 Research Article
Research Article
Phytoremediation Potential of Ficus benjamina on Soils Contaminated with Crude Oil in Rivers State, South-South Nigeria
ME Ikiriko* and JA Chukwumati
Department of Crop and Soil Science, University of Port Harcourt, Rivers State, Nigeria
Corresponding AuthorMiebaka Emmanuel, Department of Crop and Soil Science, University of Port Harcourt, Rivers State, Nigeria.
Received Date:February 03, 2023; Published Date:February 21, 2023
Abstract
The primary energy source used today is crude oil, demand for it is anticipated to rise in the years to come. Due to its intensive exploitation, there may be more environmental incidents, such as spills and leaks, and there may be more environmental liabilities as a result of refining. The aim of this research was to investigate the phytoremediation potential of ficus benjamina on soils contaminated with crude oil. 10 kg of soil was manually contaminated with crude oil, the experiment consists of four treatments 0% (control), 2% (160ml), 4% (320ml) and 6% (420ml) of crude oil on a weight/volume basis with three replications, the stem of ficus benjamina plant cultivated on the contaminated soils for three months. There was significant reduction of the root dry matter weight from (6.7 -3.4 g/plant) and the shoot dry matter weight (5.4 -2.4g/plant) irrespective of the treatment, the soil pH increased significantly from 5.69 to 6.29 with increased levels of crude oil contamination. translocation factors for Cd, Ni and Pb were greater than 1 irrespective of the treatments except for Cd at 6% level of crude oil, the bioconcentration factor for Cd, Ni and Pb were also greater than 1. The total petroleum hydrocarbon loss was significantly reduced from 2.71 to 0.87 %. The implication of this study has highlighted the potential of ficus benjamina to be used for phytoremediation.
Keywords:Heavy metals; Crude oil; Translocation factor; Bioconcentration factor; Total petroleum hydrocarbon; Ficus benjamina
Introduction
Crude oil is a quick and easy source of energy that enhances standard of living and increases the bar for a living. It is naturally found in the United States, Russia, Iran, Mexico, Iraq, Saudi Arabia, Kuwait, Libya, and Nigeria, to name a few countries [1]. The oil and gas sectors produce billions of tons of crude oil, natural gas, and derivatives each year. All of these are then refined to generate refined commodities such as diesel, gasoline, petrol, and lubricants [2].
Increased oil exploration and exploitation prospecting in Rivers state of Niger Delta region, Nigeria has resulted in a hitherto unseen inadvertent leak of crude oil, contaminating the region’s land and water sources. Furthermore, illegal tampering with wellheads, flow lines, pipelines, manifolds, and flow stations has increased the overall volume of crude oil that has entered the environment. Because of the regular reports of oil spills in the Niger Delta, a practical technique to clean up crude oil-contaminated land is necessary. When crude oil is spilled on land, it affects the physicochemical properties of the soil, such as its temperature, structure, nutrient status, and pH (Udeh et al., 2013).
The issue of soil and water contamination by crude petroleum and refinery products has been on the rise in Nigeria as oil mining and refining activities have increased [3]. Oil-producing areas of Ni geria are particularly affected by this problem, which results in a loss of soil fertility and crop death. Clean-ups of contaminated sites due to crude oil spills are a widespread environmental problem [4].
Reis (1996) identifies three primary sources of petroleum contamination: the disposal of oil-based waste, blowouts of wells, and ruptures of pipelines. In the presence of crude oil spills, plants are adversely affected since essential nutrients like nitrogen and oxygen are unavailable to them. There has been evidence that oil contamination slows down plant germination. As reported by Adam and Duncan [5], this effect is probably due to the oil serving as a barrier preventing seeds from gaining access to water and oxygen.
