 Research Article
Research Article
Effects of Natural Forest, Plantation Forest and Grazing Land on Soil Microbial Biomass and Soil Enzyme Activities in Ethiopian Highland
Hodaddis Kassahun1*, Iftekhar U Ahmed2, Douglas L Godbold2 and Hans Sandéna2
1Sirinka agricultural research center, P.O.Box 74. Woldia, Amhara Regional State, Ethiopia
2Institute of Forest Ecology, Universität für Bodenkultur (BOKU), Peter Jordan Strasse 82, Vienna, Austria
Hodaddis Kassahun, Sirinka agricultural research center, P.O.Box 74. Woldia, Amhara Regional State, Ethiopia.
Received Date:April 14, 2022; Published Date:April 26, 2022
Abstract
Microbial biomass and enzyme activities play a crucial role on availability and cycling of nutrients in soil ecosystems. The main objectives of the study were to determine the effect of natural forest, plantation forest and grazing land on soil microbial biomass carbon and nitrogen and soil enzyme activities in Gelawudios, Ethiopia. To determine soil microbial biomass C and N fumigation-extraction method was followed and for enzyme activities a fluorometric enzyme assay method based on methylumbelliferone-linked (MUF) was used. Microbial biomass carbon and nitrogen were highly influenced by the land use systems following the order: natural forest>plantation forest>grazing land. Under different tree species microbial biomass carbon was not statistically differing however, microbial biomass nitrogen showed significant difference among tree species. Enzyme activities of β- xylosidase and β-Glucosidase in soils of all tree species under natural forest except Apodytes dimidiata were much higher than the plantation forest and grazing land. In our study enzyme activities had closely positive correlated with soil organic C and also pH of the soil. Natural forest area had the limitation of N, plantation forest and grazing land had the limitation of P and C.
Keywords: Natural forest; Grazing land; Microbial biomass; Plantation; Enzyme activities
Opinion
Soil is a complex heterogeneous mixture of organic and mineral materials that is recognized as one of the single-most important natural entity for ecosystem functioning [1]. Microbial community and organic substrates are vital for many ecosystem services and processes such as: nutrient cycling, respiration, decomposition, C sequestration and storage and tree species favors distinct microbial community under their canopies in forest ecosystems [2]. Microbial biomass is recognized as an indicator of soil quality because of its crucial role in decomposition, respiration, nutrient release and sensitive to alteration of management. Tropical forests have enormous influence on the cycling of global carbon(C) and it contains about 34-55% of all the carbon in the forest of the world [3]. The micro bial biomass makes up about 1 to 3 % of total soil C and also other nutrient elements and formation of microbial biomass and fixation of nutrients is encouraged by root deposits, plant residues and presumably the addition of nutrients to soil is due to the death of microorganisms [4]. Soil microorganisms and their enzymes are the most important factors for decomposition of leaves, debris, twinges and other material in the ground which improves the soil fertility. Organic matter in the soil is the main component for soil ecosystem process and microbes as a part of organic matter, is essential for decomposition and turnover [5]. Land resources of Ethiopia are facing severe anthropogenic pressure mostly due to rapidly population growth and it induces conversion of forest lands to crop land/ gazing land which causes land degradation and affect physical, chemical and biological properties in the soil [6]. Shifting the forest area to cropland or gazed land may affect the soil organic carbon process due to change in management pattern that consequently modify quality and quantity of soil organic matter accumulation. Ungulate grazing can influence soil microbial biomass through altering the amount and composition of soil organic matter. In general, grazing land receives organic inputs from vegetation and animal excreta which can contribute high level of soil organic matter and consequently positive impact on soil biological processes [7]. Soil organic matter has a direct link to soil microbes, microbial biomass in soil is affected by grazing management. However, over grazing may cause land degradation and thus negative impact on soil microbial biomass [7] reported that soil microbial biomass in grazed land was 26% higher than the adjacent forest during the rainy season. Lepcha et al. [8] found higher soil microbial biomass C in moderately grazed soil than non-grazed and heavily grazed soils in sub-tropical grassland. Microorganisms are the major source of soil enzymes; some scholars reported that plant species can influence soil enzyme activity through microbial in soil rhizosphere besides land use change and forest management practices are major factors by influencing the quantity and quality of soil organic matter [9]. Many scholars reported that there is strong relationship between land use change and soil microbial community [10].
Soil enzymes consists both intracellular and extracellular enzymatic proteins originated from microbes, plant and animal cells that catalyze various reactions in soil such as energy transfer, nutrient cycling and decomposition processes [11]. The intensity of enzyme activities in the soil considered as an indicator of soil health and quality and it facilitate and stimulate soil biochemical processes in order to plant growth and soil environment [12].
Extracellular enzymes had vital role for the decomposition of forest letters and soil organic matter, and it would increase the storage of soil carbon and has very critical role in soil organic matter oxidation process it breaks down urea into CO2 and NH3 [13]. Soil enzyme activities are sensitive to soil management and land use changes and thus can reflect the change of soil quality [14].
In general, extracellular enzymes are responsible for breaking down of complex organic macromolecules such as protein, lipid, and polysaccharide to simple’s forms of amino acids, fatty acids and monosaccharaides respectively and simple molecules could be utilized by microorganism or plant roots in the soil [15].
In the forest areas there are different microbes like bacteria and fungi which are main source of extracellular enzyme synthesis and secretion of enzyme like proteases, ureases, and pectinases [16]. Saprophytic fungi in the soil can produce extracellular enzymes to degrade substrate and adsorbed by clay minerals or occluded in association with humus substances to maintain their activities [15]. The type of tree species can be influencing soil microbial biomass and its activities due to the characteristics of their litter fall [17]. Enzyme activities could be changed because of forest management practices due to altering the basic properties of the soil such as soil moister or temperature shading or the availability of nutrient inputs [18].
