 Research Article
Research Article
Evaluation of Irrigation Frequency and Selenium Fertilization Impacts on the Nutritional Traits of Moringa oleifera and Moringa peregrina
Khalid A Abdoun1*, Osman A Altahir2, Ahmed A Alsagan3, Mohammed Y Alsaiady4, Elfadil E Babiker5, Ali M Alshaikhi1, Faisal A Alshamiry1 and Ahmed A Al-Haidary1
1Department of Animal Production, College of Food and Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia
2Naif Arab University for Security Sciences, Biostatistics Department
3King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia
4Development and Research Unit, Arabian Agricultural Services Company (ARASCO), Riyadh 12311, Saudi Arabia
5Department of Food and Nutrition Science, College of Food and Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia
Corresponding AuthorKhalid A Abdoun, Department of Animal Production, College of Food and Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia.
Received Date:October 18, 2023; Published Date:October 30, 2023
Abstract
This study was conducted to determine the combined effect of irrigation frequency and selenium (Se) fertilizer levels on the chemical composition of Moringa oleifera (M. oleifera) and Moringa peregrina (M. peregrina), with the ultimate goal of incorporating M. oleifera and M. peregrina in livestock feed. The combined effect of irrigation frequency and selenium (Se) fertilizer levels were studied in a completely randomized split plot design. The experiment included two plant species (M. oleifera and M. peregrina), four irrigation frequencies (7, 10, 15, and 20 days), and three Se levels (0.0, 12.5 and 25 mg/L). The results of the study indicated that the irrigation frequency and the foliar spray with organic aminoselenium fertilizer affected (p < 0.05) proximate analysis (crude protein, ether extract, crude fiber and ash contents), as well as the minerals contents (P, Ca, Mg and Se) of the dried leaves and upper fine stems of M. oleifera and M. peregrina in the different cutting periods. The effect of organic amino selenium fertilizer application and irrigation frequency on all studied traits allowed the classification of M. oleifera and M. peregrina vegetative plant parts as suitable for livestock feeding. Selenium foliar spray can be considered as a safe method to increase the selenium content of both M. oleifera and M. peregrina vegetative parts, which may contribute to increase the functional feeding quality of these plants.
Keywords:Moringa; Water conservation; Foliar spray; Nutrients content
Introduction
Livestock production in arid and semi-arid zones of the world is facing great difficulties, mainly due to the fact that most of the natural range areas have poor forage plants with low nutritional value, especially in regard to digestibility and crude protein content. Feed production also decreases dramatically during the dry seasons at rates of up to 80%. To counter these problems, it has been proposed to include tree species with high nutritional value in the animal diet and thus improving production standards in the animals’ production sector [1]. When range plants are capable of easily releasing their nutrients contents, they can cheaply enhance grazing animals’ performance [2–4]. The intake of forage plants by herbivores slows down the digestion process and thus allows sufficient time for the digestion and absorption of the forage nutritional components, and consequently good body growth rates. The productivity of herbivorous animals such as sheep and poultry can be improved by using high-protein feeds. Among the alternative sources of high-protein feed are Moringa plant species.
Generally, Moringa tolerates various environmental stresses such as soil acidity [5–7], ambient temperatures (-1 - 48 °C), as well as prolonged drought periods [8], which makes it suitable for cultivation in the stressed environments prevailing in the dry regions of the world. As a forage, it has excellent ability for regrowth after repeated cuttings [9] and produces high amounts of biomass [10]. All parts of the Moringa tree can be used as a source of forage, making it one of the most important shrubs or pasture trees that spreads throughout the tropics [11–13].
Moringa plant species are characterized by their nutritional value, particularly their leaves. Moringa leaves are an excellent source of minerals, vitamins and protein which ranges between 19 - 35% dry matter [14,15]. It is worth mentioning that protein represents the most important nutrients component in animal feeds [16], as well as the most expensive ingredient [17]. The amino acid content of Moringa leaves is comparable to that of soybean [18,19], with a digestibility of 79.2% [20]. Nineteen amino acids have been reported in Moringa leaves, of which 10 amino acids are essential for animal nutrition. Lysine and methionine as essential amino acids are present at a level of 19.6 and 2.9 mg/g DM, respectively [21]. In addition, Moringa leaves contain 321 - 521 g /kg DM neutral detergent fiber, 224 - 361 g/ kg DM acid detergent fiber [22], 2.27 - 2.98 Mcal metabolizable energy/kg DM, and 79% dry matter digestibility in vitro [10,23]. Moringa leaves also contain very high levels of vitamins A (6.8 mg/kg DM), B (423 mg/kg DM) and C (220 mg/kg DM), as well as 0.6 -11.2% minerals [24], 1.28 - 4.96% fats [25], and high levels of flavonoids as antioxidants [26]. The chemical composition and digestibility of Moringa plant species vary in accordance with geographical regions, type of soil, type and level of applied fertilizer, and cutting frequency [27,28].