Three types of soil remediation techniques are frequently distinguished: chemical, physical, and biological. To break down contaminants, physical methods apply heat, washing agents, and air, whereas chemical methods use oxidizing or reducing agents [6]. The biological treatment makes use of bacteria present in the soil or attached to the roots of plants as well as specific strains of bacteria introduced into the soil to break down the contaminants. Physical and chemical methods are fast but can be intrusive, resulting in changes in soil properties. The biological method though comparatively slower, does not alter the soil properties and produce harmful intermediates as with physical and chemical remediation [6]. Bioremediation, mainly phytoremediation has been gathering attention because it is inexpensive and uncomplicated in the application. Plants have been shown to mineralize heavy metals and decompose petroleum hydrocarbons via their profuse root system. The mechanisms involved in phytoremediation typically comprise of phytoextraction, phytodegradation, phytovolatilization, phytostabilization, and rhizodegradation [7]. Phytoremediation is an in-situ soil treatment that reduces contaminants as plants grow. The selection of plant species capable of surviving in and remediating soil contaminated with target contaminants is crucial [8].
The contaminated sites should have plants appropriate for the climate and soil conditions for phytoremediation (Pivet, 2001). Additionally, plants should be able to tolerate stress conditions [9]. Grass, legumes, trees, as well as a number of other monocots and dicots, have all been utilized as phytoremediation drivers [9]. In Nigeria, efforts have been undertaken to employ various plants to clean up oil pollution [10-12].
The bio-concentration and translocation factors are typically used to assess the phytoremediation potential of any plant species to remove pollutants from the soil (Eisazadeh et al. 2018; Khalid et al., 2018 and Cano-Ruiz et al., 2020). The bio-concentration factor illustrates the relationship between the linked plant species and the contaminants in the soil (BCF). By comparing the concentration of contaminants in plant shoots and roots to those in the soil, respectively, BCF calculates the chance of a plant acquiring metals in its shoots and roots at a certain level of metal contamination (Mc- Grath and Zhao 2003; Zhen et al. 2017).
Ficus benjamina (The weeping fig,) is a member of the Moraceae family and is a native to Asia and Australia. It is a well-known decorative plant that thrives in moderate temperatures but can endure both low and hot temperatures [13] sold as houseplants, generating financial gains and reducing the cost of eventual disposal significantly [14].
Ficus benjamina used in this study has the advantages of high biomass, deep roots, easy cultivation, fierce competition and broad geographic dispersion. The impact of crude oil on the potential Ficus benjamina for phytoremediation, however, has not been shown in any prior studies. Therefore, the goal of this study is to assess the bioconcentration and translocation factor as well as its’ capacity to biodegrade soil contaminated with crude oil.
Materials and Methods
Soil preparation and pot experiments
Surface soil samples were collected with the aid of a soil auger at a depth of 0-30cm behind the Department of Crop and Soil Science Building, University of Port Harcourt (latitude 4°.91cN and Longitude 6°.92cE), Rivers state, Nigeria. The crude oil was sourced from the Nigeria National Petroleum Corporation (NNPC), Eleme, Rivers State, Nigeria. 8kg of Soil was weighed with an analytical weighing balance. The soil samples were contaminated with different levels of crude oil. 0% (control), 2% (160ml), 4% (320ml), and 6% (420ml) of crude oil respectively on a volume per weight.
The experiment consists of four treatments [0% (control), 2% (160ml), 4% (320ml), and 6% (420ml) of crude oil] replicated three times in a complete randomized design. The contaminated soils were allowed to stay for two weeks before planting. This is to allow the volatile components of hydrocarbon to be volatilized, and the experiment lasted for three months under a screen house condition.
Collection and preparation of soil samples for laboratory analysis
After 3 months of planting, the plant tissues/root, stem and shoot at the soil surface were collected and washed under running tap water and air-dried. The plants’ parts were partitioned into shoots and root systems and oven-dried. The soil samples were collected from each pot and air-dried at room temperature before being taken to the laboratory for chemical analysis. The following physical and chemical properties of the soil were analyzed before and after treatments. Total Organic Carbon (TOC), Total Petroleum Hydrocarbon (TPH), Lead (Pb), Cadmium (Cd), and Nickel (Ni) in the soil and plants were analyzed.
