 Research Article
Research Article
Effects Of Glyphosate Application on Soil Ecological Health After Continuous Planting of Transgenic Glyphosate-Resistant Soybeans in Harbin, Northeast China
Hui Liu, Kuiyuan Chen and Wei Ding*
Department of Plant Protection, College of Agriculture, Northeast Agricultural University, Harbin 150030, China
Wei Ding, Department of Plant Protection, College of Agriculture, Northeast Agricultural University, Harbin 150030, China.
Received Date: August 11, 2022; Published Date:August 22, 2022
Abstract
In modern agriculture, appropriate use of transgenic glyphosate-resistant soybeans are considered to be a key measure contributing to high yield and sufficient supply, in the process of which glyphosate is unavoidable. However, understanding of the impact on soil environmental health and the linkage between soil indexes is remarkably limited. This study conducted a 28-day field trial to explore the underlying effects and correlation of three glyphosate spraying does 1.2 (L), 2.4 (M), 3.6 (H) kga.i./hm2, transgenic (T) or recipient soybean (R) planting history on soil property components, nutrient regime and enzyme activities at the transgenic experimental station of Northeast Agricultural University - Harbin in Heilongjiang, China. Specifically, results concluded that soil alkali-hydrolyzable nitrogen (AN), available phosphorus (AP) content increased significantly, while cellulase and urease activities were reduced with passage of application time in the seven trial strategies. The concentration of glyphosate had no significant effect on soil moisture. Correlation analysis further showed that glyphosate dose was a significant driving factor for soil bulk density (BD), pH, organic matter (SOM), cellulase, urease and had a significant inverse relationship with soil phosphatase and catalase activities. The highest soil available potassium (AK) was obtained in group RSL across 28 days. The responses of enzyme activities suggested that transgenic glyphosate-resistant soybeans were effectively conducive and favorable to soil urease and phosphatase, but not soil catalase activities. Furthermore, transgenic glyphosate-resistant soybeans seemed to have the potential for changing soil structure and enhancing the variations in correlation between soil factors rather than recipient soybeans. These findings highlighted the importance and provided initial insights on safe release of glyphosate-resistant soybeans and glyphosate.
Keywords: Transgenic glyphosate-resistant soybeans; Glyphosate; Soil; Properties and nutrients; Enzyme activities; Correlation analysis
Introduction
Soybeans (Glycine max (L.) Merr.) are important sources of vegetable protein and edible vegetable oil in Asian countries [1,2]. Due to the aggravation of weed damage in main soybean fields, continuous deterioration of soil environment and serious pesticide damage to soybeans, the average output of soybeans in China has declined year by year. The traditional soybean industry is not optimistic to meet the growing production demand in China. Transgenic glyphosate-resistant soybeans were massively adopted to decrease the economic risk of weed infestations, cultivation numbers, cost of herbicide treatment and spraying trips [3].
Glyphosate [N-(phosphonomethyl) glycine], which inhibits the biosynthesis pathway of aromatic amino acids [4], turns into the most used nonselective and broad-spectrum herbicide globally mainly due to the planting of transgenic glyphosate-resistant crops [5,6]. Glyphosate-containing products are successfully registered and applied in about 130 countries and has been thoroughly assessed due to a concern about its effects on the environment [7], especially the contribution to the soil [8]. Glyphosate has a faster adsorption rate in soil and is less toxic to non-target organisms [9]. Under different climatic conditions in the field, the median dissipation half-life of glyphosate was 16.5 days [10]. With large and repeated use of glyphosate, a certain amount of glyphosate has been frequently detected in different environmental settings even if the half-life of glyphosate is relatively short [11]. In fact, previous studies reported that glyphosate was detected from 77 samples of wetlands in Melbourne, Australia during year 2017 and 2018 [12]. The median and maximum concentrations of glyphosate were ranging from 9.6 to 476 mg/kg in 45 soils and sediment samples collected from Indiana and Mississipfpi states of USA [13]. Many studies demonstrate an overall negative effect of glyphosate on soil enzyme activity and fungal communities [14]. Glyphosate also defects in growth or reproduction of soil organism earthworms [15].