Moringa plant species in dry and arid regions are irrigated for the first two months for the establishment of the plants, thereafter they rarely require watering [29]. The tree with a good root system tolerates drought and needs watering only when wilting symptoms are clear. However, information regarding irrigation methods and water requirements of M. oleifera and M. peregrina in relation to the chemical composition of their leaves and upper stems parts is scary
Selenium is an essential plant micronutrient that has been shown to enhance plant growth and development as well as contributing to plant tolerance to environmental stresses [30–33]. Selenium has been detected in all living organisms and has a significant effect on plants and animals’ health [30]. Selenium can be absorbed by plants in selenate (SeO4), selenite (SeO3) or organic forms [32]. However, Se accumulation in plants depends on the form and concentration of Se, as well as the availability of competing ions [33]. Foliar application of Se has been practiced increasing Se content in different plant species [34].
To our knowledge, no information so far is available about the effect of irrigation frequencies, levels of selenium fertilizer, and cutting intervals on the chemical composition of M. oleifera and M. peregrina. Therefore, to bridge this knowledge gab, the present study was conducted with the aim of evaluating the effect of irrigation frequencies, selenium fertilizer levels and cutting periods on the chemical composition and the nutritional value of M. oleifera and M. peregrina, and to test the hypothesis that the nutritional value of both M. oleifera and M. peregrina could vary pending on the irrigation frequency, Se fertilizer level and cutting periods.
Materials and Methods
The present study was conducted during summer season 2021 at Al-Badraniya Farm located in Al-Ghat town, Central region of Saudi Arabia (26°1′36″N 44°57′39″E). The central region’s climate is characterized by hot summers, cold winters, and scarce rainfall during winter and spring. The study was conducted as split plot arrangement in a randomized complete block design with three replications. The treatments applied in the study included two plant species (M. oleifera and M. peregrina), four irrigation periods (7, 10, 15, and 20 days), and three Se fertilizer levels (0.0, 12.5 and 25 mg/L). The selenium treatments were done by foliar spray of organic amino selenium fertilizer 2.5% (Organic Standards Fertilizer Production Company, Riyadh, KSA). Each replicate was divided into two main plots, which were assigned to the two Moringa species (M. oleifera and M. peregrina). Each main plot was divided into four subplots for the four irrigation frequencies, while each subplot was divided into three sub-subplots for the three Se fertilizer treatments. Each subplot consisted of four (6m in length) furrows. The distance between the furrows is 25cm, with 20 cm between plants within each furrow. An implanted space was left between adjacent sub-subplots. Also, there was a two meters’ distance between neighboring subplots as buffer zones to prevent the horizontal seepage of water between the different subplots.
Hundred kg of triple superphosphate, 50kg of potassium sulfate and 200kg urea per ha were added to the soil. The triple superphosphate and the potassium sulfate as well as half of the urea were applied at sowing, and the other half of the urea was applied in equal amounts after the first and second plant cuts at the peak of the vegetative growth stage.
Experimental treatments (Moringa species, irrigation frequencies, selenium levels) were randomly distributed over the three replicates. The seeds of the two Moringa species were sown during the first week of March 2021 and irrigated using drip irrigation until the plants’ establishment stage (three weeks), thereafter the plants were irrigated according to the studied irrigation frequencies. The Se treatments were applied as foliar spray early in the morning.
A sample of 100g fresh leaves and upper fine stems was harvested from each treatment, oven dried at 65 °C, then ground by a grinding machine to pass through a 20-mesh sieve. The ground plant materials were stored in paper bags and placed in a desiccator to determine their chemical composition. The second and third cuts were performed at 45 days’ intervals from the preceding cut, and the same procedures were followed for samples collection and treatment. The crude protein (CP), fat (EE), ash and crude fiber (CF) contents were estimated by the methods described in AOAC [35].
The mineral contents of the leaves and the upper fine stems samples of both Moringa species: calcium (Ca) and magnesium (Mg) were estimated using atomic absorption spectrometry. Phosphorous (P) was determined chromatically. Selenium was determined according to the method of Pedrorero et al. [36] using an ICP-MS device (Pasma Quant MS Elite, Germany). All analyzes were performed in duplicate.