The total organic carbon was evaluated using was evaluated using oxidation by the dichromate digestion method [4], pH was measured in 1:1 soil/water ratio using pH meter with glass electrode [16], the heavy metals ( Pb, Cd and Ni) both in the soil and plant tissues were analysed according to the method described by [17].
Determination of total petroleum hydrocarbon in soil
The soil samples were air-dried and sieved through 1mm mesh, the TPH in the soil was first extracted with the n-hexane by shaking with a mechanical shaker for 30 minutes as was described by Okolo et al (2005). The soil crude oil-n-hexane mixture was filtered into a beaker of known weight through a Whatman No. 1 filter paper. The TPH content of the filtrate was determined after heating the beaker at 40oC to a constant weight [18]. The amount of TPH lost from the soil was determined after as the initial amount of TPH in the soil minus that in the soil at the time of analysis. The total Petroleum Loss as calculated using the formula below

Translocation factor
The Translocation Factor is also called shoot root quotient. It explains the ability of the plants to translocate the metal from the roots through shoots and leaves of a plant which is primarily responsible for phytoextraction. It is determined by calculating the ratio of elements present in the plants shoot compared to that in the plant’s roots. (Qihang et al., 2011). The translocation factor (TF) evaluates plants ability to translocate heavy metals from the roots to the harvestable aerial part [19], value of TF > 1 indicates that the plant translocate metals effectively from the root to the shoot. The Translocation factor can be calculated using the formula below.

Bioconcentration factor
The Bioconcentration Factor (BCF) was determined through the ratio of concentration of metal in the roots to that in the soil. BCF was used to estimate the plant ability to accumulate metal in the roots as to determine the potentials of the ornamental plants for phytoremediation (Yoon et al., 2006). The Bioconcentration factor can be calculated using the formula below.

Statistical analysis
The data generated were subjected to analysis of variance using the Genstat 19th Edition to generate the treatment means by the Fisher’s LSD (Least significant difference) test at 5% probability.
Results and Discussion
Selected Heavy metals, TOC and TPH Before planting
The concentration of heavy metals in the soil varied from 3.44 to 141.74 mg/kg for Ni, 77.196 to 214.28 mg/kg for Pb, 23.01 to 95.81 mg/kg for Cd, 1.5 to 6.4 mg/kg for TOC, and 0.02 - 867.30 mg/kg for TPH, respectively (Table 1). It was discovered that the high levels of cadmium in the control soil could have been caused by erosion, anthropogenic activities like the application of fertilizers (single superphosphate fertilizer), and construction activities near the study area. It was also discovered that the concentration of the heavy metals, TOC, and TPH increased with an increase in the amount of crude oil before planting, the WHO (1996) stated that the lead and nickel levels in the control soils were within permitted levels.
Table 1:Soil chemical parameters before planting.
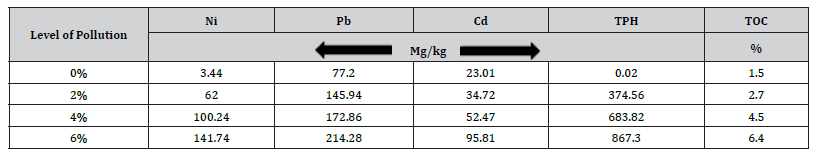
TOC; Total Organic Carbon, TPH; Total Petroleum Hydrocarbon. Cd; Cadmium, Ni; Nickel, Pb; Lead.
