 Research Article
Research Article
Proteomics of Some “Recalcitrant” Tropical Fruits: Avocado, Banana and Mango
Pier Giorgio Righetti1*, Clara Esteve2, Alfonsina D’Amato3, Elisa Fasoli1, María Luisa Marina4 and María Concepción García4
1Department of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, 20131 Milan, Italy
2Analytical Development, Janssen Biologics, Netherlands
3Department of Pharmaceutical Sciences, University of Milano, Italy
3Department of Analytical Chemistry, Faculty of Chemistry, University of Alcalá, Ctra. Madrid-Barcelona, Km. 33.600, 28871 Alcalá de Henares, Madrid, Spain
Corresponding AuthorProfessor Pier Giorgio Righetti, Department of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, 20131 Milan, Italy..
Received Date:January 06, 2023; Published Date: January 27, 2023
Abstract
The present review highlights the progress made in plant proteomics via the introduction of combinatorial peptide ligand libraries (CPLL) for detecting low-abundance species. Thanks to a variant of the CPLL methodology, namely that of performing the capture both under native and denaturing conditions, identifying plant species in the order of thousands, rather than hundreds, is now possible. We report here data on a trio of tropical fruits, namely banana, avocado and mango, classified as “recalcitrant” tissues since minute amounts of proteins (of the order of 1%) are embedded on a very large matrix of plant-specific material (e.g., polysaccharides and other plant polymers). Yet, even under these adverse conditions we could report, in a single sweep, from 1000 to 3000 unique gene products. In the case of mango, the investigation has been extended to the peel too, since this skin is popularly used to flavour dishes in the Far East cuisine. Even in this tough peel 330 proteins could be identified, whereas in soft peels, such in lemons, one thousand unique species could be detected.
Keywords:Low abundance proteome; Tropical fruits; Allergens; Combinatorial peptide ligand libraries; Mass spectrometry
Abbreviations:CPLL - combinatorial peptide ligand libraries
Introduction
The present review aims at offering a panorama of what modern pre-fractionation technologies can achieve in detecting the low- to very-low abundance proteome (LAP) in plant proteomics, an analyte fraction that is quite invisible even to the most sophisticated modern mass spectrometers (MS), whose sensitivity spans in general five orders of magnitude in relative concentrations of proteins present in a sample. Yet, in human biological fluids, such as plasma, such dynamic range can cover up to 12 orders of magnitude [1] and, in living cells, it can span at least seven orders of magnitude. Thus, it is quite obvious that MS alone cannot cover efficiently the grounds and therefore additional techniques are needed to achieve the goal of (possibly) a global coverage of any proteome. One such technique, with examples of what can be achieved in analysis of plant proteomics, is the combinatorial peptide ligand library (CPLL) technology. This methodology has now been taken at a level of maximum performance, as summarized in [2].
