 Research Article
Research Article
Modification of the Phenolic and Fatty Acid Content in Olea europaea Olives from Sardinia as a Consequence of Bactrocera oleae Infestations
Giorgia Sarais1, Francesco Corrias1, Alessandro Atzei1, Ignazio Floris2, Alberto Satta2, Piergiorgio Sedda3,Marco Campus3 and Alberto Angioni1*
1Department of Life and Environmental Science, Chemical Food Analysis Laboratory, University of Cagliari, University Campus of Monserrato S.S. 554 - Bivio Monserrato - Sestu. S.P. Monserrato - Sestu Km 0,700, Italy
2Department of Agriculture, Plant Pathology and Entomologist Section, University of Sassari, Viale Italia 39/a, 07100 Sassari, Italy
3AGRIS, Agenzia Regionale per la Ricerca in Agricoltura, Loc. Bonassai S.S. 291 Sassari-Fertilia - Km. 18,600, Italy
Corresponding AuthorAlberto Angioni, Department of Life and Environmental Science, Chemical Food Analysis Laboratory, University of Cagliari, University Campus of Monserrato S.S. 554 - Bivio Monserrato - Sestu. S.P. Monserrato - Sestu Km 0,700, Italy.
Received Date:August 24, 2024; Published Date:September 13, 2024
Abstract
Bactrocera oleae, the main pest of Olea europaea fruits, was the focus of our study. We investigated how its infestation influences the biochemical composition of olives from the Nera di Gonnos and Pitz’e Carroga cultivars, emphasizing their potential use in the table olive industry.
Olives were dissected under a microscope to assess the level of infestation and the presence of pupae. The fatty acid composition was studied by GC-FID (gas chromatography, flame ionization detector) and confirmed by GC-MS (gas chromatography-mass spectrometry). The composition of single phenols was determined by HPLC-DAD (liquid chromatography—diode array detector), and total phenols were determined using a UV (ultraviolet) spectrophotometer.
The analysis revealed a relatively low infestation level (below 10%), attributed to the agricultural practice adopted in the orchards. The levels of fatty acid and phenols were almost similar among healthy and infested samples, with less-infested olives (NGD2 and PC) showing higher values of monounsaturated (MUFA), lower saturated (SFA), and polyunsaturated fatty acid (PUFA). Multivariate statistical PLS-DA analysis of fatty acid distinguished healthy and infested fruits. Single phenols had higher amounts of oleuropein and luteolin precursor in healthy than infested olives. Total biophenols expressed as the sum of single phenols showed higher values in healthy vs infested and were positively correlated with the total number of stings, with a higher level of decrease in the samples most infected.
Keywords:Table olive; Phenolic compounds; Bactrocera oleae; Fatty acids
A Perspective
Olea europaea is an evergreen tree ubiquitous in the Mediterranean area. Olive oil and processed table olives are its main edible products. They are recognized as functional foods thanks to their high level of natural antioxidants and their high-quality composi tion of fatty acids [1,2]. Epidemiological studies have shown the essential nutritional role of Olea Europaea fruits in the Mediterranean diet and the beneficial effect of their components [3]. This property has been attributed mainly to the antioxidant and free radical-scav- enging activity of polyphenols contained in olive fruits and olive oil [4-6]. The Mediterranean population enjoys a healthy lifestyle, consuming, on average, 20 times more olive oil than Americans; their cancer risk and cardiovascular disease decrease despite the high animal fat intake [7-9]. The American Heart Association has defined recommendation guidelines reporting an association between the Mediterranean-type dietary models and cardiovascular disease decrease [10].
The phenolic fraction of olives, part of the so-called minor compounds of Olea europaea fruits, account for 1-14% of dry pulp and can vary both in quality and quantity depending on several factors, such as genetic, ripening degree of fruits, processing technologies, parasite infestation, and storage [11,12]. The major phenolic compounds identified and quantified in olive and olive oil belong to three different classes: simple phenols (hydroxytyrosol, tyrosol); secoiridoids (oleuropein, the aglycone of ligstroside and their respective decarboxylated dialdehyde derivatives) and the lignans [(+)-1-acetoxy pinoresinol & (+)- pinoresinol] [1,2].
Oleuropein glycoside, the glycoside ester of 2-(3,4-dihydroxyphenyl) ethanol (hydroxytyrosol) with the oleosidic skeleton (exocyclic 8,9-olefinic functionality, a combination of elenolic acid and a glucosidic residue), is the main phenolic compound in olive fruit and is responsible for the bitterness of fresh green olives. Oleuropein glycoside has shown bactericidal and bacteriostatic activity [13] and is not detected in the olive fruit harvested at maturity since it is deglycosylated by glycosidase enzymes, releasing the free secoiridoids [11,12] and is finally hydrolyzed in hydroxytyrosol and elenolic acid [14].