As we all know, transgenic glyphosate-resistant crops have been planted in European and American countries for more than 20 years [16]. In contrast, they have not been fully released in China. Consequently, the relatively simple agricultural soil background is beneficial for the environmental safety assessment and mechanism research. Extensive previous studies clarified the fact that more than 99% herbicides scattered to the surrounding environment instead of targeting on weeds [17]. Therefore, it is particularly important to explore the impact of genetically modified crops and glyphosate spray on soil ecology [18]. Soil nutrient carbon, nitrogen, phosphorus, potassium dynamics [19], enzyme activity, and physical or chemical properties [20,21] are the key factors that indicate the biodiversity and functions of soils due to the complex interaction between biotic and abiotic components in soil. The richness of useful micro-organisms in soil efficiency largely determines the survival and output of crop [22]. Thus, the soil ecological health is the crux of the agricultural production.
This novel study fully explored the effects of glyphosate on soil
health with long-term planting history of transgenic glyphosateresistant
soybeans compared to recipient soybeans. The results
revealed varying degrees of disturbance to soil BD, SOM content,
soil nutrient elements AN, AP, AK and enzyme activities with
different glyphosate spraying rates. The relationship between
various factors was also analyzed and elaborated in detail. The
purpose of this study is:
a) Evaluating the safety for the release of transgenic
glyphosate-resistant soybeans in soil;
b) Exploring the different effects of glyphosate dosage on
soil ecological indicators. This research can play a guiding role in
the release of transgenic glyphosate-resistant soybeans and is of
great significance for future use of glyphosate.
Materials and Methods
Chemicals and reagents
41% Glyphosate isopropylamine salt (Roundup) was produced by Monsanto, USA; The potassium chloride (AR) and toluene (AR) were obtained from Tianjin Kermel Chemical Reagent Co., Ltd. The sucrose (AR), citric acid (AR), glacial acetic acid (AR), glycerol (AR), absolute ethanol (AR), sodium hydroxide (AR), boric acid (AR) and sodium hydrogen carbonate (AR) were provided by Tianjin Yongda Chemical Reagent Co., Ltd. The ammonium acetate was produced by Tianjin ZhiYuan Reagent Co., Ltd. The analytically gum arabic and ammonium molybdate were purchased from Sangon Biotech (Shanghai) Co., Ltd. The calcium carbide (LR) and acetone (AR) were obtained from the Damao Chemical Reagent Factory. The potassium phosphate dibasic (AR), L-ascotbic acid (AR), charcoal active granular (AR) and sodium hypochlorite solution (AR) were provided by Tianli Chemical Reagent Co., Ltd. The hydrochloric acid (AR) was purchased from the Harbin Science and Technology Chemical Reagent Co., Ltd. The potassium permanganate (AR) was obtained from the Tianjin Taixing Chemical Reagent Factory. The urea (AR) and potassium dichromate (AR) was provided by the Tianjin Bodi Chemical Co., Ltd. The potassium carbonate crystal (AR) and 1,10-phenanthroline hydrate (AR) were purchased from the Tianjin Guangfu Fine Chemical Research Institute. The disodium phenyl phosphate was obtained from the Sinopharm Chemical Reagent Co., Ltd. The anthrone (AR) was produced by the Shanghai Zhanyun Chemical Co., Ltd.
Instruments and Equipment
The KNAPSACK Hydraulic Sprayer with four TEEJET 80015VS nozzles; The 78HW-1 Constant temperature magnetic stirrer was from Ronghua Instrument Manufacture Co., Ltd. (Jiangsu, China); The HZQ-C air bath oscillator was provided by Harbin Donglian Electronic Technology Development Co., Ltd. (Heilongjiang, China); The adjustable electric sand bath was from Beijing ever briGht medical treatment instrument Co., Ltd. (Beijing, China); The DHG- 9140A electric heating constant temperature blast drying oven was obtained from the Shanghai bluepard instruments Co., Ltd. (Shanghai, China); The PHS-3C digital pH meter was provided by Shanghai Skill Scientific Instrument Co., Ltd. (Shanghai, China); The TU-1900 UV-visible spectrophotometer was from Beijing Purkinje General Instrument Co., Ltd. (Beijing, China); The 6400A Flame photometer was from Shandong Gaomi Rainbow Analytical Instrument Co., Ltd. (Shandong, China); The TGL-16LM-B low temperature refrigerated centrifuge was obtained from Hunan Xingke Scientific Instrument Co., Ltd. (Hunan, China); The HPX- 9272 MBE Digital display electric heating constant temperature incubator was from Shanghai Boxun Medical Biological Instrument Corp (Shanghai, China); The KDN-04 Kjeldahl nitrogen analyzer and digestion furnace were provided by Shanghai Xinjia Electronics Co., Ltd. (Shanghai, China); The AL204 electronic balance was obtained from Mettler Toledo Instruments Co., Ltd. (Shanghai, China); The POGO II wifi Stevens Water Montoring Systerms was provided by Eco-Monitor (Beijing, China).