CPLLs appear to be a unique tool for exploring the “dark side” of any proteome, due to their unique property of providing millions of affinity ligands able to find a partner in any protein species present in biological materials. In most biological specimens a small set of proteins (often as few as 20–30) are present in a large excess and constitute, like in human sera, as much as 99% of the total protein mass. This would leave little room for sampling (and thus detecting) all other species therein. A solution proposed by the Anderson’s lab was immuno-subtraction, i.e., preparation of affinity resins containing antibodies against the six most abundant proteins in sera (first extended to 12 and now to 20) [3]. Believed to permit access to low-abundance species [4], in reality it did not quite live up to expectations [5,6], for a few reasons. Among them, the major issue was that much too little sample volume could be processed in a single sweep (barely 100 μL serum/plasma). Application of this methodology to plant proteomes, such as the immuno-depletion of RuBisCO, also did not lead to any major improvement [7]. The CPLL technique is immune from such drawbacks. These beads can be loaded with any volume (and quantity) of sample, since they work on an overloading principle. Additionally, they are universal, since they can be applied to any sample of any origin, whereas immuno- subtraction relies on antibodies made against specific samples, which requires a new one when changing organism. Thirdly, these beads act simultaneously by drastically cutting the concentration of high-abundance species while enriching the LAP population to the maximum possible extent. The consequence of such a situation is the detection of many novel proteins (the low-abundance ones) as a result of (1) the annihilation of the signal suppression due to concentrated species (e.g., albumin in serum) and (2) the detection of very low concentration proteins that were below the detectability level prior to sample treatment [8-10]. A brief description of the properties and mechanism of action of CPLLs here follows. CPLLs comprise several millions of hexapeptides, potentially able to recognize a complementary amino acid sequence in a bait protein, thus harvesting it from the sample matrix. Therefore, CPLLs can be envisioned as a matrix consisting of millions of bio-affinity ligands, contrary to classical affinity chromatography where, in general, a single ligand specific for a given protein is bound to a resin [2]. Their ability to capture a given protein, especially if present in very low abundance (LAP), as compared to much more concentrated proteins (HAP), depends on the relative affinities for any given bait. Thus, a LAP, having very high affinity for a given hexapeptide, can displace from it a HAP having low affinity for the same ligand. This mechanism of action can thus counterbalance the law of mass action and permit capture and much increased visibility of LAPs in presence of HAPs.
We have applied the CPLL technique to plant proteomics (as
well as to analysis of food stuff and beverages of plant and animal
origin) for at least three main reasons:
• To detect trace proteins/peptides exhibiting negative effects
on health (e.g., allergens);
• To detect trace proteins/peptides displaying positive effects
on health (e.g., anti-microbial, anti-hypertensive and anti-oxidant
activities);
• To expose frauds in commercial food products and provide a
proof of genuineness for “correct” commercial foods, as found
in supermarkets.
The present review is limited to application of the CPLL methodology to three typical tropical fruits of very large consumption, namely banana, avocado and mango, whose proteome has been largely unknown up to our investigations. Additionally, two of them (avocado and banana) represent “recalcitrant tissues” in that minute amounts of proteins (of the order of 1%) are embedded on a very large matrix of plant-specific material (e.g., polysaccharides and other plant polymers). Thus, the description and discovery of their proteomes represents a real challenge. We mention here two reviews covering this field quite extensively [11,12]. Special issues of different journals appear from time to time covering plant proteomics, including analysis of food and beverages. In Journal of Proteomics [13], one can read Boggess et al. [12], Nakamura et al. [14], Uvackova et al. [15] and Agrawal et al. [16].
Mammalian Versus Plant Proteomics
When surveying the deeds of scientists working with mammalian proteomics, one can find that today they can explore to a very large extent the proteome of any living cell line. For instance, when analyzing 11 human cell lines, Geiger et al. [17] could identify a total of 11,731 proteins and on average 10,361 ±120 proteins in each cell line, an outstanding catch, indeed. Interestingly, a very large number of them represent a common set shared by all cell lines, amounting to 8522 unique species. Each individual cell then displays from 200 to 500 proteins specific of each line. The latter probably represent proteins that characterize each individual line and ensure its specific biological activity. Likely, they could also be low-abundance species. How can there be such a discrepancy with plant proteomics, when in this last domain we are lucky if we can find a few hundred species in a single run? It should be noted that, in a way, these mammalian cell lines grown in vitro cultures are rather “easy” samples, in that they are not embedded in, e.g., fibrous tissues, muscles and other body compartments that would represent a complex matrix from which such cells would have to be extracted. On the contrary, in plant proteomics, most of the times, the proteins to be identified are dispersed into a very complex matrix and often are present in low amounts as compared to the plant biopolymers (e.g., polysaccharides, polyphenols) and metabolites constituting the specimen mass. This is the reason why it has been difficult to detect more than a few hundred species in any plant tissue. Thus, the CPLL methodology, allowing access to an order of magnitude more (up to 3000 unique gene products), represents an important advance in the field.