The olive fruit fly (Bactrocera oleae Gmelin, 1790) represents the most harmful parasite affecting olive quality. The premature fruit drop represents the primary damage, followed by the deterioration of the visual and texture quality of the olives, making them unusable for processing into table olives. The quality parameters most affected are acidity, peroxide value, ultraviolet (UV) absorbance, and organoleptic quality; negatively alter the chemical composition (sterols, phenols, fatty acid, and volatile fraction) and reduce oil yield [15-18]. The severity of the adverse effects depends on the stage of the development of the olive fly, the intensity of the attack, and the olive variety, leading to a decrease in the phenolic content and the total amount of volatile compound. On the other hand, no significant variation was observed in the fatty acid composition [19,20]. Medjkouh et al. [21] reported a decrease in the phenolic content of two cultivars from Algeria attacked by Bactrocera oleae; in contrast, Medjkouh et al. [19] studying eight olive cultivars from Algeria reported an uneven behavior on the composition of the fruits related to the attack. Moreover, no significant variations can be observed in the fatty acid composition [22,23]. Although there are numerous publications about the influence of Bactrocera oleae on the qualitative parameters of olive oil, potential variations in the phenolic profile have yet to be considered in depth when considering the preparation of table olives.
This investigation is significant as it aims to investigate the changes in the biochemical composition of olives from the Nera di Gonnos and Pitz’e e Carroga cultivars collected from different regions of Sardinia. We specifically relate the total and single phenolic compounds and fatty acid composition to the percentage of olive fly attacks, highlighting the potential impact of infestation on olive quality.
Acknowledgement
Chemicals and reagents
Tyrosol, 3-hydroxytirosol, luteolin 7 glucoside, luteolin, oloeuropein, verbascoside, p-cumaric acid, vanillic acid, and squalene were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Stock standard solutions of the analytes were prepared in methanol (1000 μg/mL). Intermediate stock standard solutions were prepared at 100 μg/mL in methanol by dilution of stock standard solutions.
Working standard solutions were prepared in methanol and were used for qualitative and quantitative analysis. The marine oil FAME mix analytical standard was purchased from Restek (Bellefonte, PA).
Sodium hydroxide and phosphoric acid were purchased from Merck (Darmstadt, Germany); sodium carbonate, potassium hydroxide, magnesium sulfate anhydrous, and Folin Ciocalteau reagent were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Double-deionised water with a conductivity of less than 18.2 MΩ was obtained with a Milli-Q system (Millipore, Bedford, MA, USA).
Methanol, ethyl acetate, hexane, and acetonitrile were analytical or HPLC grade (Sigma-Aldrich Inc, St. Louis, MO, USA).
Olive samples
Samples (6 Kg) from the cultivar “Nera di Gonnos” (NG) and Pitz’e e Carroga (PC) were collected within a week in November from four different growing areas of Sardinia: Dolianova 39°21’27.74”N/9°10’50.23”E (NGD1), and 39°23’27.82”N/9°10’49.85”E (NGD2, and PC); Villasor 39°23’21.67”N/8°51’41.51”E (NGV), and Gonnosfanadiga 39°30’4.13”N/8°40’4.81”E (NGG). Samples were collected randomly with three replicates for each area and immediately carried in the laboratory; the olives were separated between non-infested and infested. The degree of olive fly infestation was evaluated by analyzing with a stereomicroscope (Zeiss, Italy) dissection sample from 100 olives randomly selected from the main bulk. The number of oviposition scars (stings), live larvae, and pupae or larval/adult exit holes was determined.
Fatty Acid Composition: Three g of homogenized olives were extracted with 10 mL of hexane. Methyl esters were prepared by alkaline treatment, which was carried out by mixing 0.5 mL of the hexane extract with 0.1 mL of 2 N potassium hydroxide in methanol and mixing for 1 min with a vortex alternating heating with stirring, according to Christie et al. [24]. A GC Thermo 8000 TOP, coupled with a flame ionization detection (FID), a capillary column Supelco 24111 – SPTM (60 m x 0.25 μm, film 0.2 μm), was used. Nitrogen was the carrier and make-up gas at 200 KPa and 100 KPa, respectively. Oxygen and hydrogen were at 95 and 70 KPa, respectively. The injector and detector temperatures were 220 and 250 °C, respectively. The oven program was as follows: T=0 90 °C (1 min.), till 100°C (2 °C/min) held 3 min, till 245 °C (3 °C/min) held 20 min. GC-MS confirmed the methyl esters. A gas chromatography-mass spectrometry Thermo DSQ (GC-MS DSQ, Thermo Finnigan, Milan, Italy) with a Select Fame column (Agilent Technologies, 100 m, 0.25mm, 0.2 μm) was used. GC conditions were T=0 100 °C (1 min.), till 160 °C (3 °C/min) held 3 min, till 198 °C (1 °C/min), till 250°C (5 °C/ min) held 15 min. The injector and transfer line were at 250 °C.
MS analysis was carried out in EI+ with an m/z scan rate from 50-500 amu. Computer matching against a commercial library [25] and homemade library mass spectra made from pure substances and MS literature data were used to identify.
Phenolic Fraction: Phenolic compounds were extracted from olives according to the method for olive oil of COI [26] with some minor changes. Three grams of homogenized olives were extracted twice with 15 mL of methanol/water (80/20, v/v) solution and 10 mL of hexane. The tubes were agitated for 20 min in a rotatory shaker, and the organic layer was separated. The two extracts were combined, filtered through a 0.45 μm PTFE syringe filter (Whatman Inc., Clinton, NJ, USA), and dried in rotavapor (t= 30 °C). The residue was dissolved in 15 mL of ethyl acetate plus 2 g of anhydrous MgSO4 to remove the remaining water fraction. One mL of the ethyl acetate solution was dried under a gentle nitrogen stream, recollected with 1 mL of methanol, and injected in HPLC for the analysis. An HPLC 1100 (Agilent Technologies, Milan, Italy) coupled with a DAD detector UV 6000 (Thermo Finnigan, Milan, Italy). The column was a Varian Polaris C18 (5 um, 300 A, 250 mm x 4.6 mm). The analysis was carried out at λ 280 and 360 nm in gradient elution. Solvents were phosphoric acid 0.22 M (A), acetonitrile (B), and methanol (C). The gradient used for the separation and analysis was: T=0 A 96%, B 2%, C 2%; T=40 A 50%, B 25%, C 25%; T=45 A 40%, B 30%, C 30%; T=60 A 0%, B 50%, C 50%, hold 10 min; post time 15 min. Flow 1 mL/min. Calibration graphs were prepared with five points from 5 to 50 μg/mL.