Influence of Crude Oil on Selected Heavy Metals, TOC after planting
The concentrations of cadmium, nickel and lead were significantly (p<0.05) decreased in all the crude oil contaminated soils by Ficus benjamina. The lowest concentration varied from 1.20 mg/ kg for Cd and 0.43 mg/kg for Ni at 4% crude oil respectively. The lowest lead concentration was recorded at 2% crude oil. The pH of the soil ranged from 5.69 (control) to 6.29 at 6% contaminated, and it dropped as the level of crude oil increased, proving that more crude oil tends to cause soil pH to drop. However, there is a positive link between crude oil level and soil pH and it has been reported that raising the level of crude oil in the soil causes an increase in soil pH [12]. The pH was thought to be decreased by organic acid produced by the degradation of crude oil during phytoremediation [18] (Table 2).
Table 2:Effects of crude oil on Cd, Ni, Pb, TOC and pH in soil after the experiment.
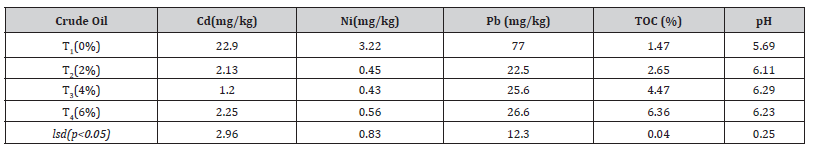
Cd; Cadmium, Ni; Nickel, Ni; Nickel, Pb; Lead, TOC; total organic carbon, T1; control, T2; 2% crude oil; T3; 4% crude oil and T4; 6% crude oil.
With increasing levels of crude oil pollution, the total organic carbon content of the soil increased significantly (p <0.05) and varied from 1.47 in control(0%) to 6.36 at 6% level of contamination.
Influence of Crude oil on Concentration of some selected heavy metals in plant tissue
Plants cultivated in soils rich in metals absorb metal ions to variable degrees. The availability of metals, which is controlled by both soil-associated and plant-associated variables, is a significant role in how readily plants absorb metals. Poor relationships between metal concentrations and plant metal uptake have been found in the majority of earlier research [11].
With increasing levels of crude oil contamination, Cd, Ni, and Pb content reduced significantly (p<0.05) in root and shoot systems. For both the root and the shoot, the three heavy metal concentrations were found to be at their greatest levels at 2% crude oil contaminate (Table 3). The shoot Cd, Ni, and Pb contents varied from 0.60 to 11.81, 66.2 to 153, and 91.6 to 192.7, respectively, with the control having the lowest 0% and 6% levels crude oil, but the root Cd, Ni, and Pb ranged from 0.43 to 7.3, 31.7 to 211.8, and 79.4 to 164.2, respectively. The Cd and Pb contents were higher in the shoot than root in plants grown on soil contaminated with different levels of crude oil, but the Ni content of the root was higher than in the shoot. However, there was no significant difference in Ni content in the plant’s shoot.
Table 3:Concentration of Cd, Ni, and Pb in Ficus benjamina. at different percentages of crude oil.
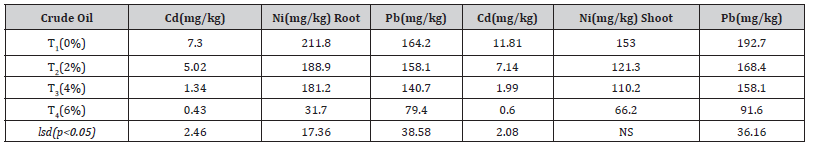
Cd; Cadmium, Ni; Nickel, Ni; Nickel, Pb; Lead, TOC; total organic carbon, T1; control, T2; 2% crude oil; T3; 4% crude oil and T4; 6% crude oil.
Mechanistic insights into tolerance and toxicity are provided by the accumulation of Cd, Ni, and Pb in root and shoot systems [20]. The distribution of Ni in the roots and shoots indicates that Ni accumulates mainly in the roots while Cd and Pb accumulate in the shoots. It is similar to Yaping et al., (2019) who found that root accounts for more than 70% of Ni in Althaea rosea Cavan roots. Because of the interactive role that the root system plays between environmental pollutants and plants, the majority of heavy metals that enter the plant are first retained in the root cells, greatly reducing the displacement of the organ to the ground and shielding the leaf tissue from damage from heavy metals (Tauqeer et al. 2016) (Table 4).