There is a reason, though, for this major advance in mapping of fruit proteomics. Up to these investigations, in fact, we applied the CPLL technology under “orthodox” conditions, namely by capturing proteomes under native conditions, since it was believed (correctly) that CPLLs would not be compatible with denaturing milieus, such as the classical cocktail of 2 M thiourea, 7 M urea and 2% CHAPS adopted for solubilizing proteins in view of two-dimensional mapping. Such conditions, indeed, are used to elute the proteins captured by CPLLs, so they could hardly be applied for their capture! Yet, we found an escape route: after solubilizing and capturing proteins under native conditions, the remaining sample could be treated with 2% SDS, but not under boiling conditions, only at room temperature. Under these conditions, plenty of additional species could be solubilized. Yet the presence of 2% SDS would be incompatible with further sample treatment via CPLLs. However, by diluting the sample to barely 0.1% SDS and adding a larger excess of surfactants compatible with CPLLs (up to 1% of Triton X 100 or CHAPS) this denatured sample is now amenable to CPLL capture. This is illustrated in Figure 1. Thus, by subjecting the sample to this double capture, under native and denaturing conditions, the number of proteins that can be identified increases substantially to well above 1000 species, a major increment over the past. There are more ways for both capturing and eluting proteins from CPLL beads as reviewed by Boschetti and Righetti [2]. The readers are thus referred to this book for detailed treatments on any possible methodology related to CPLLs.
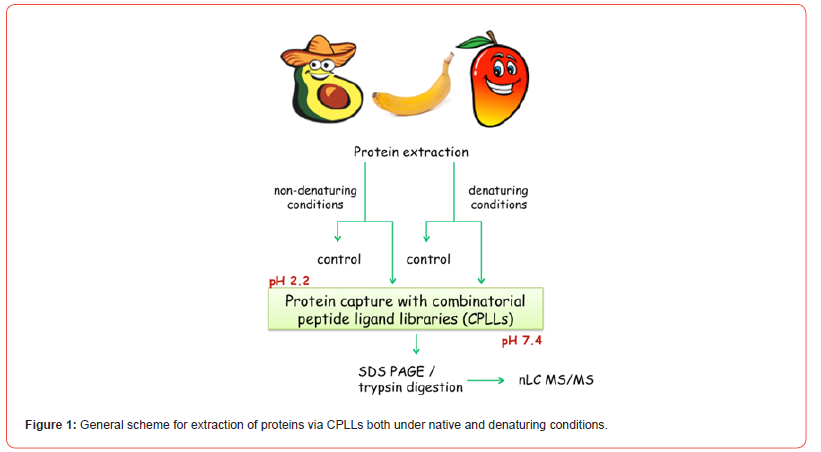
Banana Proteomics
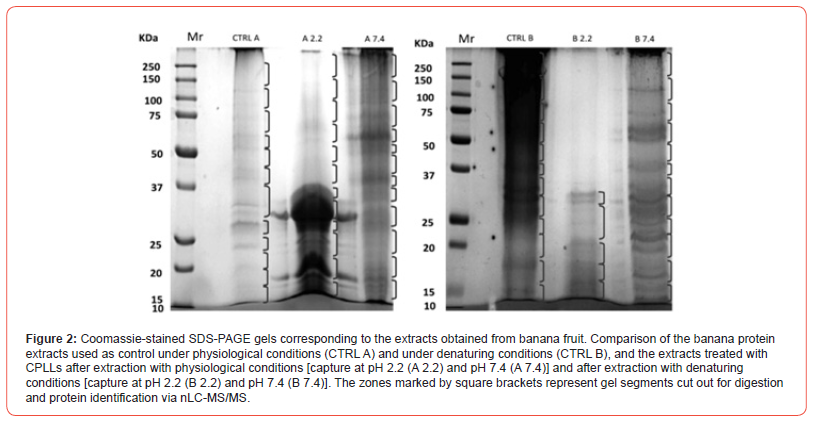
The first tropical fruit investigated, among the “recalcitrant tissues”, was the banana. Musa spp., comprising banana and plantain, is grown extensively in many developing countries and is considered to be one of the most important sources of energy in the diet of people living in tropical humid regions. Due to its antioxidant and cell anti-proliferative activities, the consumption of banana has been associated with reduced risk of chronic diseases such as cardiovascular diseases and cancer [18]. Up to date, no in-depth work has been focused on identifying the banana fruit proteome; since fresh banana pulp contains approximately 20% of carbohydrates and only 1% of proteins, this fruit has been traditionally considered as a difficult matrix for protein extraction [19]. Fruits, as every biological source, contain highly-abundant proteins, which are often of limited interest for proteome analysis, whereas other proteins may be orders-of-magnitude less abundant, although still of high importance. Here too, by using CPLLs, advanced mass spectrometry techniques and Musa mRNAs database in combination with Uniprot_ viridiplantae database, we could identify 1131 proteins [20]. Among this large amount of species found, several already known allergens such as musa a 1, pectinesterase, superoxide dismutase and potentially new allergens have been detected. Additionally, several enzymes involved in degradation of starch granules and strictly correlated to ripening stage were identified. These results constitute