Experimental Design
The study was conducted in the genetically modified soybean test field of Northeast Agricultural University (Harbin, Heilongjiang, China). Direct spraying of glyphosate on bare soil was performed after continuous planting of transgenic glyphosate-resistant soybeans (Hujiao 06-98) and recipient soybeans (Mengdou 12) for more than 3 years. A completely randomized block design was adopted with each treatment repeated for 3 times. The 21 plots, every area of which was 12.0 m2 with clear boundaries were installed. The sowing date and density were consistent with the local field. Experiments were designed according to (Table 1). Sample collection was conducted by the five-spot-sampling method at 7 d, 14 d, 21 d and 28 d after application of glyphosate and preserved at -20°C.
Table 1: The experimental design.
Soil properties
Soil moisture was measured by Stevens POGO II. The national industry standard method “NY/T 1121.2-2006 Soil Testing-Part 4: Method for determination of soil bulk density” was applied for the detection of soil bulk density. Soil pH values were determined by the national industry standard method “NY/T 1121.2-2006 Soil Testing-Part 2: Method for determination of soil pH” of China. SOM content was detected by the potassium dichromate volumetric method, which referred to the national industry standard method “NY/T 1121.2-2006 Soil Testing-Part 6: Method for determination of soil organic matter”.
Soil nutrients
Soil AN, AP and AK content was determined by the alkaline hydrolysis diffusion, sodium bicarbonate extraction-molybdenum antimony colorimetric and ammonium acetate extractionflame spectrophotometry method, respectively. AN detection referred to the standard document “LY/T 1228-2015 Nitrogen determination methods of forest soils” and “DB13/T 843- 2007 Soil available nitrogen determination”. AP detection was performed according to the standard document “LY/T 1232-2015 Phosphorus determination of forest soils”, “HJ 704-2014 Soil quality-Determination of available phosphorus-Sodium hydrogen carbonate solution-Mo-Sb anti spectrophotometric method” and “NY/T 1121.7-2014 Soil testing-Part 7: Method for determination of available phosphorus in soil”. The standards “LY/T 1234-2015 Potassium determination methods of forest soils” and “NY/T 889-2004 Determination of exchangeable potassium and nonexchangeable potassium content in soil” were applied for AK assay.
Soil enzyme activities
Soil cellulase, urease, phosphatase and catalase activities were determined by anthrone colorimetry, indophenol blue colorimetry, phenyl disodium phosphate and potassium permanganate titration method. Soil cellulase activity was measured by the method proposed by Guan [23]. The activity is denoted by milligrams of glucose produced from 1 g dry soil for 1 d. Soil urease activity was determined with a modified method referring to the previous literature [23,24], which is expressed as milligrams of NH3-N in 1 g soil after 1 d. The method created by Guan [23] and the group standard of China “T/NAIA 012-2020 Determination of soil phosphatase activity phenyl disodium phosphate colorimetric method” raised by Ningxia Chemical Analysis and Testing Association were referenced for the soil phosphatase activity detection, of which the unit is mg P2O5/d/g soil. Soil catalase activity was tested with a modified method referring to the Guan [23] and the group standard of China “T/NAIA 013-2020 Determination of soil catalase activity-potassium permanganate titration method” raised by Ningxia Chemical Analysis and Testing Association. We use μmol KMnO4/d/g soil to represent the unit of soil catalase activity.
Statistical analysis
The Excel 2016 was used to process the original data, and DPS v9.01 software was used to analyze the significant difference of the average data. Different lowercase letters a, b, c, d, ……and their combinations indicate significant differences at the P<0.05 level. Correlation analysis was carried out with IBM SPSS statistics 20 software to obtain the correlation coefficient R at the P<0.05 and P<0.01 levels. If |R|>0.6, it indicates that there is a strong or extremely strong correlation between the two indexes.