Statistical analyses. The chemical composition of Moringa species was subjected to one-way analysis of variance. (ANOVA) according to the general linear model (GLM) using the SPSS 20.0 statistical software. In case of significant differences between the treatments means, Duncan’s multiple range test at 5% level of significance was used to compare the treatments means.
Results and Discussion
The drip irrigation frequency had significant effects (P < 0.05) on the proximate composition of dried leaves and upper fine stems of M. oleifera and M. peregrina (Table 1). The proximate composition ofM. oleifera and M. peregrina indicated significant differences between the two Moringa species with respect to all the studied traits (Table 1). The highest percentages for all studied traits were recorded in M. oleifera in the third cut with drip irrigation frequency every 20 days, and the lowest percentages were recorded in M. peregrina especially in the first cut with drip irrigation frequency every 7 days. Regardless of the cutting period and irrigation regimes, M. oleifera recorded the highest values of all studied traits.
Table 1:The effect of irrigation frequency (days) on the proximate composition of M. oleifera and M. peregrina dried leaves and upper fine stems at different cutting periods.
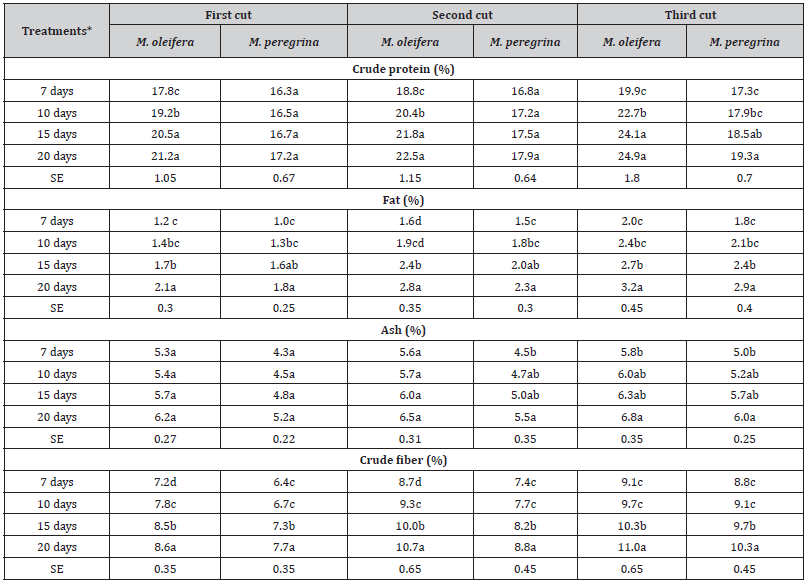
*Means within the same column for each trait with different letters are significantly different at P < 0.05.
The CP percentages ranged from 17.8 to 24.1% in M. oleifera and from 16.3 to 18.5% in M. peregrina, which were similar to those reported by Abul-Ezz et al. [37] and Offor et al. [38]. However, the CP values were lower than those recorded by Kakengi el al. [39] and Olugbemi et al. [23], and higher than those reported by Amabye and Gebrihiwot [40]. The fat percentages ranged from 1.2 to 3.2% in M. oleifera and from 1.0 to 2.9% in M. peregrina which was lower that reported in Nigeria [38]. Differences in fat content could be attributed to the plant genotype, climatic conditions and the cutting stage [41]. The ash percentages ranged from 5.3 to 6.8% in M. oleifera and from 4.3 to 6.0% in M. peregrina. The here in reported ash contents of Moringa leaves were higher than those recorded in Nigeria [21]. The crude fiber percentages ranged from 7.2 to 11.0% in M. oleifera and from 6.4 to 10.3% in M. peregrina. These values are lower than those reported by Offor et al. [38], but similar to those reported by Moyo et al. [21] and Melesse [42].
The cutting periods had positive impacts on the nutritional quality of the leaves of both M. oleifera and M. peregrina. The chemical composition was generally higher in each cutting period as compared to the previous one. The results obtained in this study contradict those reported by Nouman et al. [43] and Sánchez et al. [44] who recorded no significant changes in the chemical composition of Moringa leaves due to cutting periods. Differences in nutrient values observed in this study as compared with those of previous studies may be due to genotypic as well as the environmental differences [45,46].