the largest description so far of the banana proteome. Figure 2 summarizes the data here discussed. The upper left side gel strips represent the SDS-PAGE profiling of a sample prior and after CPLL capture. The Venn diagrams exhibit the proteins IDs as obtained in the control and in the CPLL treated sample and using CPLLs under native and denatured conditions. The GO graph on the right side displays the major Gene Ontology categories in which the various species have been classified. The use of CPLLs more than doubled (from 452 to 1131) the number of identified proteins.
Avocado Proteomics
Avocado, the fruit of the tropical tree Persea americana, native to Mexico, is nowadays grown and consumed in many parts of the world. The oil obtained from pressing the avocado fruit, already used in Mexican folk medicine in the XVI century [21], is nowadays employed for manufacturing foodstuff, cosmetics and health care products [22]. Besides this, avocado oil has been proposed as a domestic source of cooking oil to help improve the nutritional status of population in some developing countries. The consumption of both, the avocado fruit and oil, has been associated with health benefits such as the decreases of total serum cholesterol, LDL-cholesterol and triglycerides [23], the control of blood pressure and the inhibition of certain types of cancer [24]. The avocado fruit composition has been deeply studied, leading to good characterization of smallsize compounds such as fatty acids and sterols. However, up to the present, no work had been focused on identifying the avocado pulp proteome; only some reports have appeared on the avocado seed [25] and root [26] proteins, with a total identification of proteins of the order of a few dozens.
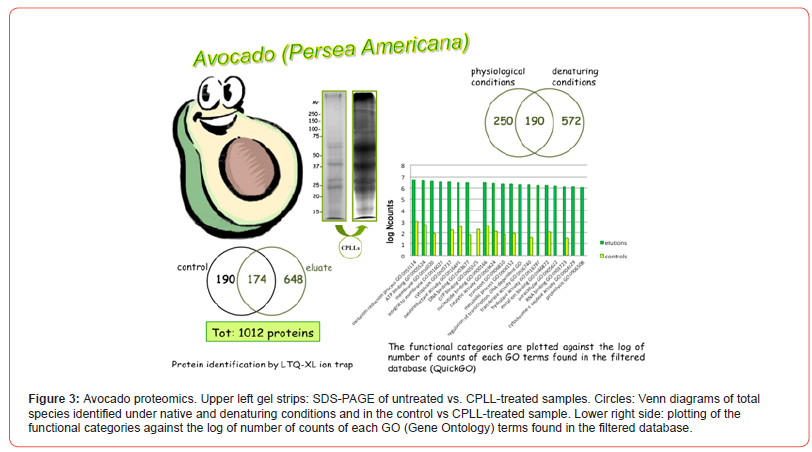
On the contrary, by using the CPLL approach, the total number of unique gene products identified amounted to 1012 proteins, of which 174 were in common with the control, untreated sample, 190 were present only in the control and 648 represented the new species detected via CPLLs of all combined eluates, likely representing low-abundance proteins. Among the 1012 proteins it was possible to identify the already known avocado allergen Pers a 1 and different proteins susceptible to be allergens like a profilin, a polygalacturonase, a thaumatin-like protein, a glucanase, and an isoflavone reductase-like protein. Figure 3 summarizes the data here discussed. The upper left side gel strips represent the SDS-PAGE profiling of the samples prior and after CPLL capture. The Venn diagrams exhibit the proteins IDs as obtained in the control and in the CPLLs treated sample and by using CPLLs under native and denatured conditions. The GO graph on the right side displays the major Gene Ontology categories in which the various species have been enriched. In this case, application of CPLLs technique almost tripled (from 364 to 1012) the number of identified proteins, many of them observed under denaturing conditions. Moreover, it can be appreciated that, in all GO categories, the number of protein species identified after CPLL capture is much higher than in the controls. Additionally, in the CPLL eluates, three novel categories, not represented in the control, could be detected [27].