Total phenol: 10 g of olives were extracted with 20 mL of methanol/ water (80/20, v/v) solution for 30 min in a rotatory shaker. The extracting solution was centrifuged for 10 min at 4000 rpm and diluted ½ with methanol before the analysis. Total phenols were determined using the Folin-Ciocalteu method. 100 μL of the diluted extract solution were put in a 10 mL calibrated flask with 500 μL of Folin-Ciocalteu reagent, 3 mL of a sodium carbonate solution 20% (p/v), and MilliQ water till 10 mL. The mixture was agitated for 1 minute in a vortex and incubated for 80 min at room temperature (18 °C) in the dark. Before UV analyses, samples were centrifuged at 4000 rpm for 10 min. A Varian Cary50 spectrophotometer with a 1 cm quartz cuvette, set at λ 725 nm, was used. Quantitative analyses were made using the external standard method, using gallic acid as a standard reference and correlating absorbance to concentration. Calibration graphs were made between 200 and 2000 μg/g; the results were expressed as μg/g of gallic acid.
Statistical Analysis: Data were analyzed using Statistica 6.0 (Statsoft, Tulsa, OK, USA) statistical software. Unless otherwise stated, the reported values are averages of at least three repetitions (n= 3). Tukey’s honest significant difference (HSD) multiple comparisons (one-way ANOVA) and Pearson’s linear correlations are both at p < 0.05.
GC-MS data sets were imported into SIMCA 13 (Umetrics AB, Umea, Sweden) for processing using principal component analysis (PCA) and partial least squares regression (PLS-DA). Additionally, R2 (the multiple correlation coefficient) and Q2 (the cross-validated correlation coefficient) were used as touchstones for the robustness of a pattern recognition model [27].
Results and Discussion
Olive’s infestation
Olives were classified as infested when bearing stings and healthy olives. The data showed a generally low infestation of total olives harvested, with higher values for NGV and NGG (8%) and lower for PC and NGD2 (2%). These data are compatible with olive oil production, which tolerates values of infestation ≤ 10%; however, a degree of infestation greater than 1% prevents the use of the fruits as table olives [28]. Among the samples tested, NGV>NGG> NGD1 showed the higher infestation, while PC and NGD2 showed the lowest (Table 1). NGV samples were the most affected, with 597 total stings, a maximum number of stings for olives of 47, and 30% with stings ≥ 7. NGV was the only sample showing larvae L2 and L3, accounting for 19 total exit holes. The samples NGG and NGD1 had 572 and 470 total stings, maximum stings, and exit holes of 18 and 10 and 21 and 10, respectively (Table 1).
The percentage of olives with stings ≥ 7 was 32, and 25% for NGG and NGD1, respectively. The samples of NGG showed an old infestation with the absence of larvae or pupae in the pulp but 21 exit holes, while two pupae were detected in the samples NGD1 and NGV. The samples of PC and NGD2 showed the lowest level of olive fly attack with only 297 and 243 total punctures and 6 and 5 exit holes, respectively. The maximum number of stings per olive was 7 and 8, with 2% of olives with stings ≥ 7. No larvae or pupae were detected in PC and NGD2 samples (Table 1).
Table 1:Infestation parameters of the olives collected during the experiment.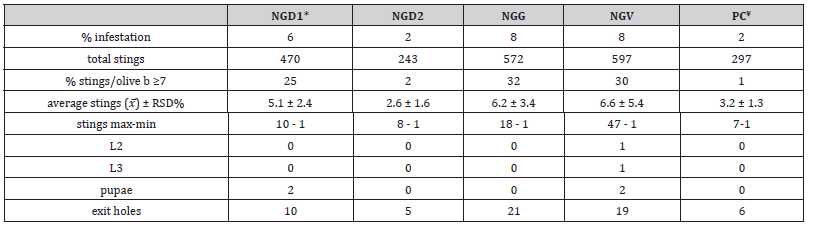
* NG – Nera di Gonnos
¥ PC – Pitz’e e Carroga
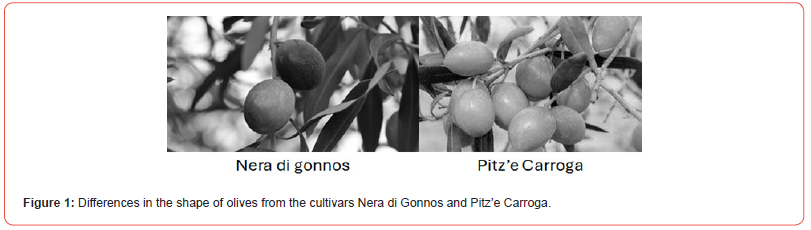
Olive fruit fly adults exhibit, under field conditions, a preference for large fruit [20,29]. Olives of the cv Nera di Gonnos (NG) and Pitz’e Carroga (PC) have similar weights (around 5 g) but different shapes; NG is elliptic, while PC is asymmetric with a sharp tip (Figure 1). PC is susceptible to Bactrocera oleae because of its size and earliness.