Table 2 presents the approximate composition of dried M. oleifera and M. peregrina leaves and fine upper branches at different cutting periods. The studied traits included CP, fat, ash and CF. Application of selenium fertilizer increased (P < 0.05) the contents of the studied traits in both M. oleifera and M. peregrina as compared to the control. Plants that received Se fertilizer at the level of 25 mg/L recorded the highest significant values for all studied traits in all plant cutting periods (Table 2). The lowest contents of all studied traits were recorded in M. peregrina as compared to M. oleifera regardless of the Se level or the cutting period (Table 2). Several studies have indicated the beneficial effects of Se at low concentrations on the growth rate of higher plants [47–50], and in increasing plant resistance against oxidative stress [51–53]. Furthermore, Se increased the net assimilation rate of soluble sugars and enhanced protein synthesis [48,54].
Table 2:The effect of selenium fertilization (mg/L) on proximate compositions of M. oleifera and M. peregrina dried leaves and upper fine stems at different cutting periods.
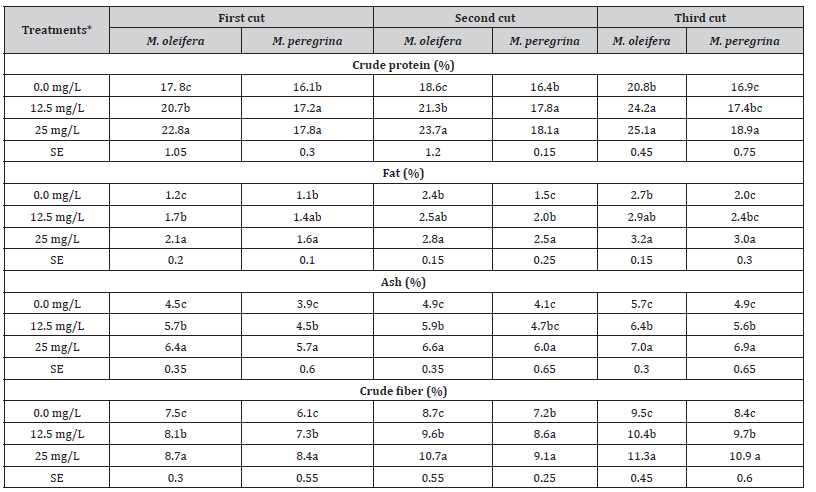
*Means within the same column for each trait with different letters are significantly different at P < 0.05.
The mineral content of the leaves and the upper fine stems of M. oleifera and M. peregrina are shown in Table 3. Statistical analyzes showed differences (P < 0.05) in mineral contents of the two species due to the effects of drip irrigation frequency and cutting periods. It was clearly observed that on the basis of the means of each of the three cutting periods, the content of P, Ca, Mg and Se in the dry leaves and fine stems were highest when the two Moringa plant species were drip irrigated every 20 days. The contents of N, P, K, Ca, Mg and Se were higher in the third cut than the two other cutting periods (Table 3). The minerals (P, Ca, Mg and Se) contents of the dry leaves and upper fine stems were the highest in the third cut under 20 days’ drip irrigation frequency for both M. oleifera and M. peregrina (Table 3). In general, the lowest minerals contents were recorded in plants subjected to 7 days’ drip irrigation frequency, and the mineral contents were lower in M. peregrina compared to M. oleifera regardless of the cutting period (Table 3). The herein obtained results support the findings of the previous reports [55–57].
Table 3:The effect of irrigation frequency (days) on the mineral contents of M. oleifera and M. peregrina dried leaves and upper fine stems at different cutting periods.
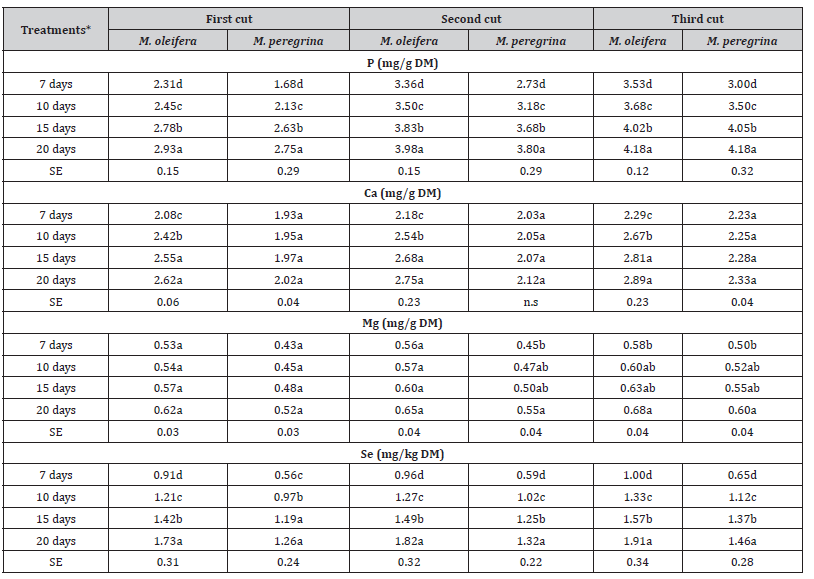
*Means within the same column for each trait with different letters are significantly different at P < 0.05.