Table 4:Biomass, Translocation factor, and Bioconcentration factor of Ficus benjamina.
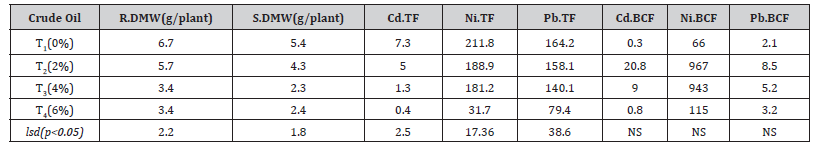
DMWR; root dry matter weight, SDMW; shoot dry matter weight, Cd.TF; cadmium translocation factor, Ni.TF; nickel translocation factor,Pb.TF; lead translocation factor, CdBCF; cadmium bioconcentration factor, Ni.BCF; nickel bioconcentration factor, Pb.BCF; lead bioconcentration factor T1; control,T2; 2% crude oil; T3; 4% crude oil and T4; 6% crude oil contamination.
When plants are exposed to heavy metals at excessive concentrations, several of the plant’s metabolic processes suffer severe damage, eventually leading to the plant’s death. According to Gadd [21], excessive metal exposure inhibits physiologically critical enzymes, deactivates photosystems, and harms mineral metabolism [22]. As previously stated, a high removal rate of harmful heavy metals is heavily dependent on the biomass of the plant used in the phytoremediation process.
Plants appeared healthy in the control and soil contaminated with a low percentage of crude oil in this experiment, however, plants growing in soils with higher quantities of crude oil displayed yellowing to browning of the leaves and stunted growth. There were also greater changes in shoot and root biomass between the same plant growing on soil contaminated with varying amounts of crude oil (Table 4). Crude oil was more effective in reducing plant biomass of growing plants when compared to the same plants grown on control and 2% crude oil. The dry weights of the plant were reduced by 49.7 % in the root and by 55.9 % in the shoot when cultivated on soil laced with 6% crude oil (Table 4).
Table 5:Total petroleum hydrocarbon and total petroleum hydrocarbon loss from the soil.
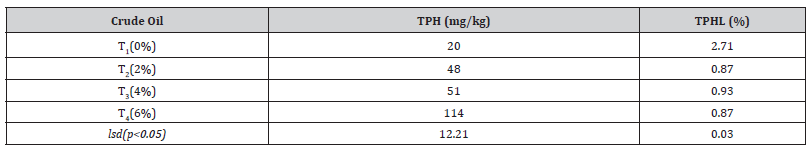
LSD; least significant difference, T1; control, T2; 2% crude oil; T3; 4% crude oil and T4; 6% crude oil, TPH; total petroleum hydrocarbon and TPHL; total petroleum hydrocarbon loss.
The Ficus benjamina development and metabolism were influenced variably by increased percent crude oil stress, which resulted in stunted growth, lower biomass output, and created recognizable visual symptoms comparable to those documented by previous workers in different plant species (Zhou and Qiu, 2005; Gajewska and Sklodowska, 2007). A considerable increase in crude oil levels in plant tissue supports these findings. The observed decrease in dry matter production due to metal stress is consistent with previous findings in other plants (Pandey and Pathak, 2006; Ryser and Sauder, 2006) [23,24].