Results and Discussion
Soil properties
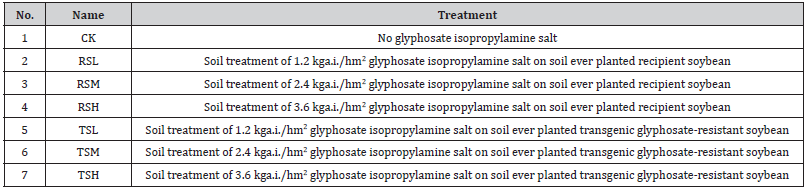
Soil general characteristics for the studied sites and glyphosate treatments are shown in (Table 2). During the studied period of 7 days, the soil moisture showed significant differences between different glyphosate treated groups. The significant differences disappeared from 14 to 28 days, which indicated that glyphosate had no long-term effect on soil moisture. Lower values of soil moisture, as compared with 0 d, is related to glyphosate spray at 7 d. After 14 days of glyphosate application, the soil moisture in every group dropped below 21.25% and maintained at 24.09%-25.90% after 28 days. The transgenic glyphosate-resistant soybeans planting history allowed the soil with better water retention capacity than that of the recipient soybeans at 7 days. No significant effects of soybean varieties under the use of recommended 1.2 kga.i./hm2 glyphosate were observed on soil moisture. From our study, although soil moisture was not significantly correlated with glyphosate application concentration, the on-site soil glyphosate pollution is more likely to occur with low soil moisture based on previous results concluding that glyphosate persisted longer in dry soils [25,26].
Table 2:The soil properties under different glyphosate treatment.
The soil BD of each group was evident ranging from 0.8447 to 1.2753 g/cm3 in our study. Our results showed the “increasingdecreasing- increasing” trend of soil BD under 1.2 kga.i./hm2 glyphosate treatment at 7-28 d. The soil BD in RSM and TSM group first rose and then fell with the employment of glyphosate. Similar trends were observed between TSH and TSM group about soil bulk density. This difference was, however, soil BD in RSH group performed the trend of “decreasing-increasing-decreasingincreasing” from 7 to 28 days. The increments of BD observed may negatively attributed to the number of soil microorganisms based on previous research [27], which revealed that the adverse impact of glyphosate on the soil microbial community should be paid special attention after applying glyphosate despite normal recommended dosage.
According to our present study, the soil pH in all groups was basically neutral to weakly acidic with values from 6.67 to 7.90. High concentration (3.6 kga.i./hm2) of glyphosate promoted larger soil pH compared with CK group. Overall, the effect of glyphosate application on soil pH exhibited less significant in soil with transgenic glyphosate-resistant soybeans cultivation history than that of recipient soybeans. At the initial stage of glyphosate spraying for 7 days, soils were more acidic than control except for the high concentration of glyphosate (3.6 kga.i./hm2) groups. The possible reason for this phenomenon is that a decrease of pH could urge the sorption of glyphosate to minerals and benefit glyphosate degradation [28,29] during the best degradation cycle of glyphosate. Over time, higher soil pH was observed than CK group at 21 and 28 days, in general, indicating that spraying glyphosate didn’t adversely affect the acidity of the soil complied with the previous results [30,31].
It is noteworthy that SOM content was determined to range from 4.1417 to 5.5792%. Highest SOM content based on planting transgenic glyphosate-resistant and recipient soybeans was reflected in RSL and TSH groups after application of glyphosate for 7 days. The SOM content was positively correlated to the glyphosate spraying concentrations in soil that had ever been planted with transgenic glyphosate-resistant soybeans at 7 and 28 days. The medium concentration of glyphosate resulted in higher SOM content than the other two spraying concentrations during 14 and 21 days. As evidenced by previous studies, spraying glyphosate had an adverse effect to decrease organic carbon [32,33], probably leading to reducing soil nutrition, fertility and contributing to the increase of CO2 in the atmosphere [34]. Another finding is that the adverse effects of planting transgenic glyphosate-resistant soybeans on SOM were less obvious. Therefore, the transgenic glyphosate-resistant soybeans were more preferably selected to achieve soil health.
Soil AN, AP and AK
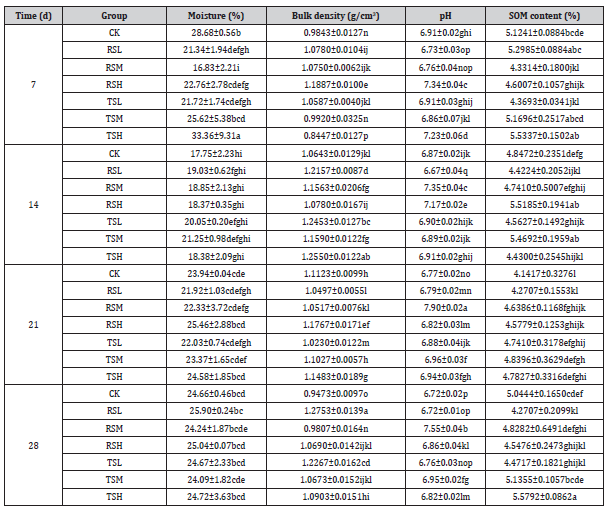
The soil AN content varied widely among different groups (Figure 1A). A rise in AN content was observed in group CK at 14 d compared to 7 d and maintained stable at 21 and 28 days. Previous study implied that the content of AN was negatively correlated with glyphosate dosage within a certain range [35]. AN content in both RSL and TSL groups illustrated an increasing trend at 14 d compared to 7 d, while declined at 21 d and 28 d. Overall, high doses of glyphosate (2.4 kga.i./hm2 and 3.6 kga.i./hm2) were beneficial for the increase of AN content. The most notable phenomenon is that AN in RSH and TSM groups significantly decreased at 14 and 21 days, merely, with values of 138.14 and 133.51 mg/kg, respectively. The effect of glyphosate spraying on AN content was more significant in fields ever planted recipient soybeans than that of transgenic soybeans. This meaned that the varieties of crops could lead to differences in the content of AN, thereby affecting the uptake of nitrogen by plants.