The P, Ca, Mg and Se contents in the leaves and the upper fine stems of both M. oleifera and M. peregrina in the control samples were lower (P < 0.05) compared to the samples treated with Se fertilizer (12.5 and 25 mg Se/L) foliar spray (Table 4). Both Se fertilizer levels and the cutting stage affected (p < 0.05) the contents of P, Ca, Mg and Se in M. oleifera and M. peregrina (Table 4). In all cases, the leaves and upper fine stems samples of M. oleifera that sprayed with Se fertilizer had higher minerals contents compared to M. peregrina (Table 4). The highest P, Ca, Mg and Se contents due to Se fertilizer application were recorded in the third cut for both plant species.
The results of the current study indicate that there are no negative effects of selenium fertilization on the mineral contents of M. oleifera and M. peregrina. This is consensus with the findings of other studies, which included different fertilization methods and selenium levels [58–60]. The present study has proven that selenium promoted plant growth and didn’t cause any negative impact on the studied minerals contents in the two Moringa species that can pose threats to humans or animals health. The herein reported selenium content in the upper vegetative parts of both Moringa species were within the safe “not toxic” ranges [61,62]. Thus, it can be concluded that the bio fortification process for both M. oleifera and M. peregrina was successfully completed and the target safe levels were obtained.
Table 4:The effect of selenium fertilization (mg/L) on the mineral contents of M. oleifera and M. peregrina leaves and upper fine stems at different cutting periods.
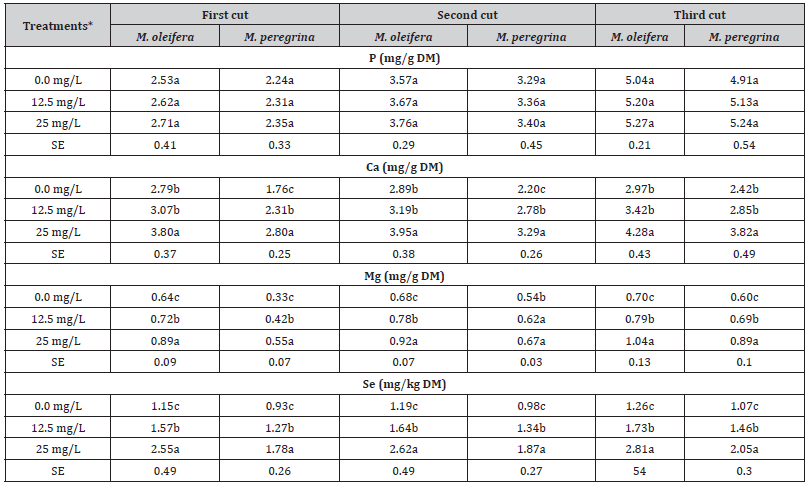
*Means within the same column for each trait with different letters are significantly different at P < 0.05.
Conclusion
The results of the current study indicated that the most effective method for both irrigation and application of Se fertilizer was the irrigation frequency every 20 days, and the foliar spray of Se fertilizer at the maximum vegetative growth of the plants. The highest concentrations of all studied traits were observed in the dry leaves and upper fine stems at drip irrigation frequency of 20 days for both M. oleifera and M. peregrina in the third cutting period. Foliar spraying of Se fertilizer can be considered as a safe method to increase the Se content in M. oleifera and M. peregrina , which may contribute to increasing their nutritional value for livestock farming.
Author Contributions
All authors contributed to the study conception and design. Data curation and formal analysis Elfadil Babiker, Ali Ashaikhi and Faisal Alshamiry; funding acquisition Osman Altahir, Ahmed Alsagan, Khalid Abdoun and Mohammed Alsaiadi; investigation Mohammed Alsaiadi, Ahmed Al-Haidary. and Ali Ashaikhi; methodology Elfadil Babiker, Ali Ashaikhi and Faisal Alshamiry; project administration Ahmed Al-Haidary; supervision, Mohammed Alsaiadi and Ahmed Al-Haidary; validation, Osman altahir, Ahmed Alsagan and Khalid Abdoun; writing - review and editing, Osman altahir, Ahmed Alsagan and Khalid Abdoun. All authors read and approved the final manuscript.
Acknowledgement
The authors extend their sincere gratitude to Organic Standard Company LLC, Riyadh, Saudi Arabia for providing the fertilizer “Amino Selenium 2.5” that used in this research.