Environmental conditions whether natural or anthropogenic factors could be influences enzyme activities either directly or indirectly, these consists of soil physico-chemical properties, organic matter accumulation, and texture of the soil and land use management, environmental pollution, use of inorganic fertilizer, insecticide, pesticide [17]. The surrounding temperatures also affect enzymatic activities in the soil by influencing the dynamics and stability of enzymes [1]. Thus, Extracellular enzymes in soil are sensitive to temperature and it is changing the structure of protein availability and the metabolic rate of microorganism that producing extracellular enzymes in soil is higher with increasing temperature over the range of 5-40 °C [19].
In Ethiopia there is gap of knowledge and information related to soil ecology and the impact of land uses i.e. natural forests, plantation and grazing land on the soil and its contribution to enhance the soil health as well as biophysiochemical properties of the soil ecosystem. Therefore, the aim of this study was to determine the effect of natural forest, plantation forest and grazing land on soil microbial biomass carbon and nitrogen and soil enzyme activities in Gelawudios, Ethiopia.
Material and Methods
Site description
The study was conducted at a natural remnant forest at Gelawdiwos, a Eucalyptus plantation and grazing land the Amhara National Regional State (11°38’25’’ N 37°48’55’’ E) in North- West Ethiopia. The forest type is classified as Afromontane dry tropical. The altitude of the study area is 2500 m above sea level. The area has a monsoonal climate with mean annual temperature of 19 °C and the mean annual precipitation is about 1200 mm with the main rainy season between June and September [20]. The majority of the soils in the study area are Cambisols [21] with weak horizon differentiation and rocks below 50 cm depth . The forest at Gelawdios has an area of 19 ha and is a remnant of natural pristine forest composed of mostly an intimate mixture of indigenous tree species. Dominant tree species in the study area are Albizia schimperiana, Apodytes dimidiata, Calpurnia aurea, Croton macrostachyus, Ekebergia capensis, Maytenus arbutifolia, and Schefflera abyssinica. The adjacent grazing land is highly degraded due to erosion of the topsoil and characterized by scattered bushes, grass and large patches of bare soil. Eucalyptus globules plantation was established on previously grazing land around 1985 and was consecutively thinned.
Collection of soil samples: In the natural forest four the most dominate tree species were randomly selected, Chionanthus- mildbraedii-, Apodytes dimidiata, Teclea nobilis and Combretum molle and as plantation forest Eucalyptus globulus and Cupressus lusitanica were taken and eight trees were randomly chosen from each species and also adjacent grazing lands were selected. Soil samples were collected from the areas under the canopy of each individual tree. Sampling points were 1.5 m from the tree stem. The sampling depth was 0-6-cm as this layer has the highest levels of soil organic matter. In the plantation forest, eight soil and litter samples were collected from forest floor and topsoil under each two species as stated above. .The distance between two samples was 20m; the soil sampling depth and litter collection technique were similar as in natural forest sampling. The soil and litter samples were collected at 1.5 m distance from the main stem. The distance between two samples was 10m and the samples were taken to a depth of 6 cm. All soil samples were stored in plastic bags and transported to laboratory.
Determination of microbial biomass and enzyme activity in the laboratory
The enzyme activities were determined in each soil samples. There were six types of enzyme activities. Leucine amino peptidase, β-xylosidase, Cellobiohydrolase, N- Acetylglucosaminidase, β-Glucosidase and Acid phosphatase.
Sample preparation: In the laboratory, soil samples were sorted into litter, roots and stones, and half of the soil was air-dried at room temperature, ground and sieved using a 2 mm sieve. Another half of the sample was kept at field moisture. All samples were stored at 4 °C in a cool room until further analysis.
Procedures applied for fumigation extraction method: 0.5 M K2SO4 solution was prepared by dissolving 87g of K2SO4 in 1L of deionized water. Preparation of ethanol free chloroform was prepared by removing ethanol by washing 100ml chloroform with 100ml of 5% H2SO4 using a separating funnel and draining the chloroform from the bottom by using separating funnel into a beaker. Finally, the chloroform was washed three times with 100ml deionized water [22].
0.5gram of soil from each sample was taken and placed in a glass via and the exact mass of the soils was recorded using a fine balance. A separated portion of soils was put into the oven for measurement of the moisture content. The soil samples were put into the dissector with 25ml ethanol free chloroform in a glass beaker at the center of the desiccator. A piece of paper towel soaked with water was put inside desiccator. The desiccator was connected with suction pump. Some anti-dumping granules were put into chloroform beaker to prevent the spillage of boiling chloroform. The air inside desiccator was slowly removed using suction pump until the chloroform boiled and after 1-2 min fumigation, the outlet of the desiccator was closed, and the suction pump was disconnected. The desiccator was covered with black cover and kept for 24 h. After the fumigation, the soils were transferred in to 50ml centrifuge tubes and 25ml of 0.5 molar K2SO4 were added to each sample in centrifuge tubes and shaken for 6 hours by using an orbital shaker, and then centrifuged for 5 min. at 4000 revolutions per minutes (rpm). The supernatant was filtered through filter paper (What man 42) and kept in 20ml plastic scintillation vials at below 4 °C until analysis. Non- fumigated samples were extracted as described above.
Calculations:
The following formula has applied to calculate microbial biomass
C and N.
MBC= (FC-NFC)/ 0.45
Where,
MBC= Microbial Biomass Carbon 0.45= Extraction efficiency for
carbon FC=Fumigated Carbon and,
NFC= Non-Fumigated Carbon
MBN= (FN-NFN)/0.54
Where,
MBN= Microbial Biomass Nitrogen 0.54= extraction efficiency
for Nitrogen FN=Fumigated Nitrogen and,
NFN= Non-Fumigated Nitrogen
Soil Moisture Content and Loss on Ignition: Approximately 5g soil from each sample was weighed into the pre-weighted aluminum cup and dried in an oven at 105 °C for 24 hours. After oven drying, the samples were cooled in a desiccator then weighed it to calculate the percentage of moisture content in the soil. To determine the amount of organic matter by loss on ignition, the same sample was ignited in a muffle furnace at 450 °C overnight. After ignition, the samples were cooled in desiccator and the final weight was recorded Per cent organic matter was calculated as follows:
Weight of OD sample-Weight of ignited sample
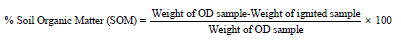
The procedures used for substrate preparation: For the enzyme analysis stock solutions of substrates were prepared in Na-acetate buffer pH 6.5 were prepared as shown in Table 1 and frozen at -18 °C until needed. For all substrate were used methoxyethanol to dissolve but for leucine amino peptidase was not used because the substrate was easily dissolved (Leucine-AMC substrate) (Table 2).