Mango Proteomics
The mango is a fleshy stone fruit belonging to the genus Mangifera, consisting of numerous tropical fruiting trees in the flowering plant family Anacardiaceae. It is native to South Asia, wherefrom it has spread worldwide to become one of the most cultivated fruits in the tropics. Among the different species Mangifera indica – the ‘common mango’ or ‘Indian mango’ – is the only mango tree commonly cultivated in many tropical and subtropical regions. It is the national fruit of India, Pakistan and the Philippines, and the national tree of Bangladesh. The ripe fruit varies in size and colour. Cultivars are typically yellow, orange, red or green, and carry a single flat, oblong pit that can be fibrous or hairy on the surface, and which does not separate easily from the pulp. Mango is used to make juices, smoothies, ice cream, fruit bars, raspados, aguasfrescas, pies and sweet chili sauce, or mixed with chamoy, a sweet and spicy chili paste. In Central America, mango is either eaten green mixed with salt, vinegar, black pepper and hot sauce, or ripe in various forms. Some people also add soy sauce or chili sauce. Pieces of mango can be mashed and used as a topping on ice cream or blended with milk and ice as milkshakes.
In mango fruit pulp, the antioxidant vitamins A and C, vitamin B6 (pyridoxine), folate, other B vitamins and essential nutrients, such as potassium, copper and amino acids, are present. Mango peel and pulp contain other compounds, such as carotenoids and polyphenols, and omega-3 and -6 polyunsaturated fatty acids [28]. Additionally, mango peel pigments seem to have important biological effects [29], including carotenoids, such as the provitamin A compound, beta-carotene, lutein and alpha-carotene [30], polyphenols [31] such as quercetin, kaempferol, gallic acid, caffeic acid, catechins, tannins, and the unique mango xanthonoid, mangiferin [32], any of which may counteract free radicals in various disease processes. Notwithstanding all data reported above on mango metabolites, not much is known on this fruit proteome. Only a two-dimensional (2D) mango pulp analysis has been reported, aiming at identifying modulation of protein expression associated with ripening [33]. A total of 373 spots could be visualized in the 2D map, leading to the identification of 51 unique gene products. In another report, via database searches of mango-derived ESTs and proteins along with proteins from six other closely related plant species, Renuse et al. [34] could identify 1001 peptides that matched to 538 proteins. However, this set of proteins applied to mango leaves, not to pulp nor peel.