Funding
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number 3-17-07-001-0006.
Conflict of Interest
The authors declare no conflict of interest.
References
- Lombo Ortiz, Ibrahim M, Villanueva Najarro C, Tamara B, Skarpe C, et al. (2013) Disponibilidad de biomasay capacidad de rebrote de leñosas forrajeras en potreros del trópico seco de Nicaragua. Agrofor Amer 50: 62-68.
- Pamo ET, Boukila B, Fonteh FA, Tendonkeng F, Kana JR, et al. (2007) Nutritive values of some basic grasses and leguminous tree foliage of the Central region of Africa. Anim Feed Sci Technol 135(3-4): 273-282.
- Meyer K, Hummel J, Clauss M (2010) The relationship between forage cell wall content and voluntary food intake in mammalian herbivores. Mamm Rev 40(3): 221-245.
- Niraj CB, Vardhan HB (2012) Impact of Moringa leaves on erythrocytes maturation in a mammal Cavia porcellus. Indian J Fundam Appl Life Sci 2(2): 26-29.
- Muhl QE, du Toit ES, Robbertse PJ (2011) Moringa oleifera (Horseradish Tree) Leaf Adaptation to Temperature Regimes. Int J Agric Biol 13: 1021-1024.
- Padilla C, Fraga N, Suárez M (2012) Efecto del tiempo de remojo de las semillas de moringa (Moringa oleifera) en el comportamiento de la germinación y en indicadores del crecimiento de la planta. Rev Cub Cien Agríc 46(4): 419-421.
- Nouman W, Siddiqui MT, Basra SMA, Khan RA, Gull T, et al. (2012) Response of Moringa oleifera to saline conditions. Int J Agric Biol 14: 757-762.
- Abdulkarim SM, Long K, Lai OM, Muhammad SKS, Ghazali HM, et al. (2007) Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem 105(4): 1382-1389.
- Nouman W, Basra SMA, Siddiqui MT, Yasmeen A, Gull T, et al. (2014) Potential of Moringa oleifera L. as livestock fodder crop: a review. Turk J Agric Forest 38(1): 1-14.
- Reyes SN, Ledin S, Ledin I (2006) Biomass production and chemical composition of Moringa oleifera under different management regimes in Nicaragua. Agrofor Syst 66: 231-242.
- Fuglie L (2001) Natural Nutrition for the Tropics. CTA/CWS, Dakar, Senegal, pp. 103-115.
- Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 21(1): 17-25.
- Rashid U, Anwar F, Moser BR, Knothe G (2008) Moringa oleifera oil: A possible source of biodiesel. Bioresour Technol 99(17): 8175-8179.
- Adeyinka SM, Oyedele OJ, Adeleke TO, Odedire JA (2008) Reproductive performance of rabbits fed Moringa oleifera as a replacement for Centrosema pubescens. In the proceedings of the 9th World Rabbit Congress. Verona, Italy.
- Alvarado-Ramírez ER, Joaquín-Cancino S, Estrada-Drouaillet B, Martínez-González JC, Hernández-Meléndez J, et al. (2018) Moringa oleifera Lam.: Una alternativa forrajera en la producción pecuaria en Mé Agro Productividad 11(2): 106-110.
- Román-Miranda ML, Martínez-Rosas LA, Mora-Santacruz A, Torres-Morán P, Gallegos-Rodríguez A, et al. (2013) Leucaena lanceolata S. Watson ssp. lanceolata, especie forestal con potencial para ser introducida en sistemas silvopastoriles. Rev Chapingo Ser Cienc Ambiente 19: 103-114.
- Hofmann P, Siegert W, Kenez A, Naranjo VD, Rodehutscord M, et al. (2019) Very low crude protein and varying glycine concentrations in the diet affect growth performance, characteristics of nitrogen excretion, and the blood metabolome of broiler chickens. J Nutr 149(7): 1122-1132.
- Bau HM, Villaume C, Lin CF, Evvrard J, Quemener B, et al. (1994) Effect of a solid-state fermentation using Rhizopus oligosporus sp. T-3 on elimination of anti-nutritional substances and modification of biochemical constituents of defatted rapeseed meal. J Sci Food Agric 65(3): 315-322.
- Makkar HPS, Becker K (1996) Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim Feed Sci Technol 63(1-4): 211-228.
- Ly J, Samkol P, Preston TR (2001) Nutritional evaluation of tropical leaves for pigs: pepsin/pancreatin digestibility of thirteen plant species. Livest Res Rural Dev 13(5): 37.