Figure 1: Impact of different glyphosate treatment on soil AN (A), AP (B) and AK (C) content
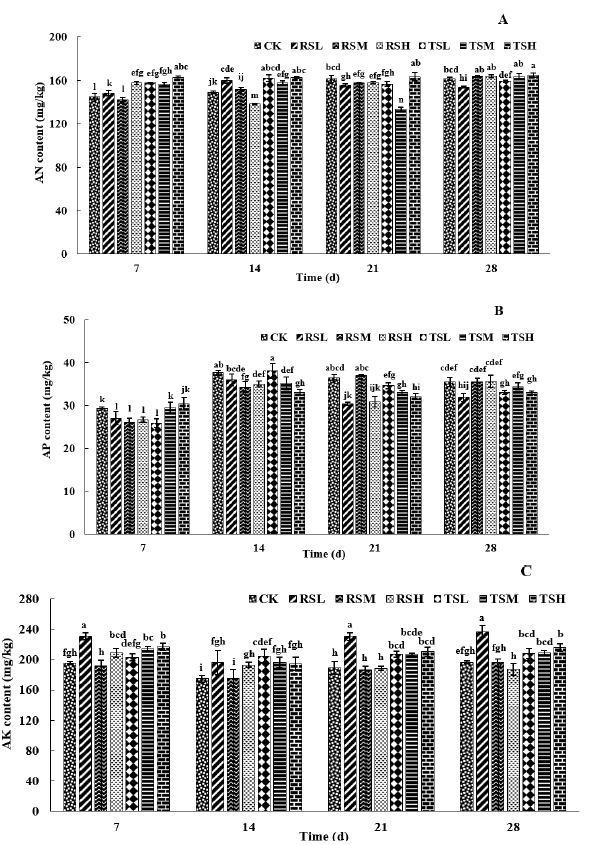
Regarding soil AP (Figure 1B), the results revealed that the content in CK treatment significantly raised after 7 days. Because of the warming up environment and gradually increasing rainfalls during this period, it is implied that account for P in natural soil was faster and stronger under warmer and wetter conditions [36]. Our results clearly showed that the concentrations of soil AP were mainly closely related to the continuous planting of genetically modified crops and long-term spray of glyphosate, which was consistent with the results of Obour et al. [37]. During the experiment, the content of AP in soil generally reached the highest at 14 days, declined at 21 days and remained stable at 28 days. Data from EFSA at year 2021 about glyphosate reported that DT50 value ranged from 1.1 to 13.7 days in fields [38]. Glyphosate may compete as phosphate fertilizers for binding sites in soil due to chemical similarity since it contains phosphorus [39]. These results could explain why AP occurred most at 14 days with the rapid degradation of glyphosate to provide sources of phosphorus in soil. It could be attributable to the competition between the soybean roots and rhizosphere microbial community for AP [40].
Our results pointed out that the AK content in group CK decreased at 14 d and resumed at 21 d and 28 d (Figure 1C). It may be due to the directly negative effects of high temperature and precipitation on soil K concentrations [41]. In analogy to the results of previous studies, glyphosate generally did not induce a reduce of exchangeable K or affect non-exchangeable K [42, 43]. However, it disagreed with the negligible positive effect of glyphosate application in the RSL group. Noteworthy is that a strong relationship between the application amount of glyphosate and AK content in the soil with a planting history of receptor soybeans, instead, not transgenic soybeans was observed. It can be speculated that the nutritional structure and properties of transgenic glyphosate-resistant soybean fields were not easily disturbed by glyphosate spraying.