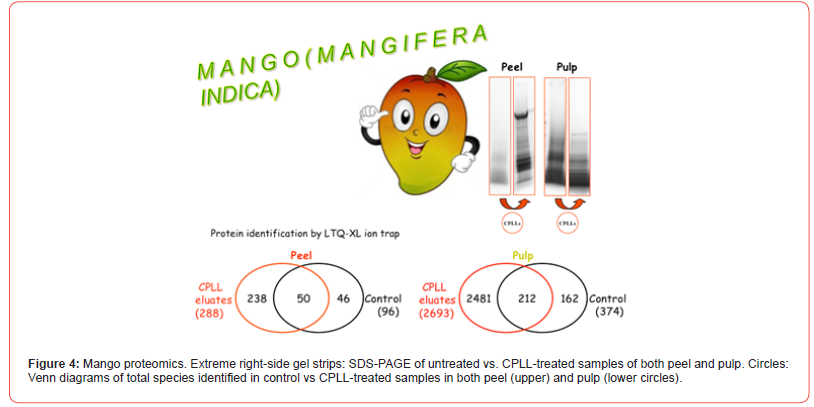
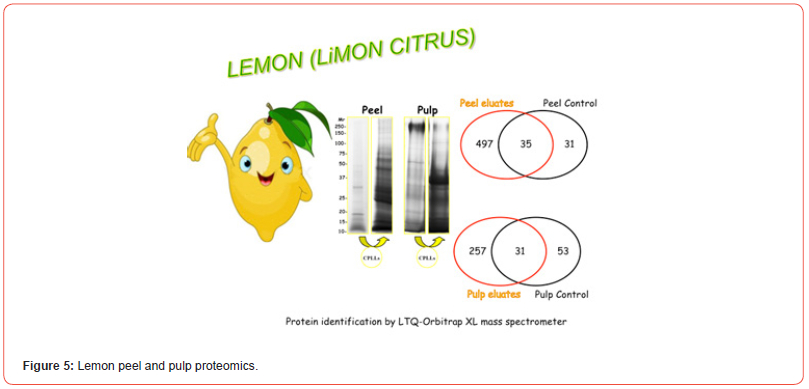
In the case of this fruit, our research has taken a sudden twist: we have also explored the proteome of the peel, since this part of the fruit is largely used in the Far East cuisine to flavor plenty of dishes. Additionally, we have also captured its peptidome, after protein removal, via C18 resins [35]. This idea of also exploring the peel proteome of fruits has been extended to other plant samples as well. For instance, we have reported the proteome of lemon peel (just the flavedo), since this part of the fruit is used, in the Mediterranean area, not only for flavouring dishes, but also for producing a very popular liqueur called, aptly, “Limoncello”, indeed an infusion of the lemon flavedo [36]. Recently, we have applied this strategy also to the peels of oranges and clementines since these skins too are used in cuisine. By performing the capture here too under both native and denaturing conditions, a total of 334 unique protein species have been identified in the peel vs. 2855 in the pulp, by acting at two different pH values (2.2 and 7.2). These data are presented in Figure 4, where the gel strips on the right-side exhibit SDS-PAGE profiles prior and after CPLL capture and the Venn diagrams show the total species identified in both peel and pulp. The GO graph of Figure 5 displays the major Gene Ontology categories in which the various species have been classified in both compartments. It is of interest to note that, although in the peel only a bit more than 10% of the proteins detected in the pulp are present, yet at least 8 GO categories are unique to this organ and apparently absent in the pulp, suggesting that they have a special biological role confined to the skin. Conversely, in the pulp, another 8 GO categories appear which do not seem to have a counterpart in the peel. In regard to potential mango’s allergies, the responsible allergens have not yet been identified, due also to the fact that the mango’s proteome is not completely known because its genome has not yet been sequenced. For this reason, in allergens databases we could not find specific proteins referred to mango but in our identified species we could verify the presence of well-known allergens, referred to the same plant order (Sapindales), belonging to the same taxonomic group (Plantae Magnoliopsida). By consulting the IUIS allergen nomenclature databases (http://www.allergen.org/), we obtained a list of allergens, of which some are present in our list. In particular in CPLLs eluates we recognized: non-specific lipid transfer protein, superoxide dismutase, germin-like protein and profilin.
Discussion
We hope that the data here summarized will show the unique potential of CPLLs in detecting those low-abundance species sorely missing the roll call in plant proteomics. It can also be appreciated that, via the use of CPLLs, coupled to modern MS instrumentation, also in plant proteomics an important step forward has been made, permitting, in a single sweep, identifications ranging now in the thousands, vs. barely in the hundreds up to recent times. Yet, we cannot ignore that mammalian proteomics is quite ahead as compared to plant proteomics, since in this domain it is not uncommon to see papers reporting at once IDs above 10 thousands in a single run (another important reason, of course, is the fact that mammalian genomes, and especially the human genomes, have been much more extensively sequenced and annotated than plant genomes).