- Moyo B, Masika PJ, Hugo A, Muchenje V (2011) Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr J Biotechnol 10(60): 12925-12933.
- Mendieta-Araica B, Sporndly E, Reyes-Sanchez N, Salmeron-Miranda F, Halling M, et al. (2012) Biomass production and chemical composition of Moringa oleifera under different planting densities and levels of nitrogen fertilization. Agrofor Syst 87: 81-92.
- Olugbemi TS, Mutayoba SK, Lekule FP (2010) Effect of Moringa (Moringa oleifera) inclusion in cassava-based diets fed to broiler chickens. Int J Poult Sci 9(4): 363-367.
- Mbora A, Mundia G, Muasya S (2004) Combating nutrition with Moringa oleifera. World Agroforestry Centre, Nairobi, Kenya.
- Peñalver R, Martínez-Zamora L, Lorenzo JM, Ros G, Nieto G (2022) Nutritional and antioxidant properties of Moringa oleifera leaves in functional foods. Foods 11(18): 1107.
- Adebayo IA, Arsad H, Samian MR (2018) Total phenolics, total flavonoids, antioxidant capacities, and volatile compounds gas chromatography-mass spectrometry profiling of Moringa oleifera ripe seed polar fractions. Pharmacogn Mag 14(54): 191-194.
- Isaiah MA (2013) Effect of inorganic fertilizer on the growth and nutrient composition of Moringa oleifera. J Emerg Trends Eng Appl Sci 4(2): 341-343.
- Méndez Y, Suárez FO, Verdecia DM, Herrera RS, Labrada JA, et al. (2018) Bromatological characterization of Moringa oleifera foliage in different development stages. Cuba J Agric Sci 52(3): 337-346.
- Palada MC, Chang LC (2003) Suggested cultural practices for Moringa. Asian Vegetable Research and Development Center, Shanhua, Taiwan.
- Nawaz F, Ahmad R, Ashraf MY, Waraich EA, Khan SZ, et al. (2015) Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol Environ Saf 113: 191-200.
- Anjum K, Cheema SA, Farooq M, Hafeez ur Rehman, Haider FU, et al. (2019) Exploring the potential of selenium (Se) and Moringa (Moringa oleifera L.) leaf extract on the production and performance of Triticum aestivum L. J Res Ecol 7: 2390-2402.
- Zhang Z, Gao S, Shan C (2020) Effects of sodium selenite on the antioxidant capacity and the fruit yield and quality of strawberry under cadmium stress. Sci Hortic 260: 108876.
- Wu C, Dun Y, Zhang Z, Li M, Wu G, et al. (2020) Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol Environ Saf 190: 110091.
- Teimouri S, Hasanpour J, Tajali AA (2014) Effect of selenium spraying on yield and growth indices of Wheat (Triticum aestivum L.) under drought stress condition. Int J Adv Biol Biomed Res 2(6): 2091-20103.
- AOAC (2005) Official Methods of Analytical Chemist. Association of Official Analytical Chemists, Inc., Gaithersburg, MD, USA.
- Pedrero Z, Madrid Y, Camara C (2006) Selenium species bioaccessibility in enriched radish (Raphanus sativus): A potential dietary source of selenium. J Agric Food Chem 54(6): 2412-2417.
- Abou-Elezz FMK, Sarmiento-Franco L, Santos-Ricalde R, Solorio-Sanchez F (2011) Nutritional effects of dietary inclusion Leucaena leucocephala and Moringa oleifera leaf meal on Rhode Island Red hen’s performance. Cuba J Agric Sci 45(2): 163-169.
- Offor IFI, Ehiri RCI, Njoku CN (2014) Proximate nutritional analysis of dried Moringa oleifera leaves from Oshiri Onicha L.G.A., Ebonyi State, Nigeria. J Environ Sci Toxicol Food Technol 8(1): 57-62.
- Kakengi AMV, Shem MN, Sawartt SV, Tujiahara T (2005) Can Moringa oleifera be used as protein supplements for ruminants? Asian-australas J Anim Sci 18(1): 42-47.
- Amabye GT, Gebrehiwot K (2015) Chemical composition and nutritional value of Moringa oleifera available in the market of Mekelle. Int J Food Sci Nutr 3: 187-190.
- Yang RY, Chang LC, Weng BBC, Palada MC, Chadla ML, et al. (2006) Nutritional and functional properties of Moringa leaves: From Germplasm to plant, to food, to health. In Proceedings of the workshop Moringa and other highly nutritious plant resources: strategies, standards and markets for a better impact on nutrition in Africa. Accra, Ghana.