Table 1:Working solution concentration were used for the six different substrates.
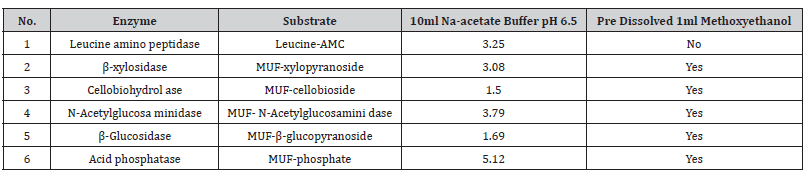
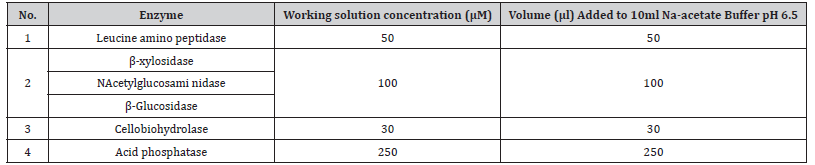
Table 2:The standard four working concentration solution were used for the standard curves.
Slurry Preparation
Initially 0.5 g of fresh soil from each sample were taken and placed into a small beaker, and then 50ml tris 6.5 pH buffers were added. The soil slurry was stirred in both a magnetic stirrer and a hydrosonic stirrer for three times for each sample.
The soil enzyme activity analysis:For the enzymes β-xylosidase, Cellobiohydrolase, N-Acetylglucosaminidase and β- Glucosidase 200 μl of slurry and 50 μl of substrate were used. Leucine amino peptidase 25 μl and acid phosphatase 100 μl of slurry were used. As a control 200 μl slurry and 50 μl 50ml tris 6.5 pH buffers were added, and for the substrate control 200 μl buffers and 50 μl substrates were added. Each analysis was carried out using three replications per plate. To measure quenching, 200μl of soil slurry and 200 μl buffer were used. To these 5 μl, 10 μl, 15 μl, 20 μl, 25 μl, 30 μl or 35 μl of the working standard solution were added. For the standard curve 5 μl, 10 μl, 15 μl, 20 μl, 25 μl, 30 μl or 35 μl of the working standard solution were added buffer to a volume of buffer to make up 250 μl. After pipetting the plate was placed in 20oC incubator for 2 hours and then analyzed. The amount of fluorescence was determined in a fluorimeter (Multimode Plate Reader, EnSpire) at 365 nm excitation and 460 nm emission using 20 flashes.
pH analysis:Approximately 5ml of soil was transferred to glass tube and 20 ml of deionized water was added. The sample was vortexes for 5 minutes and allowed to settle at 4oC overnight. After adjusting to room temperature, the pH was measured by using a pH meter (inolab®pH/ION.735, WTWSeries).
Statistical analyses:To compare and contrast all collected data obtained from laboratory results were analyzed by applying Tukey hoc one-way ANOVAs by using R software at p<0.5 significant different level and figures were constructed by using Microsoft Excel and R software.
Results and Discussion
Effects of land use systems to soil microbial biomass carbon and nitrogen
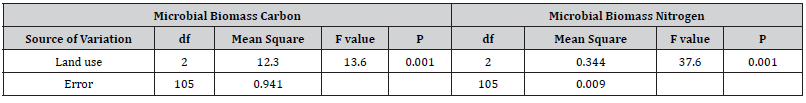
Soil microbial biomass carbon under natural forest on average the highest microbial biomass carbon was observed than grazing land and plantation forest soil which was followed by plantation forest and grazing land (Table 3). Grazing land contained the lowest quantity of soil microbial biomass carbon which scored 80% and 70% lower than natural and plantation forests respectively. The results of ANOVA revealed that these variations were statistically highly significant. Further multiple comparison test (Tukey) showed that difference between natural forest and grazing land (p=0.001) and plantation forest and grazing land (p=0.002) were statistically significant but not between natural forest and plantation forest (p=0.246).
Table 3:land use system with soil microbial biomass carbon and nitrogen, Mean ± standard error (SE), Similar letters in the columns are not statistically significant different whereas different indices indicated that they significantly different at (p<0.05).
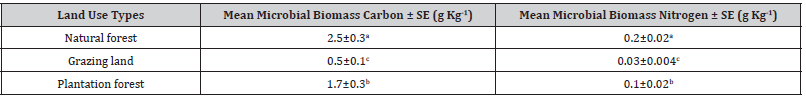
Overall result had no impacts on soil microbial biomass carbon irrespective of land use types. However, in natural forest slightly lower soil microbial biomass carbon was observed in wet season and in other two land-uses no seasonal variation was found. Our multivariate analysis showed significant interaction effects of land use and seasons (p=0.034). Microbial biomass nitrogen content in three land use types followed the same trend as shown in microbial biomass carbon: natural forest > plantation > grazing land. However, grazing land had much lower nitrogen content (85% lower than natural forest) and plantation forest remain intermediate level and the variations in soil microbial biomass nitrogen over three land use types were statistically significant (Table 4).
Table 4:Summery of multivariate analysis for soil microbial biomass carbon and nitrogen in relation to land use system.
Effects of tree species on soil microbial carbon and nitrogen
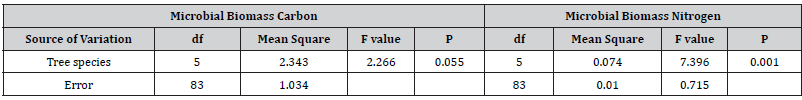
Microbial biomass carbon showed no significant variations over different tree species in natural and plantation forests however, among different tree species, Chionanthus- mildbraedii and Combretum molle showed the highest soil microbial biomass carbon and C. lustianica was the lowest (58% lower than Combretum molle). On the other hand, seasonal variations in soil microbial biomass carbon under different tree species were statistically showed significant variation (Table 5). Post Hoc test indicated that the variation between Chionanthus- mildbraedii and Teclea nobilis was significant different at (p=0.001) (Table 6).