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Anderson NL, Anderson NG (2002) The human plasma proteome: History, character, and diagnostic prospects. Mol Cell Proteomics 1: 845-867.
- Boschetti E, Righetti PG (2013) Low-Abundance Protein Discovery: State of the Art and Protocols; Elsevier: Amsterdam, pp. 1-345.
- Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, et al. (2003) The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics 3: 1345-1364.
- Fang X, Zhang WW (2008) Affinity separation and enrichment methods in proteomic analysis. J Proteomics 71: 208-303.
- Tu C, Rudnick PA, Martinez MY, Cheek KL, Stein SE, et al. (2010) Depletion of abundant plasma proteins and limitations of plasma proteomics. J Proteome Res 9: 4982-4991.
- Shen Y, Kim J, Strittmatter EF, Jacobs JM, Camp DG, et al. (2005) Characterization of the human blood plasma proteome. Proteomics 5: 4034-4045.
- Widjaja I, Naumann K, Roth U, Wolf N, Mackey D, et al. (2009) Combining sub-proteome enrichment and RuBisCO depletion enables identification of low abundance proteins differentially regulated during plant defense. Proteomics 9: 138-147.
- Boschetti E, Lomas L, Citterio A, Righetti PG (2007) Romancing the “hidden proteome”, Anno Domini two zero zero six. J Chromatogr A 1153: 277-290.
- Righetti PG, Boschetti E, Lomas L, Citterio A (2006) Protein Equalizer technology: The quest for a “democratic proteome”. Proteomics 6: 3980-3992.
- Righetti PG, Boschetti E, Kravchuk AV, Fasoli E (2010) The proteome buccaneers: How to unearth your treasure chest via combinatorial peptide ligand libraries. Expert Rev Proteomics 7: 373-385.
- Agrawal GK, Sarkar A, Righetti PG, Pedreschi R, Carpentier S, et al. (2013) A decade of plant proteomics and mass spectrometry: Translation of technical advancements to food security and safety issues. Mass Spectrom Rev 32: 335-365.
- Boggess MV, Lippolis JD, Hurkman WJ, Fagerquist CK, Briggs SP, et al. (2013) The need for agriculture phenotyping: “Moving from genotype to phenotype”. J Proteomics 93: 20-39.
- Jorrín-Novo J, Valledor Gonzalez L (2013) Special Issue: Translational Plant Proteomics. J Proteomics 93: 1-368.
- Nakamura R, Teshima R (2013) Proteomics-based allergen analysis in plants. J Proteomics 93: 40-49.
- Lubica Uvackova L, Skultety L, Bekesova S, McClain S, Hajduch M (2013) The MSE-proteomic analysis of gliadins and glutenins in wheat grain identifies and quantifies proteins associated with celiac disease and baker’s asthma. J Proteomics 93: 65-73.
- Agrawal GK, Timperio AM, Zolla L, Bansal V, Shukla R, et al. (2013) Biomarker discovery and applications for foods and beverages: Proteomics to nano-proteomics. J Proteomics 93: 74-92.
- Geiger T, Wehner A, Schaab C, Cox J, Mann M (2012) Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics 11: M111.014050.
- Sun J, Chu YF, Wu X, Liu RH (2002) Antioxidant and anti-proliferative activities of common fruits. J Agric Food Chem 50: 7449-7454.
- Saravanan RS, Rose JKC (2004) A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 4: 2522-2532.
- Esteve C, D’Amato A, Marina ML, Concepción García M, Righetti PG (2013) In-depth proteomic analysis of banana (Musa spp.) fruit with combinatorial peptide ligand libraries. Electrophoresis 34: 207-214.