Effects of different glyphosate treatments on soil enzyme activities
Soil enzyme activity of CK group has a more significant connection with climate. Warming was observed to limit both the number of substrates and enzyme activity. With the arrival of rainy season, the enzyme activity recovered again [44].
Soil cellulase plays important roles in the decomposition and transformation of SOM. Large numbers of findings have suggested that most insecticides had promoting effects, moreover, fungicides and herbicides inhibited or had no effect on soil cellulase activity [45]. The reaction of soil cellulase enzyme activity in every group was shown in (Figure 2A). Highest cellulase activity was noticed after application of glyphosate for 7 days within the detection time. Whether the soil has ever been planted with recipient or genetically modified soybeans, a significant reduction of soil cellulase activity treated with glyphosate at rates of 2.4 kga.i./hm2 was observed. This finding of the lowest soil cellulase activity was in good accordance with the reported previous studies [46]. In addition, no differences in the effect of transgenic and non-transgenic soybeans on soil cellulase enzyme activities were found in this study as Nakatani et al. [47] verified in their research.
Figure 2:The soil cellulase (A), urease (B), phosphatase (C) and catalase (D) activities under different glyphosate treatment.
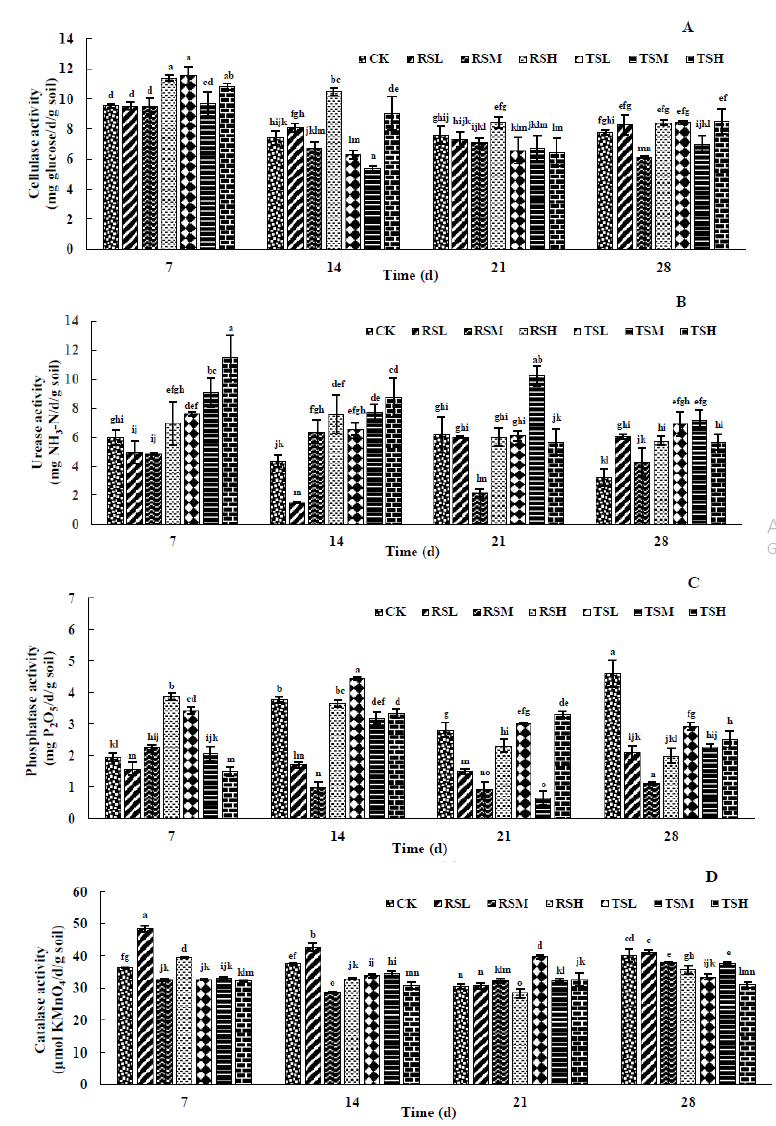
Urease is an important component involved in the soil nitrogen cycle and can catalyze the hydrolysis of urea into ammonia and carbon dioxide. Some referenced studies reported that herbicides appeared to reduce the soil urease activity [48] or had no effect [49]. Our study (Figure 2B) found that soil urease activity showed a very strong and statistically significant positive correlation with spraying concentration of glyphosate at 7 d and 14 d. It is stated that interesting and different results were obtained about urease activity of soil that ever-planted recipient (RSM) and transgenic glyphosate-resistant (TSM) soybeans at 21 and 28 days. The urease activities of RSM and TSM were concluded as the lowest and highest during sample collection, respectively. The research of Nieder et al. [50] also confirmed that soil urease activity was stimulated by glyphosate. In addition, transgenic glyphosate-resistant soybeans were indicated a beneficial effect on maintaining soil urease activity, on account of helping preserve the plant nitrogen.