Table 5:Soil microbial biomass carbon and nitrogen among different tree species, Mean ± standard error (SE), the similar letters in the columns are not statistically significant different and different letters showed significant different at (p<0.05). Ns (non-significant).
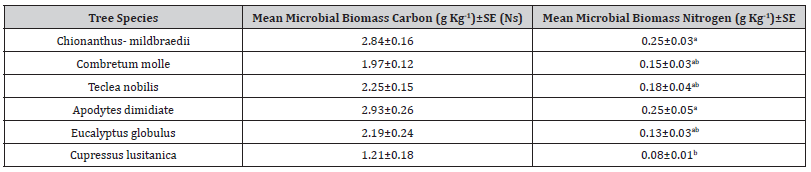
Table 6:Summary of Post Hoc multivariate significant variation analysis of soil microbial biomass carbon and nitrogen among different tree species at p<0.05.
C: N ratio in soil and microbial biomass
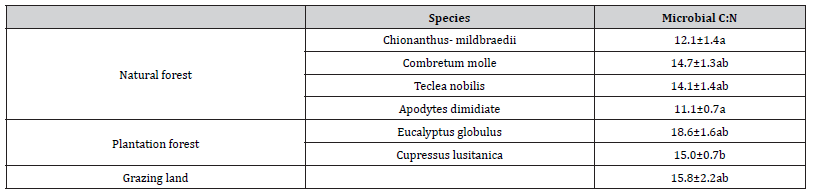
Consistently higher C: N ration was observed in soils of two plantation species compared with soils of species in natural forest and grazing land. C: N ratio in soil microbial biomass was affected land use during wet season, no effect species identity was observed in natural and plantation forest. There no significant variation among tree species and grazing land except Cupressus lusitanica which had variation with Chionanthus- mildbraedii (Table 7).
Table 7:Soil microbial biomass carbons and nitrogen ratio under different land use system. Mean ± standard error (SE), value with similar letters in the column has no significant different whereas different indices showed significant different at (p=0.05).
Correlations
The relationship between soil pH and soil microbial biomass N and C in soil have weak positive relation at R2= 0.49 and 0.34 (Table 8 ). Microbial biomass C:N ratio with pH there was also weak positive relation. Soil organic carbon with microbial biomass carbon has strong positively related (Table 8). The relationship between microbial biomass C and N was very strong at R2=0.88. A strong relationship between soil MB C and N was observed at ( R2= 0.88). So, when microbial biomass C increased with increased microbial biomass N (Table 8).
Table 8:correlation between soil pH, soil organic carbon (%) with microbial biomass C, N and C: N ratio at R2=Correlation coefficients.

Enzyme activities among different tree species and grazing land
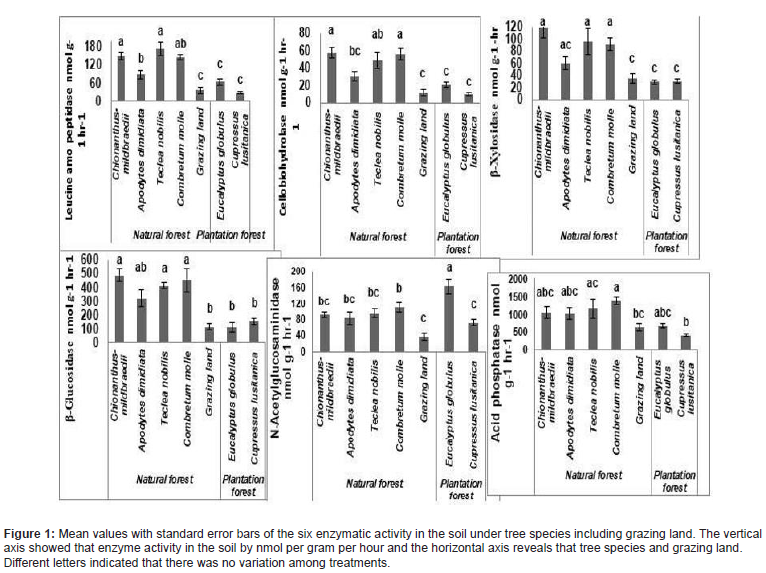
In all types of enzyme activities were showed significant differences among tree species and grazing land (Figure 1). In general, the enzyme activity was lower in the grazing land and plantations tree species than under natural forest tree species the result revealed that the rate of enzyme activity in the sequence of natural forest>plantation forest>grazing land. In Leucine amino peptidase, mobilizing N, the enzyme activity in the soil below Apodytes dimidiata was lower than Teclea nobilis and Chionanthus- mildbraedii but all the trees in the natural forest had higher enzyme activity than in the soil from the grazing land and the plantations. The grazing land and the plantations did not differ in Leucine amino peptidase activity. In Cellobiohydrolase enzyme, which is mobilizing C in the soil under Chionanthus- mildbraedii, Combretum molle was higher than under Apodytes dimidiata. The grazing land soil and the plantation soils did not differ in enzymatic activity just as for Leucine amino peptidase and was also significantly lower than the under the trees in the natural forest except for Apodytes dimidiate. β-xylosidase and β-Glucosidase showed the same pattern in enzyme activity between the species and grazing land and the enzyme activity under the natural forest trees was once again higher than in the grazing land and the plantations except for Apodytes dimidiate. There were however no differences between the soil under the trees in the natural forest. There were any differences between the grazing land and the plantations. N-Acetylglucosaminidase enzymes, which is mobilizing N and C from chitin showed a very different pattern compared to the other C and N-mobilizing enzymes. Eucalyptus globulus had here significant higher enzyme activity in the soil than any other tree species and the grazing land. However, the trees in the natural forest there was no difference and only Chionanthus- mildbraedii showed significant higher activity than the grazing land and the Cupressus lusitanica plantation. The grazing land and the Cupressus Lusitania did not differ in N-Acetylglucosaminidase activity. In acid phosphatase enzyme there were no differences between the soil under the species in the natural forest. Only Combretum molle showed a higher enzymatic activity in the soil than the grazing land and the plantations. The other tree species in the natural forest tended to be higher than the plantations and the grazing land but this was not significant. In all enzymes except N-Acetylglucosaminidase there was in phosphatase also no difference between the grazing land and the plantations.