The samples of NGD2 and PC belonged to the same orchard and were subjected to the same field management. The plants followed a meticulous protocol of treatments with plant protection products, which decreased the population of Bactrocera oleae and, consequently, fly attack in these samples. The resulting low level of damage on the olives confirms this behavior.
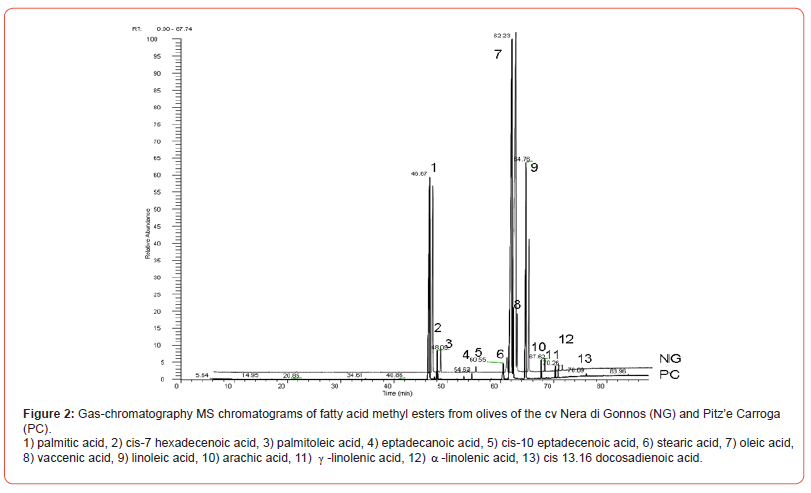
Thirteen fatty acids were detected and subsequently identified by GC-MS. No qualitative differences were observed among healthy and infested olives, neither among different samples of the same cultivar nor from cultivars.
Concerning data, little differences were observed in the quantitative composition of fatty acid among olive samples from healthy or infested cultivars. Oleic acid was the most concentrated methyl ester in all samples, with average values of 56.84 ± 3.33% and 56.38 ± 4.99 in healthy and infested, respectively, followed by linoleic acid (15.14 ± 11.27% healthy and 14.52 ± 27.06% infested), palmitic acid (18.30 ± 4.49% healthy, and 18.58 ± 3.64 infested), all other fatty acids accounted for less than 4% (Table 2).
However, comparing healthy and infested samples from each orchard, we identified two patterns: NGD1, NGG, NGV, and PC and NGD2 (Table 2).
Table 2:Fatty acid composition (%±RSD%) in the samples of healthy and infested olives collected in Sardinia during the experiment.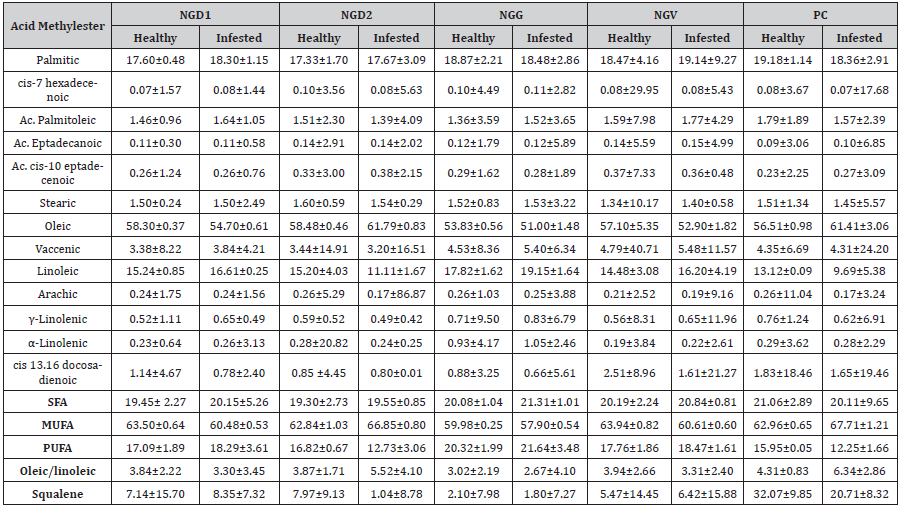
The former had higher levels of oleic acid and lower palmitic and linoleic acid in healthy olives, whereas the second had higher palmitoleic and linoleic and lower oleic acid in healthy olives related to infested. All samples showed higher levels of cis 13, 16 docosadienoic acid in healthy vs infested. Montedoro et al. [30] reported that fatty acid composition could be influenced by various factors such as environmental factors, infestation, and agronomic techniques; on the contrary, Mraicha et al. [31] observed that also very severe infestation did not cause essential changes in the fatty acid composition. More recently, Valencic et al. [32] reported that olive oils affected by damaging infestation had lower amounts of oleic acid and higher amounts of myristic, linoleic, and linolenic acids. Our data confirmed the slight differences in the fatty acid composition among healthy and infested olives; however, the results showed a reversed behavior compared to the literature data.