Translocation and bioconcentration parameters are employed to evaluate plant phytoremediation capacity, according to Wei and Zhou (2004). The use of bioaccumulation and translocation factors has proven to be useful methods for determining plant metal uptake capabilities. The translocation factor (TF) values for Cd, Ni, and Pb ranged from 7.3 to 0.4, 211.8 to 31.7, and 164.2 to 79.4 correspondingly, with the TF decreasing significantly (p<0.05) as the quantity of crude oil in the soil increased. Although the TF value for Cd at 6% crude oil was less than one, it was the lowest of the three heavy metals (Table 4). The bioconcentration factors (BCF) for Cd, Ni, and Pb varied from 20 to 0.3, 967 to 66, and 8.5 to 2.1, respectively [25].
Except for Cd at 6% crude oil, both the BCF and TF values are larger than 1; the elevated BCF and TF could be related to Cd, Ni, and Pb mobility in the soil, which implies they are less absorbed by the soil and hence more freely available to plants (Kashem et al. 2007). Pollutant uptake is also influenced by environmental factors, pollutant chemical properties, soil conditions, and plant type (Noman and Aqeel, 2017). The low Cd BCF could be attributed to Ficus benjamina’s restricted ability to deposit Cd metal under current environmental conditions. According to Yoon et al., plants having BCF values greater than one is optimal for phytoextraction (2006).
Crude oil pollution is becoming more of a problem, affecting several oil-producing countries, most notably Nigeria. Crude oil contamination has harmed plants and the environment (Odukayo et al., 2019). Although crude oil is mostly made up of aliphatic and aromatic molecules, it also contains trace elements like nickel, iron, and aluminium, as well as heavy metals like lead, cadmium, and chromium (Wilberforce, 2016) [26]. Each treatment’s starting TPHL level was greater than the end TPHL level (p0.05). The TPH loss decreased as crude oil added to the soil rose. According to research, plants use many ways to improve crude oil remediation, including degradation, rhizospheric action, containment, and transfer of volatile components. Ficus benjamina’s possible method for enhancing TPH removal from the soil in this study could be one or a mixture of both.
Conclusion
This study aimed to evaluate the phytoremediation potential of ficus benjamina on crude oil-contaminated soil. The results showed that total petroleum hydrocarbon was successfully degraded to 0.87 % at 6% crude oil contamination. The phytoremediation potential parameters (BCF and TF) were all greater than one except for Cd at 6% crude oil contamination. There are few research and publications on Ficus benjamina’s bioaccumulation, phytoremediation capabilities, and heavy metal uptake capacity. As a result, this study may serve as a foundation for future research to increase the plant’s efficiency and capability in the heavy metal buildup and crude oil remediation.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Ezeji U, Anyadoh SO, Ibekwe VI (2007) Cleanup of crude oil-contaminated soil. Terrestrial and Aquatic Environmental Toxicology 1(2): 54-59.
- Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011: 941810.
- Vidal J (2010) Impact of Oil Spill along Nigerian Coast. Journal of Environmental Science 30(5): 22-26.
- Bundy JG, Paton GI, Campbell CD (2002) Microbial communities in different soil types do not converge after diesel contamination. J Appl Microbiol 92(2): 276-288.
- Adam GI, Duncan H (2002) Influence of diesel on seed germination. J Environ Pollut 120(2): 363-370.
- Adetitun D, Akinmayowa V, Atolani O, Olayemi A (2018) Biodegradation of jet fuel by three Gram negative Bacilli isolated from kerosene contaminated soil. Pollution 4(2): 291-303.
- Tang KHD, Juan A (2018) Phytoremediation of crude oil-contaminated soil with local plant species. Paper presented at the 11th Curtin University Technology, Science and Engineering (CUTSE) International Conference, Curtin University Malaysia, Miri, Sarawak, Malaysia.
- Kumar A, Bisht BS, Joshi VD, Dhewai T (2011) Review of bioremediation of polluted environment: A Management Tool. International Journal of Environmental Science 1(6): 1079-1093.
- Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, et al. (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plant. Nat Biotechnol 13(5): 468.
- Efe SI, Elenwo IE (2012) Management of petroleum impacted with phytoremediation and soil amendments in Ekpan Delta State, Nigeria. Journal of Environmental Protection 3(5): 59-67.