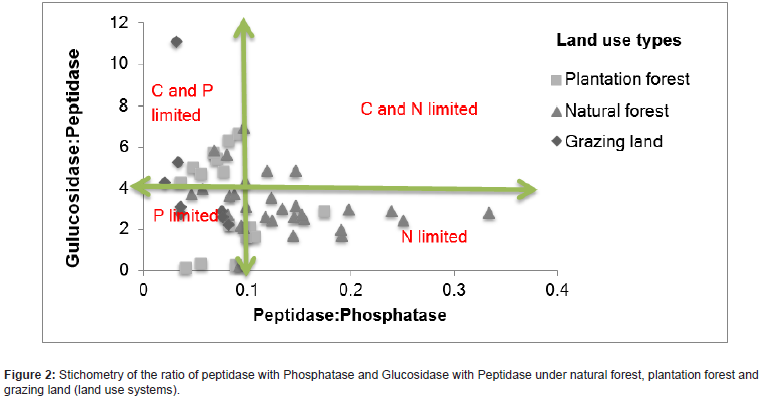
According to the result showed that what type of nutrients were limited among tree species. Plantation forest and grazing land had much higher limitation on C and P , under natural forest trees species soil showed that higher limitation on the availability of nitrogen and they had slight limitation on soil C (Figure 1).
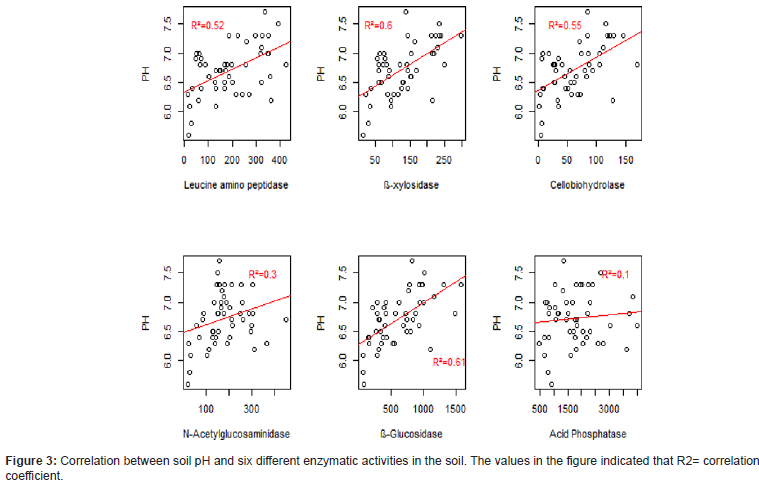
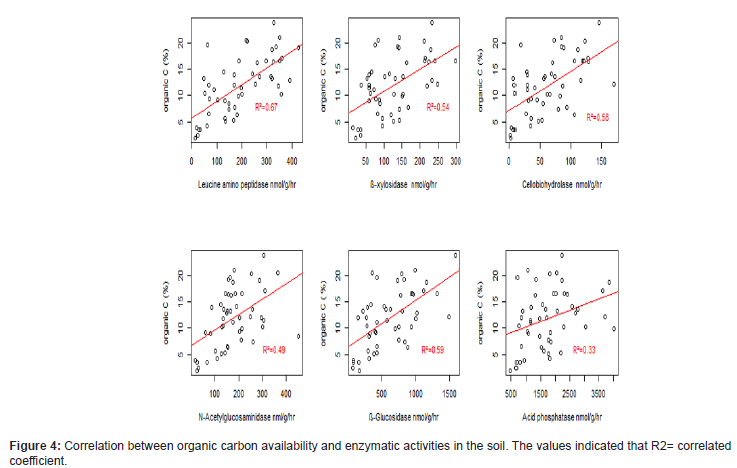
There is a positive relation in both pH and organic carbon with all enzymatic activities. Thus, when the pH increases the enzyme activities increases. Especially ß- glucosidase had showed highest or strong positive relationship with soil pH and soil organic carp on the others (R2=0.61 and 0.59, respectively) while in Acid phosphatase enzyme activity showed very weak relationship (R2= 0.1 and 0.33, respectively) (Figures 2-4).
Discussion
Change of the forest to cropland or grazing land may affect the soil organic carbon process due to change in management pattern that consequently modify quality and quantity of soil organic matter accumulation. Ungulate grazing can influence soil microbial biomass through altering the amount and composition of soil organic matter. In general, grazing land receives organic inputs from vegetation and animal excreta which can contribute high level of soil organic matter and consequently positive impact on soil biological processes [23]. Soil organic matter has a direct link to soil microbes; microbial biomass in soil is affected by grazing management. However, over grazing may cause land degradation and thus negative impact on soil microbial biomass [7] reported that soil microbial biomass in grazed land was 26% higher than the adjacent forest during the rainy season. Lepcha et al. [8] found higher soil microbial biomass C in moderately grazed soil than non-grazed and heavily grazed soils in sub-tropical grassland.
Consistent with our findings, Ajema [24] reported significantly higher soil microbial biomass in natural mixed forest (1350 mg kg-1) than other land use such as crop lands and plantation forest. In another study [17] found that significant reduction in soil microbial biomass C due to conversion of natural broad-leaved forest to plantation forest in sub-tropical China. There are many factors that can explain the influence of land use change on soil microbial biomass C such as soil organic matter content, soil C , pH etc. however, Ajema [24] suggested that soil organic C might be the most influential factor. Because the labile fraction of soil organic C such as glucose and sucrose are readily available to soil microorganism to rapidly propagate and increase their activities. Therefore, soil microbial biomass C is effectively limited by availability of soil organic C. This was supported by our soil C data that grazing land with the lowest C content than other two land use types [25] found that grazing decreased soil microbial biomass. In our study sites, land was degraded due to over grazing which might have influence on quantity of microbial biomass. Overall, land use change in our experiment sites altered the soil properties considerably which presumably affected soil microbial composition as previous studies confirmed that soil physico chemical properties particularly soil pH and nutrient elements had a profound impact on soil microbial communities and eventually on soil microbial biomass [10]. Our results showed significantly lower microbial biomass N in grazing soil than forest which indicated severe depletion of soil fertility due to land use change. During decomposition of soil organic matter, part of released N immobilized in the body of soil microorganisms, however the turnover rate this microbial biomass N is ten times faster than the N plant litter [17]. Therefore, the reduction of soil microbial biomass N can affect the productivity of soils.