- Argueta-Villamar A, Cano L, Rodarte M (1994) Atlas de las Plantas de la Medicina Tradicional Mexicana. Ed Instituto Nacional Indigenista, México, pp. 55.
- Swisher HO (1993) Avocado oil from food use to skin care. J Am Oil Chem Soc 65: 1704-1706.
- Lopez-Ledesma R, Frati-Munari AC, Hernandez-Dominguez BC, Cervantes-Montalvo S, Hernandez-Luna MH, et al. (1996) Monounsaturated fatty acid (avocado) rich diet for mild hypercholesterolemia. Arch Med Res 27: 519-523.
- Lu QY, Arteaga JR, Zhang Q, Huerta S, Go VLW, et al. (2005) Inhibition of prostate cancer cell growth by an avocado extract: role of lipid-soluble bioactive substances. J Nutr Biochem 16: 23-30.
- Sánchez-Romero C, Perán-Quesada R, Barceló-Muñoz A, Pliego-Alfaro F (2002) Variations in storage protein and carbohydrate levels during development of avocado zygotic embryos. Plant Physiol Biochem 40: 1043-1049.
- Acosta-Muñiz CH, Escobar-Tovar L, Valdes-Rodriguez S, Fernandez-Pavia S, Arias-Saucedo LJ, et al. (2012) Identification of avocado (Persea americana) root proteins induced by infection with the oomycete Phytophthoracinnamomi using a proteomic approach. Physiol Plant 144: 59-72.
- Esteve C, D'Amato A, Marina ML, García MC, Righetti PG (2012) Identification of avocado (Persea americana) pulp proteins by nanoLC-MS/MS via combinatorial peptide ligand libraries. Electrophoresis 33: 2799-2805.
- Ajila CM, Prasada Rao UJ (2008) Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L. peel extract Food Chem Toxicol 46: 303-309.
- Berardini N, Fezer R, Conrad J, Beifuss U, Carle R, et al. (2005) Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol-O - and xanthone C-glycosides, anthocyanins, and pectin. J Agric Food Chem 53: 1563-1570.
- Gouado I, Schweigert FJ, Ejoh RA, Tchouanguep MF, Camp JV (2007) Systemic levels of carotenoids from mangoes and papaya consumed in three forms (juice, fresh and dry slice). Eur J Clin Nutr 61: 1180-1188.
- Singh UP, Singh DP, Singh M, et al. (2004) Characterization of phenolic compounds in some Indian mango cultivars. Int J Food Sci Nutr 55: 163-169.
- Andreu GL, Delgado R, Velho JA, Curti C, Vercesi AE (2005) Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium-induced permeability transition. Arch Biochem Biophys 439: 184-193.
- De Magalhães Andrade J, Torres Toledo T, Beserra Nogueira S, Cordenunsi BR, Lajolo FM, et al. (2012) 2D-DIGE analysis of mango (Mangifera indica L.) fruit reveals major proteomic changes associated with ripening. J Proteomics 75: 3331-3341.
- Renuse S, Harsha HC, Kumar P, Acharya PK, Sharma J, et al. (2012) Proteomic analysis of an unsequenced plant-Mangifera indica. J Proteomics 75: 5793-5796.
- Fasoli E, Righetti PG (2013) The peel and pulp of mango fruit: a proteomic samba. Biochim Biophys Acta 1834: 2539-2545.
- Fasoli E, Colzani M, Aldini G, Citterio A, Righetti PG (2013) Lemon peel and Limoncello liqueur: A proteomic duet. Biochim Biophys Acta 1834: 1484-1491.
-
Pier Giorgio Righetti*, Clara Esteve, Alfonsina D’Amato, Elisa Fasoli, María Luisa Marina and María Concepción García. Proteomics of Some “Recalcitrant” Tropical Fruits: Avocado, Banana and Mango. World J Agri & Soil Sci. 8(4): 2023. WJASS. MS.ID.000692.
-
Low abundance proteome, Tropical fruits, Allergens, Combinatorial peptide ligand libraries, Mass spectrometry
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