- Melesse A, Banerjee S, Meskel DH, Abebe A, Sisay A, et al. (2016) Carcass and meat quality characteristics of Arsi-Bale goats sup-plemented with different levels of air-dried Moringa stenopetala leaf. J Agric Rural Develop Trop Subtrop 117(2): 233-242.
- Nouman W, Siddiqui TM, Basra AMS, Farooq H, Zubair M, et al. (2013) Biomass production and nutritional quality of Moringa oleifera as a field crop. Turk J Agric Forest 37(4): 410-419.
- Sánchez RN, Ledin S, Ledin I (2006) Biomass production and chemical composition of Moringa oleifera 343 under different management regimes in Nicaragua. Agrofor Syst 66:231-344 242.
- Osman MA (2004) Chemical and nutrient analysis of baobab (Adansonia digitata) fruit and seed protein solubility. Plant Foods Hum Nutr 59(1): 29-33.
- Chadare FJ, Linnemann AR, Hounhouigan JD, Nout MJR, Van Boekel MAJS, et al. (2009) Baobab food products: a review on their composition and nutritive value. Crit Rev Food Sci Nutr 49(3): 254-274.
- Hawrylak-Nowak B, Matraszek R, Pogorzelec M (2015) The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol Plant 37: 41-53.
- Hajiboland R, Sadeghzadeh N, Ebrahimi N, Sadeghzadeh B, Mohammadi SA, et al. (2015) Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agric Slov 105(2): 175-191.
- Hawrylak-Nowak B, Dresler S, Rubinowska K, Matraszek-Gawron R, Woch W, et al. (2015) Selenium biofortification enhances growth and alters the physiological response of lamb's lettuce grown under high temperature stress. Plant Physiol Biochem 127: 446-456.
- Chen H, Cheng Q, Chen Q, Ye X, Qu Y, et al. (2022) Effects of selenium on growth and selenium content distribution of virus-free sweet potato seedlings in water culture. Front Plant Sci 13: 965649.
- Saini RK, Shetty NP, Giridhar P (2014) Carotenoid content in vegetative and reproductive parts of commercially grown Moringa oleifera Lam. cultivars from India by LC–APCI–MS. Eur Food Res Technol 238: 971-978.
- Tang C, Luo S, Rui W, Yan H, Yin B, et al. (2020) Migration and transportation of selenium in Moringa oleifera Lam.-soil system. J Food Saf Qual 11(19): 7135-7141.
- Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178: 84-91.
- Hajiboland R, Sadeghzadeh N (2014) Effect of selenium supplementation on CO2 and NO3 – assimilation under low and adequate N supply in wheat (Triticum aestivum L.) plants. Photosynthetica 52(4): 501-510.
- Al-Fraihat AH, Al-Dalain SYA, Al-Rawashdeh ZB, Abu-Darwish MS, Al-Tabbal JA, et al. (2011) Effect of organic and biofertilizers on growth, herb yield and volatile oil of marjoram plant grown in Ajlounregion. Jordan J Med Plant Res 5(13): 2822-2833.
- Ali HAM, Yagoub SO, Hamza NB (2015) Chemical analysis of Moringa oleifera and M. peregrina and their growth responses to water stress under semi- desert conditions of Sudan. J Appl Life Sci Int 3(1): 7-14.
- El-Sayed MM, Mahmoud AM (2018) Irrigation and fertilization practices for Moringa plant growth under Upper Egypt conditions. Middle East J Appl Sci 8(1): 145-156.
- Ellis DR, Salt DE (2003) Plants, selenium, and human health. Curr Opin Plant Biol 6(3): 273-279.
- Boldrin PF, Faquin V, Ramos SJ, Boldrin KVF, Avila FW, et al. (2013) Soil and foliar application of selenium in rice biofortification. J Food Comp Anal 31(2): 238-244.
- Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7: 2074.
- White PJ (2015) Selenium accumulation by plants. Ann Bot 117(2): 217-235.
- Bañuelos GS, Freeman J, Arroyo I (2019) Accumulation and speciation of selenium in biofortified vegetables grown under high boron and saline field conditions. Food Chem X 5: 100073.
-
Khalid A Abdoun*, Osman A Altahir, Ahmed A Alsagan, Mohammed Y Alsaiady, Elfadil E Babiker, et al. Evaluation of Irrigation Frequency and Selenium Fertilization Impacts on the Nutritional Traits of Moringa oleifera and Moringa peregrina. World J Agri & Soil Sci. 9(1): 2023. WJASS.MS.ID.000704.
-
Moringa, Water conservation, Foliar spray, Nutrients content
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