Effect of tree species on soil microbial biomass C and N
Our results indicated no effect of tree species on soil microbial biomass C in natural forest. Tree species could influence the composition and function of soil microbial community structure by changing forest microclimates, quality and quantity of above and below ground litter production, production of root exudates, symbiotic association with mycorrhiza and other fungi [26]. Thus, in general, it is presumed that tree species can influence soil microbial biomass C and N. However, Liu et al [17] reported that the influence of individual species identity on microbial communities of soil and rhizosphere could be pronounced when the trees were grown in monoculture [24] suggested that effects of tree species and coexistence were more pronounced on soil microbial composition than total biomass. Therefore, it was uncertain to identify the impacts of individual tree on soil microbial biomass in natural mixed forest as in our study site. This was supported by our findings of microbial biomass in plantation forests where two monoculture species E. globulus, C. lusitanica soils are significantly differ microbial biomass C.
Soil microbial biomass N significantly differed under tree species of both natural and plantation forests. Soil microorganisms are involved in nitrogen mineralization and nitrification processes to provide nitrogen supply. Our results indicated that C. mildbraeddi of natural forest had the highest microbial biomass N and C. lusitanica of plantation forest had the lowest. We did not analyze the chemical composition of leaf litter from different tree species. However, variation in soil microbial biomass N under different tree species might be attributed to litter quality and subsequent N mineralization. Kacálek et al. [27] found that the litter quality (lignin: N) might have more influence in controlling nitrogen mineralization in organic and mineral soil. Tree species in our study showed no effect on soil microbial C because microorganisms differ more widely in their N content than C depending on age. As a result, small variation in soil microbial community structure can results in larger change in biomass N than C [28].
Response enzyme activities on land use change
The type of tree species may have influence on soil microbial biomass and its activities. The quantity and quality of organic matter is depending on the natural characteristics of tree species [24]. Tree species and land use systems had a significant effect on the potential enzymatic activity in the soil. The enzyme activities increase in the order: forest>plantation forest> grazing land. In all types of enzyme activities there were significant differences among tree species and grazing land as shown (Figure 1). In general, the enzyme activity was lower in the grazing land and plantations than under the trees in the natural forest. In Leucine amino peptidase, mobilizing N, the enzyme activity in the soil below A. dimidiate was lower than T. noblies and C. mildbraedii but all the trees in the natural forest had higher activity than in the soil from the grazing land and the plantations. The grazing land and the plantations did not differ in Leucine amino peptidase enzyme activity.
Microbial biomass highly influenced enzyme activities in the soil and there is variation in enzyme activities in different land use. Least microbial biomass and enzyme activities was found under grazing land. Likely due to low organic carbon or organic matter input. The Low C availability causes less microbial biomass which leads to less enzymatic activities. Forests has been found to have higher enzyme activities than grazing land and agricultural land [29]. As microbial biomass, enzyme activities were positively relat ed with organic C. It explains that if there is sufficient availability of substrate in the soil for microbes the potential of enzyme activities would be more. The similar finding reported that organic C in the soil had strong correlation with enzyme activities and nutrient availability raised leads to the improvement of soil microbial biomass in the soil [15].
Correlation between microbial biomass C and N and soil properties
Soil pH is one of the influential factors for soil microbial C and N. It is closely related to some abiotic factors such as availability of C and nutrients and solubility of metals that have great impacts on growth of soil microorganisms. We have a positive correlation with soil pH and microbial biomass C and N (Table 8). Averill et al. [30] reported the decrease in bacterial growth and increase in fungal growth by decreasing soil pH. Microbial biomass C is strongly and positively correlated with microbial biomass N (Table 8). This is in agreement with Jin et al (2007) who found a significant positive correlation between microbial biomass C and N (r2=0.864) in calcareous surface soil. The addition of substrate organic matter can influence C and N through comprehensive mineralization and immobilization processes. Microbial biomass in soil are linked with both quality and quantity of available organic matter, however, bacterial community composition was more influenced by quality of organic matter [31,32].
Enzymatic activity was in general also positively correlated to the pH of the soil (Figure 3). Similar study reported that Enzyme activities are rising with increase soil pH and it has high effect on microbial community compositional structure and organic and inorganic ameliorants on soil enzyme activities in contrast, enzyme activities under the soil pH value at 5.2, 6 and 7 indicated that more or less similar schemes, while the soil pH is 8.2 significantly lower, most probably due to conformational alteration in protein arrangement close to or somewhat above physiological pH values of the soil, which assist denaturation [23,33]. The similar finding showed that where the area has low soil fertility and the soil pH value like Oxisols and Ultisols have actually maximum enzymatic activities than incomplete weathered tropical soils type Inceptions, most probably in the cause of its maximum availability of organic matter and better texture and these enzyme activities influenced with agricultural management system which reduced the essential biochemical reaction in the soil and its effectiveness [34,35].
Acid phosphatase had no strong correlation with pH and a week with C (Figure 3). This is in accordance with [13] who found that Soil pH had a significant negative on acid phosphatase activity. The lack of a positive correlation may depend on the plants also exudates acid phosphatase and that the plant activity is not regulated by carbon and pH as microbes.