Soil phosphatase enzyme is responsible for the mineralizationimmobilization processes of organic phosphorus and supplying potential capability [51]. Since the degradation of glyphosate in soil is closely related to the breaking of C-P bond [52], the activity of soil phosphatase varied greatly with the change of glyphosate spraying concentration (Figure 2C). These results demonstrated that the phosphatase activity in soil that recipient soybeans ever planted increased by extending glyphosate concentrations at 7 d. Conversely, the soil phosphatase activity was negatively correlated to glyphosate applied concentrations after cultivating transgenic glyphosate-resistant soybeans. The overall performance of phosphatase activity was the best in RSL and TSL groups, instead, the least active in RSM and TSM groups. The most obvious inhibition of glyphosate on phosphatases was observed at 28 days in our present study [53]. Consequently, this study stated clearly that applying glyphosate at the recommended dosage showed the minimal disturbance and impact on soil microorganisms. High concentrations of glyphosate may provide sufficient P element, which better activated the soil phosphatase activity. Planting transgenic glyphosate-resistant soybeans can better promote soil phosphatase activity instead of recipient soybeans.
Soil catalase is an important enzyme for soil microbial metabolism and removing the H2O2 to protect plants from oxidative stress [54,55]. In the soils where receptor soybeans were ever planted, catalase activity was conformed to the same discipline: RSL > RSH > RSM after applying glyphosate for 7 and 14 days (Figure 2D). Highest soil catalase activity was exhibited in group RSM for 21 days. Until 28 days, soil catalase activity was inversely proportional to the dosage of glyphosate. In the soils with planting history of transgenic glyphosate-resistant soybeans, highest soil catalase activity occurred in group TSL and TSM at 21 and 28 days, respectively. Excessive glyphosate precipitated the lowest soil catalase activity in group TSH at 14 and 28 days. As shown by the previous study, electron transport chain and synthesis of oxygen radicals in the microorganism cells could be induced by glyphosate, thence, the soil catalase activity increased reacting against them [56]. However, availability for growth of soil catalase activity is also closely related to the application dose of glyphosate and the planting varieties of soybeans.
Correlation analysis
The results revealed different correlations among the soil properties, available nutrients and enzymatic activities (Table 3) in the soil with planting history of receptor soybeans (RS groups) and transgenic soybeans (TS groups). In RS groups, AN (R=0.390, P<0.01) and cellulase (R= −0.609, P<0.01) changed significantly with the extension of time after glyphosate’s application. The correlation between soil cellulase and AP was visibly strong (R= −0.619, P<0.01). In TS groups, soil AK was confirmed positively related to glyphosate dosage (R=0.601, P<0.01). In addition, urease had beneficial correlation with pH (R=0.612, P<0.01). The correlations were observed between soil cellulase and AP (R= −0.707, P<0.01), followed by urease and phosphatase (R= −0.652, P<0.01). Overall, the correlation between soil indexes in soil with transgenic soybeans planting history was more complex than that of recipient soybeans, despite the release of genetically modified soybeans seemed to have no impact on soil microbial biomass and communities [57]. The negative relationship between soil BD and moisture (TS groups, R= −0.588, P<0.01), SOM (RS groups, R= −0.395, P<0.01; TS groups, R= −0.478, P<0.01), pH (TS groups, R= −0.367, P<0.05), respectively, suggested that soil properties would affect and achieve each other [58,59]. Hence, soils with high moisture, SOM, pH with low BD are less resistant to compaction, however, can recover better. From the research results, the application dose of glyphosate had a greater impact on each index compared with application time to attract more attention. Correlations between nutrient content and soil properties were determined in our study, such as AN and moisture (RS groups, R=0.377, P<0.01); AN and SOM (RS groups, R= −0.403, P<0.01); AP and moisture (TS groups, R= −0.379, P<0.01), BD (TS groups, R=0.360, P<0.05), pH (TS groups, R=0.320, P<0.05); AK and moisture (TS groups, R=0.465, P<0.01), pH (TS groups, R=0.315, P<0.05). According to previous studies, soil nutrient content was more associated with soil physical properties [60]. With respect to the relation with chemical properties (SOM, etc.), it is more indirectly caused by the reaction of soil microbial biodiversity [61,62].