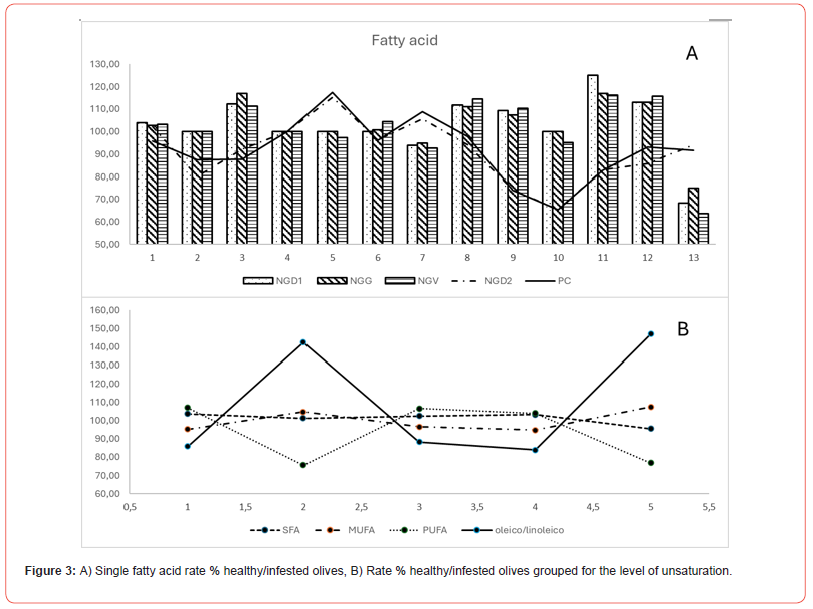
The rate of oleic/linoleic acid showed higher values in the most damaged olives (NGV, NGG, and NGD1) than in the less infested (NGD2 and PC) (Table 2).
Grouping fatty acid for the level of unsaturation evidenced MUFA as the main fraction in all samples, followed by SFA and PUFA. NGD1, NGG, and NGV had average values in infested olives, slightly higher for saturated (SFA) and polyunsaturated (PUFA) and lower values for monounsaturated (MUFA) compared to healthy.
On the contrary, in healthy olives, NGD2 and PC showed lower SFA and PUFA values and higher MUFA values (Table 2) than infested ones (Figure 3). Moreover, the oleic/linoleic acid ratio was almost even in all samples of NG, while it was higher in the infested samples from PC and NGD2.
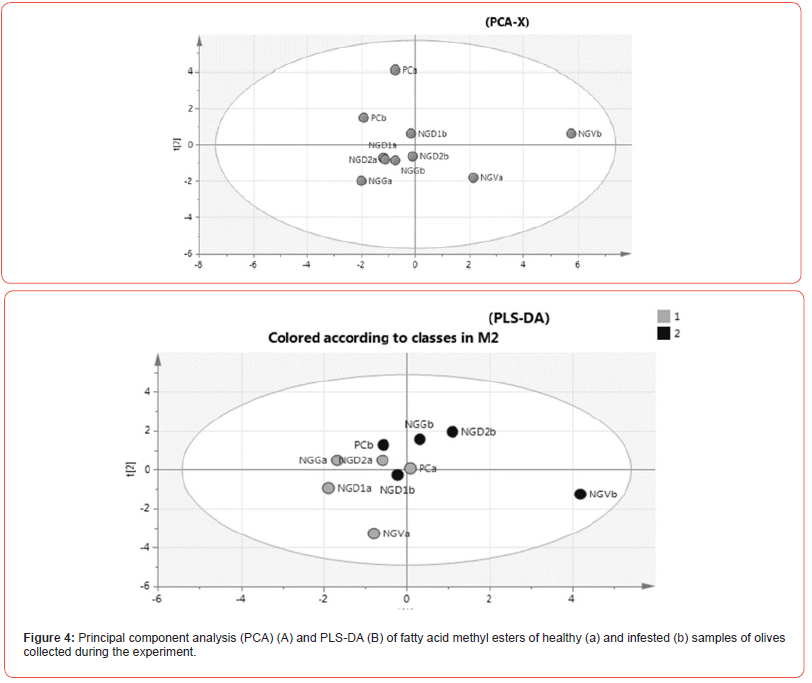
Fatty acid data were analyzed using multivariate statistical analysis (Figure 4). A PCA model was created using all samples. Healthy samples were identified by the letter “a” while infested by the “b”. The scores plot of the principal component 1 versus the principal component 2 confirmed the similarity among the samples (Figure 4A). However, PC samples were well separated from NG samples. NGVb samples were probably distinguished from the others for a higher infestation level.
The PLS-DA model, created to evaluate the significance of the difference between healthy and infested olives, showed poorly differentiated classes. Only NGVb, the most affected by olive fly, was separated from the other samples (Figure 4B).
The importance of the variables in distinguishing the two groups was ranked according to their VIP scores in the PLS-DA model. The results indicated that oleic acid (variable 8) had the highest correlation with the grouping of the infested olives, followed by palmitoleic (variable 3) and linolenic acid (variable 11).
Squalene showed uneven amounts among the different samples. The trend was similar, with lower amounts in infested vs. healthy in NGD2 and PC, while NGD1, NGG, and NGV samples showed an even amount between healthy and infested. PC showed values notably higher, accounting for 32.07 ± 9.85 and 20.71 ± 8.32 in healthy and infested, respectively (Table 2).