Many studies have found that the addition of nutrients can have both positive and negative influence on C, N and P acquiring enzymes. The activity also depends on the type of tree species in the area [34]. We found that under natural forest area the microbes are more N limited, whereas in both plantation forest and grazing land the microbes were more P and C limited. That the microbes under
N-Acetylglucosaminidase enzyme activities in the soil of Eucalyptus globulus plantation was observed too have much higher potential activity than all other trees. N- Acetylglucosaminidase enzyme activities are important for mobilizing C and N from chitin groups [25]. Chitin is a structural compound in the fungi cell wall. A high N-Acetylglucosaminidase activity could indicate a high abundance of fungal material probably derived form mycorrhiza belonging to Eucalyptus globulus. Tree species which have fast decomposition characteristics of litter are increases carbon dependent enzyme activities in the soil [36].
Eucalyptus globulus had high P limitation because they are fast growing tree species with recalcitrant litter. Their Eucalyptus globulus might be able to allocate C belowground punching the system into P limitation and fast decomposition litter type tree species increases carbon dependent enzyme activities in the soil [37].
In Leucine amino peptidase enzyme activity Teclea nobilis had a higher activity compared to the other tree species in the forest [38]. However, In our result both Acid phosphatase and Leucine amino peptidase enzyme activities were much higher in the soil under different tree species with compared to other enzyme activities. Cellobiohydrolase enzyme activities under Chionanthus- mildbraedii-, Teclea nobilis and Combretum molle had significant different with plantation forest and the cellobiohdrolase enzyme activity is highly affected by both ozone and carbon dioxide interaction [39]. Chionanthus- mildbraedii- and Combretum molle had significant different with Apodytes dimidiata while, Apodytes dimidiata had no significantly different with Eucalyptus globulus and Cupressus lusitanica. In both β-xylosidase and β-Glucosidase enzyme activities in the soil almost all-natural forest species showed that high value except Apodytes dimidiata. Chionanthus- mildbraedii-, Teclea nobilis and Combretum molle were significantly varied with plantation forest, while Apodytes dimidiata species had no significant variation with plantation forest (Figure 1) [40,41].
Conclusion
Land use change has great influence where due change of the type of vegetations on the process of soil microbial biomass activities because it has highly influenced by the quantity and quality litter inputs on the surface of the soil. Because high amount of microbial biomass indicated that the sustainability potential of the soil to the soil ecosystem and important to design land use management systems. . Under natural mixed forest, there was little variation in soil microbial biomass C and N under different tree species. However, variations were observed between the trees in mixed natural and monoculture plantation forests. Land use change and subsequent degradation of soil properties was found as the major cause for depletion of soil microbial biomass. Effects of tree species was more pronounced mainly under monocultural plantation forest while, soil enzyme activities among different tree species were not consistent over types of enzymes. Furthermost of the enzyme activities from the sex enzymes where under plantation forest exhibited much lower than the natural forest. Enzyme activities had significant positive correlation with both organic C. Enzyme activities had strong relationship with both organic C which told that enzymes are more dependent on the availability of microbial biomass in the soil. We have also observed natural forest tree species had the limitation of nitrogen while, plantation forest tree species had the limitation of phosphorus as well as carbon. limitation of phosphorus in the soil might have revealed the area has exposed to topsoil erosion (the soil might have less infiltration capacity). The results on soil enzyme activities were not consistent over different land use and tree species, rather some enzymes were sensitive to particular tree species. Therefore, it cannot be generalized that land use had more influence on soil enzyme activities than tree species.
Acknowledgement
First of all, I would like to say thank you for my Almaty God. Next to that I have grateful for my advisors Professor Douglas L. Godbold, and my co-advisors Dr. Iftekhar Ahmed and Hans Sandéna, they were gave me a fruit full and appreciable comments on my thesis work and assisted every time without exhausting and great thank for the Austrian government and people who allowed me to study my education. I have grateful for BOKU University and all my classmate students we were spent a great time with closed friendly and nice relationship and Amhara Agricultural Research Institute (ARARI) whose gave me a chance to do my MSc program in mountain forestry and also they provided vehicles and necessary equipment’s during my field work and Carbon Project whose supported me in finance until I completed my study and also all Forest ecology institute staffs whose were assisting me in different directions by dedicating their time, specially Dr. Hans he helps me a lot by devoting his time and laboratory technicians and experts whose help me to did my laboratory works Dr. Katrin, Marcel, Ferawuka, and Lenda. Special thanks for my families for my mam Adanech Gelagay, my sister Melesech Kassahun and for my brothers Belalu Kassahun and Berihun Berehanu and all my colleagues.
Conflict of Interest
No conflict of interest.
References
- Matulich KL, Weihe C, Allison SD, Amend AS, Berlemont R, Goulden ML, Kimball S, Martiny AC, Martiny JBH (2015) Temporal variation overshadows the response of leaf litter microbial communities to simulated global change. The ISME Journal 9: 2477-2489.
- Soil W, Reports R (2015) World reference base for soil resources 2014 International soil classification system.
- Gautam TP, Mandal TN (2016) Effect of disturbance on biomass , production and carbon dynamics in moist tropical forest of eastern Nepal. For Ecosyst.
- Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol Biochem 19(6): 697-702.
- Corsi S, Friedrich T, Kassam A, Pisante M (2012) Soil Organic Carbon Accumulation and Greenhouse Gas Emission Reductions from Conservation Agriculture : A literature review Soil Organic Carbon Accumulation and Greenhouse Gas Emission Reductions from Conservation Agriculture.
- Gurmessa B, Demessie A, Lemma B (2016) Dynamics of soil carbon stock, total nitrogen, and associated soil properties since the conversion of Acacia woodland to managed pastureland, parkland agroforestry, and treeless cropland in the Jido Komolcha District, southern Ethiopia Biyensa Gurmessa, Ambachew Demissie & Bekele Lemma. Journal of Sustainable Forestry 35(5).
- Liu N, Zhang Y, Chang S, Kan H, Lin L (2012) Impact of Grazing on Soil Carbon and Microbial Biomass in Typical Steppe and Desert Steppe of Inner Mongolia. 7(5).
- Lepcha NT, Devi NB (2020) Effect of land use , season , and soil depth on soil microbial biomass carbon of Eastern Himalayas.
- Gregorich EG, Carter MR, Angers DA, Monreall CM, Ellerta BH (1994) Towards a minimum data set to assess soil organic matter quality in agricultural soils.
