillus megaterium strain B153-2-2 to soybean root and seed exudates. Physiol Mol Plant Pathol 48: 21-35.
- Zhang P, Ma Q, Naconsie M, Wu X, Zhou W, (2017) Advances in genetic modification of cassava. Burleigh Dodds Science Publishing Limited.
- Zhao S, Dufour D, Sánchez T, Ceballos H, Zhang P (2011) Development of waxy cassava with different biological and physico-chemical characteristics of starches for industrial applications. Biotechnology and Bioengineering 108 (8): 1925-1935.
- Zhongyong C, Xinglu L, Jiang S, Hexia X, Minqing C, Yuanlan H, Yinghua YP (2006) The effects of bio-organic fertilizer on plants growth and root tubers yield of cassava. Chin. Agric. Sci. Bull. 22(11): 202-206.
- Zidenga T, Leyva Guerrero E, Moon H, Siritunga D, Sayre R (2012) Extending cassava root shelf life via reduction of reactive oxygen species production. Plant Physiology 159(4): 1396-1407.
-
Otaiku AA, Mmom PC, Ano AO. Biofertilizer Impacts on Cassava (Manihot Esculenta Crantz) Rhizosphere: Soil Microbiome Engineering, Genetic and Sustainable Agroecosystems, Igbariam, Nigeria. World J Agri & Soil Sci. 6(1): 2020. WJASS.MS.ID.000630.
-
Biofertilizer, Rhizosphere engineering, Cassava genetics, Niger-Delta, Sustainable development goals (SDG) , Biodegradation, Remediation-to-biofuel development, Re-generative agriculture, Bio-ethanol
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