Phenolic fraction composition
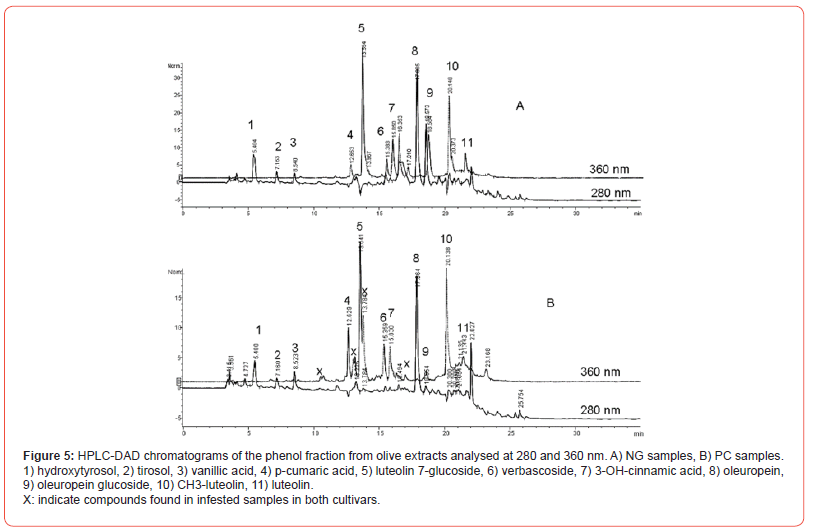
The analysis of HPLC-DAD chromatograms at 280 and 360 nm allowed us to identify 11 main phenols: hydroxytyrosol, tyrosol, vanillic acid, vanillin, p-cumaric acid, luteolin 7-glucoside, verbascoside, 3-OH-cinnamic acid, oleuropein, luteolin, methyl-luteolin (Figure 5). All cultivars belonging to Nera di Gonnos (NG) had overlapped chromatographic profiles (Figure 5A), while PC showed a specific profile at 360 nm with the presence of three undefined peaks and the absence of the peak at 16.35 (Figure 5B).
PC samples showed notably less amounts of Oleuropina glycoside. The 280 nm and 360 nm analyses showed minor differences between healthy and infested samples.
Hydroxytyrosol, tyrosol, luteolin, and CH3-luteolin had lower values in healthy olives samples, while oleuropein, oleuropein glucoside, and luteolin-7-glucoside had higher values in healthy samples (Table 3).
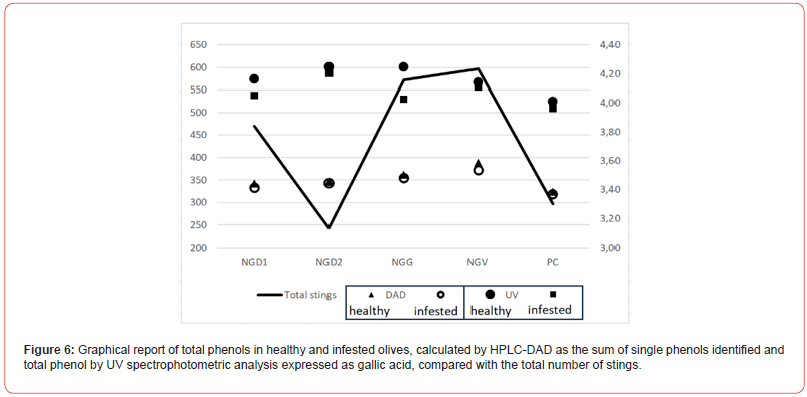
NGV, NGD1, and NGG showed the most remarkable differences between healthy and infested samples (Table 3). At the same time, NGD2 and PC had almost overlapping values except for oleuropein glucoside, luteolin, and methyl-luteolin. The data of total phenols corresponded to the number of stings, with NGD2 and PC almost overlapping (Figure 6). Spectrophotometric analysis of total phenols showed a reversed amount in NGD2 and PC, with less significant differences compared to HPLC DAD analysis.
Table 3:Phenolic composition (μg/g ± RSD%), and total phenols (μg/g ± RSD) in the samples of healthy and infested olives collected in Sardinia during the experiment.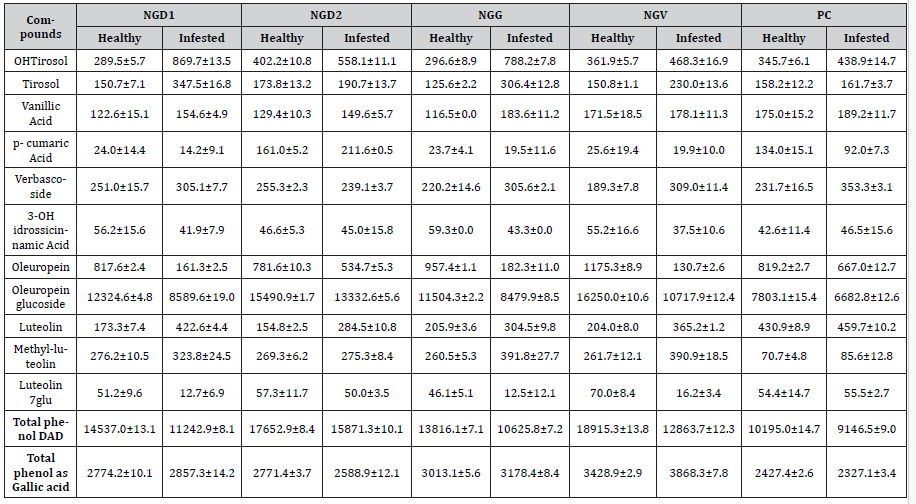
The two analyses are performed at different wavelengths, and the analytical responses cannot be compared.