- Gu C, Zhang S, Han P, Hu X, Xie L, Li Y (2019) Soil Enzyme Activity in Soils Subjected to Flooding and the Effect on Nitrogen and Phosphorus Uptake by Oilseed Rape. Front Plant Sci 10: 1-9.
- Ullah S, Ai C, Huang S, Zhang J, Jia L, et al. (2019) The responses of extracellular enzyme activities and microbial community composition under nitrogen addition in an upland soil. PLoS ONE 14(9): e0223026.
- Maboeta M, EN Cele (2016) Response of soil enzyme activities to synergistic effects of biosolids and plants in iron ore mine soils. Int J Environ Sci Technol 13(9): 2117-2126.
- Elley ALMK, Ay PHAF, Olley HWAP, Ill RIAG, Al KET (2011) Atmospheric CO2 and soil extracellular enzyme activity : a meta-analysis and CO2 gradient experiment. 2: 1-20.
- Kujur M (2020) Comparative Assessment of Microbial Biomass and Soil Enzyme Activities as Potential Indicators of Soil Quality in Different Mine Spoil , Odisha.
- Kujur M, Kumarpatel A (2014) Kinetics of soil enzyme activities under different ecosystems: An index of soil quality. Chilean J Agric Res 74(1).
- Gómez EJ, Delgado JA, González JM (2020) Persistence of microbial extracellular enzymes in soils under different temperatures and water availabilities. Ecology and Evolution 10(18): 10167-10176.
- Liu Y, Shen X, Chen Y, Wang L, Chen Q, et al. (2019) Litter chemical quality strongly affects forest floor microbial groups and ecoenzymatic stoichiometry in the subalpine forest Litter chemical quality strongly affects forest floor microbial groups and eco-enzymatic stoichiometry in the subalpine forest.
- Błońska E, Lasota J, Gruba P (2017a) Soil Science and Plant Nutrition Enzymatic activity and stabilization of organic matter in soil with different detritus inputs. Soil Sci Plant Nutr 63(3): 242-247.
- Burns RG, Wallenstein M (2010) Microbial extracellular enzymes and natural and synthetic polymer degradation in soil : Current research and future prospects Microbial extracellular enzymes and natural and synthetic polymer degradation in soil : current research and future prospects.
- Wassie A, Sterck FJ, Teketay D, Bongers F (2009) Tree regeneration in church forests of Ethiopia: effects of microsites and management. Biotropica 41(1):110-119.
- WRB (2014) World Reference Base for Soil Resources 2014. Food and Agriculture Organization of the United Nations, Rome, Italy.
- Witt C, Gaunt JL, Galicia CC, Neue JCGOH (2000) A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biology and Fertility of Soils volume 30: 510-519.
- Technologies E (2019) Simple measurements in a complex system: soil community responses to nitrogen amendment in a Pinus taeda forest. 10(4).
- Ajema L (2018) Effects of Biochar Application on Beneficial Soil Organism Review. 5(5): 9-18
- Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trends in Ecology and Evolution 20(11): 634-641.
- Odinga ES, Waigi MG, Gudda FO, Wang J, Yang B, Hu X, Li S, Gao Y (2020) Occurrence , formation , environmental fate and risks of environmentally persistent free radicals in biochars. Environ Int 134: 105172.
- Kacálek D, Dušek D, Novák J, Bartoš J (2013) The impact of juvenile tree species canopy on properties of new forest floor. J For Sci 59: 230-237.
- Campbell JL, Gower ST (2000) Detritus Production and Soil N Transformations in Old- Growth Maple Stands. Ecosystems 3: 185-192.
- Acosta-martinez V, Mikha MM, Stahlman PW, Benjamin JG (2011) Multi-Location Study of Soil Enzyme Activities as Affected by Types and Rates of Manure Application and Tillage Practices 1(1): 4-21.
- Averill C, Brzostek ER (2019) extracellular enzyme activities under long-term N fertilization. 24(6): 2721-2734.
- Błońska E, Lasota J, Zwydak M (2017b) The relationship between soil properties , enzyme activity and land use. Forest research work 78(1): 39-44.
- Paolo D, Lonardo D (2019) The microbial side of soil priming effects.
- Kucharski J (2019) Microbiological and biochemical properties of soil polluted with a mixture of spiroxamine, tebuconazole, and triadimenol under the cultivation of Triticum aestivum L.
- Bowles TM, Acosta-martínez V, Calderón F, Jackson LE (2014) Soil Biology & Biochemistry Soil enzyme activities , microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape q. Soil Biol Biochem 68: 252-262.
- Martina Š, Baldrian P (2011) Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant and Soil 338: 99-110.
- Gruba P (2016) Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecology and Evolution 31(5): 655-664.
- Awulachew SB, McCartney M, Steenhuis TS, Ahmed AA (2009) A review of hydrology, sediment and water resource use in the Blue Nile Basin. International Water Management Institute (IWMI), Colombo, Sri Lanka.
- Betrie GD, Mohamed YA, Griensven A van, Srinivasan R (2011) Sediment management modelling in the Blue Nile Basin using SWAT model. Hydrol Earth Syst Sci 15(3): 807- 818.
- Environ PS, Li Q, Liang JH, He YY, Hu QJ, et al. (2014) Effect of land use on soil enzyme activities at karst area in Nanchuan, Chongqing, Southwest China. Plant Soil Environ 60(1):15-20.
- Klepeis P, Orlowska IA, Kent F, Cardelús CL, Eshete AW (2016) Ethiopian Church Forests: A Hybrid Model of Protection. Human Ecology volume 44: 715-730.
- Publishing B (2005) Nutrient limitation and enzyme activities during litter decomposition of nine wetland species in relation to litter N:P ratios 19: 582-593.
-
Hodaddis Kassahun, Iftekhar U Ahmed, Douglas L Godbold and Hans Sandéna. Effects of Natural Forest, Plantation Forest and Grazing Land on Soil Microbial Biomass and Soil Enzyme Activities in Ethiopian Highland. World J Agri & Soil Sci. 7(5): 2022. WJASS.MS.ID.000674.
-
Natural forest, Grazing land, Microbial biomass, Plantation, Enzyme activities
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