- Greger M (1999) Metal availability and bioconcentration in plants. In: Prasad MNV, Hagemeyer J (Eds.), Heavy Metal Stress in Plants-From Molecules to Ecosystems. Springer Press, Berlin, pp. 1-27.
- Njoku KL, Akinola MO, Oboh BO (2009) Phytoremediation of crude oil contaminated soil: the effect of growth of Glycine max on the physico-chemistry and crude oil contents of soil. Nature and Science 7(10): 79-87.
- Kim KJ, MJ Kil, JS Song, EH Yoo, KC Son, et al. (2008) Efficiency of volatile formaldehyde removal by indoor plants: contribution of aerial plant parts versus the root zone. J Am Soc Hortic Sci 133(4): 521-526.
- Liu JN, Zhou QX, Sun T, Wang XF (2007) Feasibility of applying ornamental plants in contaminated soil remediation. Chin J Appl Ecol 18(7): 1617-1623.
- BW Avery, CL Bascomb (1982) Soil Survey Laboratory Methods, Soil Survey of England and Wales, Harpenden, UK. No 6.
- B Minasny, AB McBratney, DM Brough, D Jacquier (2011) Models relating soil pH measurements in water and calcium chloride that incorporate electrolyte concentration, Eur. J Soil Sci 62(5): 728-732.
- Gode C, Yola ML, Yilmaz, Atar N, Wang S (2017) A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: application to simultaneous determination of Fe(III), Cd(II) and Pb(II) ions. J Colloid Interface Sci 508: 525-531.
- Merkl N, Schutze-Kraft R, Infante C (2005) Assessment of tropical grasses and legumes for phytoremediation of petroleum contaminated soils. Water, Air and Soil Pollution 165(1-4): 195-209.
- Mattina MI, Lannucci-Berger W, Musante C, White JC (2003) Concurrent plant uptake of heavy metals and persistent organic pollutants from soil. Environ Pollut 124(3): 375-378.
- Zhang J, Wang L, Yang J, Liu H, Dia J (2015) Health risk to residents and stimulation to inherent bacteria of various heavy metals in soil. Sci Total Environ 508: 29-36.
- Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res 111(1): 3-49.
- Janas KM, Zieli A, Ska-Tomaszewska J, Rybaczek D, Maszewski J, et al. (2010) The impact of copper ions on growth, lipid peroxidation, and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol 167(4): 270-276.
- MA Khan, H Khan, A Rauf, S Perviz (2014) Antioxidant Potential Of Extracts Of Artimisia Scoparia: Expression In-Vitro Studies. Global Journal Of Pharmacology 8(3): 410-414.
- ML Badea, M Zamfirache (2011) Anatomical Research On Artemisia Santonica And Artemisia Scoparia (Asteraceae). Analele Stiintifice Ale Universitatii, Al I Cuza, Din Iasi 57(2): 21.
- Robinson JP, Kingman SW, Lester EH, Yi C (2012) Microwave remediation of hydrocarbon contaminated soils - Scale-up using batch reactors. Separation and Purification Technology 96: 12-19.
- Yaw Duah Boakye, Sofia Shaheen, Haq Nawaz, Shafaq Nisar, Muhammad Waqar Azeem (2017) Artemisia scoparia: A review on traditional uses, phytochemistry and pharmacological properties. International Journal of Chemical and Biochemical Sciences 12: 92-97.
-
ME Ikiriko* and JA Chukwumati. Phytoremediation Potential of Ficus benjamina on Soils Contaminated with Crude Oil in Rivers State, South-South Nigeria. World J Agri & Soil Sci. 8(4): 2023. WJASS.MS.ID.000693.
-
Heavy metals, Crude oil, Translocation factor, Bioconcentration factor, Total petroleum hydrocarbon, Ficus benjamina
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