Hydroxytyrosol and tyrosol derive from oleuropein and ligstroside hydrolysis, and each process that may cause hydrolysis of oleuropein and ligstroside can increase these two metabolites.
Literature data reported that the infestation of olives by Bactrocera oleae can cause extensive damage to the tissue and the cells of the olive pulp, affecting the phenol fraction and the aromatic profile [17,19,32-34].
The data reported in this paper confirmed the general decrease of phenol fraction; a significant decrease of oleuropein and oleuropein glucoside can be noted, with a resulting increase of OHtyrosol and tyrosol.
The damage related to the infestation against the cells increases the release of enzymes, which leads to the degradation of the secoiridoids compounds, growing the amount of the smaller phenols. This condition can be characterized in the samples NGV, NGG, and NGD1, most affected by the parasite (Table 3). A decrease in the total content represents the trend of the bisphenols; however, each single phenol follows a proper dissipation rate.
The rates were well related to the level of infestation; NGD2 and PC showed the lowest differences among the reported phenols.
Conclusion
It has been reported that olive infestation could affect olives and olive oil quality. In this paper, we examined the changes in some olive quality parameters concerning Bactrocera oleae infestation. The samples analyzed showed a level of infestation ranging from 10 to 2% and were above the level accepted (1%) for the technological processing of table olives. However, the biochemical analysis showed beneficial characteristics, with only minor differences from healthy olives. The olives most affected by infestation, both for the number of stings and the total level of infestation, were represented by the samples of NGV, NGG, and NGD1. Infestation influenced only slightly the fatty acids fraction; however, it was possible to distinguish two sets of data, constituted by NGG, NGD1, and NGV, which showed higher levels of SFA and PUFA and lower MUFA in healthy vs infested olives. In comparison, NGD2 and PC showed higher MUFA levels and lower SFA and PUFA. The oleic/linoleic acid rate confirmed the presence of two distinguished patterns, which were well separated by multivariate analysis. This fact may indicate a degradative process of MUFA to load in two opposite directions, the increase of unsaturation and the saturation of individuals’ double bonds. Single phenols showed higher amounts of oleuropein and luteolin precursors in healthy than infested olives, data confirmed by the analysis of total phenols, which had higher values in healthy vs infested. The differences among single and total phenols (as the sum of single phenols) were positively correlated with the total number of stings, decreasing in the samples most infected (Figure 6). The data demonstrates that new studies are needed to understand better the biochemical reaction at the base of phenols and fatty acid modifications in response to pest attacks.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Alves E, do Rosário Domingues M, Domingues P (2023) Chapter 4 - Olive oil, Ed(s): Zabetakis I, Tsoupras A, Lordan R, Ramji D, Functional Foods and Their Implications for Health Promotion. Academic Press, Pgs. 97-129.
- Rocha J, Borges N, Pinho O (2020) Table olives and health: a review. Journal of Nutritional Science 9: e57.
- Sivakumar G, Uccella N (2010) Olive biophenols and conventional biotechnology from Mediterranean aliment culture. In: V. R. Preedy, & R.R. Watson, (Eds.), Olives and olive oil in health and disease prevention. Academic Press, Oxford, pp 333-347.
- International Olive Oil Council (IOOC).
- Saghir SA, Rajabi RK (2017) The secret miracle of oil. International Food Research Journal 7: 68-84.
- Carluccio MA, Calabriso N, Sconditti E, Massaro M, De Caterina R (2015) Mediterranean diet polyphenols. In Preedy, V.R., Watson, R.R. (Eds). The Mediterranean diet. Academic press, Elsevier Inc.
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, et al. (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med 113: 71S-88S.
- Owen RW, Haubner R, Wurtele G, Hull WE, Spiegelhalder B, et al. (2004) Olives and olive oil in cancer prevention. Eur J Cancer Prev 13: 319-326.
- Boskou G, Salta FN, Chrysostomou S, Mylona A, Chiou A, et al. (2006) Antioxidant capacity and phenolic profile of table olives from the Greek market. Food Chem 94(4): 558-564.
- Gardner CD, Vadiveloo MK, Petersen KS, Anderson CAM, Springfield S, et al. (2023) Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance: A Scientific Statement from the American Heart Association. Circulation 147(22): 1715-1730.
- Romero C, Brenes M, Yousfi K, Garcia P, Garcia A, et al. (2004) Effect of cultivar and processing method on the contents of polyphenols in table olives. J Agric Food Chem 52: 479-484.
- Morello JR, Vuorela S, Romero MP, Motilva MJ, Heinonen M (2005) Antioxidant activity of olive pulp and olive oil phenolic compounds of the Arabequina cultivar. J Agric Food Chem 53: 2002-2008.
- Soler-Rivas C, Espin JC, Wichers HJ (2000) Oleuropein and related compounds. J Sci Food Agric 80(7): 1013-1023.
- Gutierrez-Rosales F, Paz Romero M, Casanovas M, Motilva MJ, Mínguez-Mosquera MI (2012) β-Glucosidase involvement in the formation and transformation of oleuropein during the growth and development of olive fruits (Olea europaea L. cv. Arbequina) grown under different farming practices. J Agric Food Chem 60: 4348−4358.
- Lynda Medjkouh-Rezzak L, Tamendjaria A, Mettouchi S, Bouarroudj-Hamici K, Oliveira MB (2023) Influence of olive fly (Bactrocera oleae) on the phenolic composition and antioxidant activity of four Algerian olive cultivars. La rivista Italiana delle sostanze grasse - Vol. C - gennaio/Marzo: 19-28.
- Notario A, Sánchez R, Luaces P, Sanz C, Pérez AG (2022) The Infestation of olive fruits by Bactrocera oleae (Rossi) modifies the expression of key genes in the biosynthesis of volatile and phenolic compounds and alters the composition of virgin olive oil. Molecules 27(5): 1650.
- Evangelisti F, Zunin P, Calcagno C, Tiscorina E, Petacchi R (1994) Dacus oleae infestation and its consequences on the phenolic compounds of virgin olive oil. Riv It Sost Grasse 74: 507-511.
- Gomez-Caravaca AM, Cerretani L, Bendini A, Segura-Carretero A, Fernandez-Gutierrez A, et al. (2008) Effects of fly attack (Bactrocera oleae) on the phenolic profile and selected chemical parameters of olive oil. J Agric Food Chem 56(12): 4577-4583.
- Medjkouh L, Tamendjari A, Alves RC, Laribi R, Beatriz M, et al. (2018) Phenolic profiles of eight olive cultivars from Algeria: effect of Bactrocera oleae attack. Food Funct 9(2): 890-897.
- Burrack HJ, Zalom FG (2008) Olive fruit fly (Diptera: Tephritidae) ovipositional preference and larval performance in several commercially important olive varieties in California. J Econ Entomol 101(3): 750-758.
- Medjkouh L, Tamendjari A, Alves RC, Araújo M, Beatriz M, et al. (2016) Effect of Bactrocera oleae on phenolic compounds and antioxidant and antibacterial activities of two Algerian olive cultivars. Food Funct 7(10): 4372-4378.
- Tamendjari A, Angerosa F, Bellal MM (2004) Influence of Bactrocera oleae infestation on olive oil quality during ripening of Chemlal olives. Ital J Food Sci 3: 343-354.
- Pereira JA, Alves MR, Casal S, Oliveira MBPP (2004) Effect of olive fruit fly infestation on the quality of olive oil from cultivars cobrancosa, madural and verdeal transmontana. Ital J Food Sci 16(3): 355-365.
- Christie WW (1998) The Preparation of Derivatives of Fatty Acids. In Gas Chromatography and Lipids, The Oily Press: Ayr, Scotland, pp 64-84.
- (2023) NIST / EPA / NIH Mass Spectral Library.
- (2017) International Olive Council, COI/T.20/Doc. No 29/Rev.1, Determination of biophenols in olive oils by HPLC.
- Atherton HJ, Bailey NJ, Zhang W, Taylor J Major H, Shockcor J Clarke, et al.(2006) A combined 1H NMR spectroscopy and mass spectrometry-based metabolomic study of the PPAR-α null mutant mouse defines profound systemic changes in metabolism linked to the metabolic syndrome. Physiological Genomics 27: 178-186.
- Segura Buitrago MD (2002) Anàlisis poblacional y evolutivo en “Bactrocera oleae” (GMELIN) mediant eel uso de marcadores moleculares. Memoria para optar al grado de dotoctor en Ciencias Biologicas. Universidad Complutense de Madrid.
- Yokoyama VY, Miller GT, Stewart-Leslie J, Rice RE, Phillips PA (2006) Olive fruit fly (Diptera: Tephritidae) populations in relation to region, trap type, season, and fruit availability. J Econ Entomol 99: 2072-2079.
- Montedoro GF, Servili M, Baldioli M, Miniati E (1993) Simple and hydrolysable compounds in virgin olive oil. 3. Spectroscopic characterization of secoridoid derivatives. J Agric Food Chem 41: 2228-2234.
- Mraicha F, Ksantini M, Zouch O, Ayadi M, Sayadi S, et al. (2010) Effect of olive fruit fly infestation on the quality of olive oil from Chemlali cultivar during ripening. Food Chem Toxicol 48(11): 3235-3241.
- Valencic V, Butinar B, Podgornik M, Bucar-Miklavcic M (2021) The Effect of Olive Fruit Fly Bactrocera oleae (Rossi) Infestation on Certain Chemical Parameters of Produced Olive Oils. Molecules 26(1): 95.
- Bendini A, Cerretani L, Cichelli A, Lercker G (2008) Effect of Bactrocera oleae infestation on the aromatic profile of virgin olive oils. Riv Ital Sost Grasse 85(3): 167-177.
- Tamendjari A, Angerosa F, Mettouchi S, Bellal MM (2009) The effect of fly attack (Bactrocera oleae) on the quality and phenolic content of Chemlal olive oil. Grasas Aceites 60(5): 507-513.
-
Giorgia Sarais, Francesco Corrias, Alessandro Atzei, Ignazio Floris, Alberto Satta, Piergiorgio Sedda, Marco Campus and Alberto Angioni*. Modification of the Phenolic and Fatty Acid Content in Olea europaea Olives from Sardinia as a Consequence of Bactrocera oleae Infestations. World J Agri & Soil Sci. 9(2): 2024. WJASS.MS.ID.000709.
-
Table olive, Phenolic compounds, Bactrocera oleae, Fatty acids
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