Table 3:Correlations among the soil properties, available nutrients and enzymatic activities.
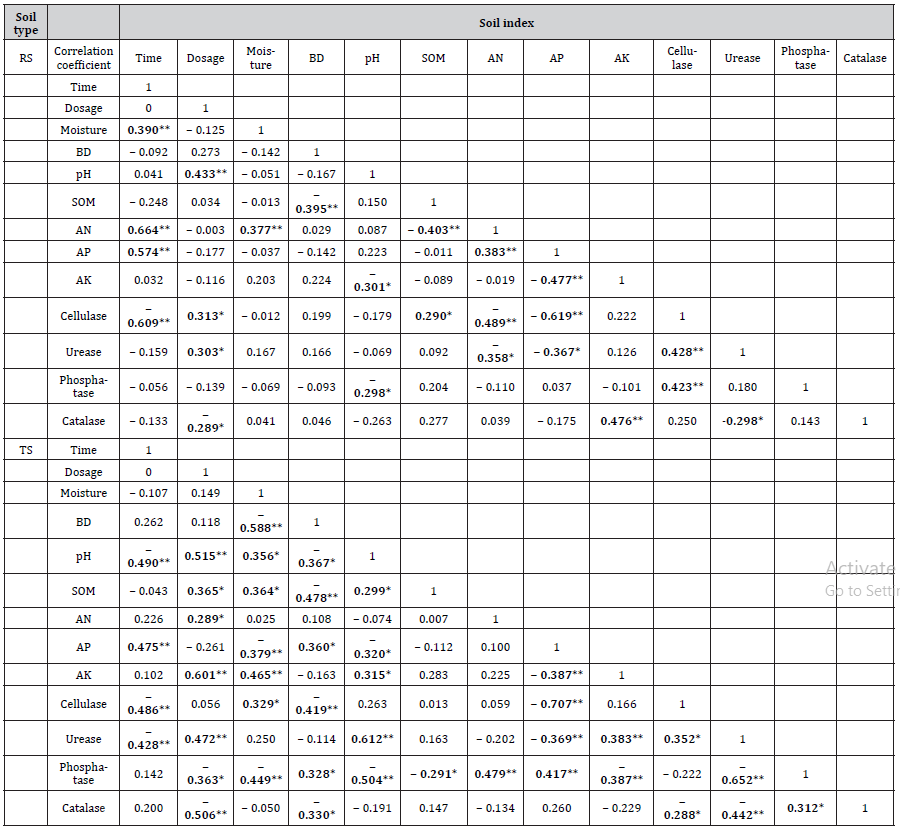
**Significant correlation at 0.01 level (bilateral). * Significant correlation at 0.05 level (bilateral).
Soil enzymes drove by microorganisms represented a critical role in maintaining soil structure and nutrient cycling. The SOM and soil nutrients rather than the active microorganisms were demonstrated to control soil enzyme kinetics [63]. Cellulase was most significantly affected by SOM (RS groups, R=0.290, P<0.05), AN (RS groups, R= −0.489, P<0.01) and AP, the conclusions of which were not completely consistent compared to Liu et al.’ research [64]. The pH, AN (RS groups, R= −0.358, P<0.05), AP (RS groups, R= −0.367, P<0.05; TS groups, R= −0.369, P<0.01), AK (TS groups, R=0.352, P<0.05) were the factors most significantly correlated with soil urease. Previous observations had indicated that AN [65] and pH [66] contributed to changes in the activity of urease. The relationship between phosphatase and soil indicators was completely different in RS and TS groups. In RS groups, only pH was the strong regulator of phosphatase activity (RS groups, R= −0.298, P<0.05). Almost all soil indexes were significantly related to phosphatase, of which pH value was still the most prominent (TS groups, R= −0.504, P<0.01) in TS groups. Low soil moisture could nullify the positive effects of elevated temperature to limit in enzyme activity [67], thus, phosphatase was calculated to be negatively relevant to soil moisture (TS groups, R= −0.449, P<0.01). The positive correlation between soil P level and phosphatase activity was reported by Wei et al. [68], which fitted the results obtained by our study (TS groups, R=0.417, P<0.01). The conclusion that phosphatase activity was driven by microbial C acquisition [69] could be obtained through the relationship between phosphatase and SOM (TS groups, R= −0.291, P<0.05). Since soil catalase reflects the ability of soil to resist adverse external effects, it was calculated as more negatively related to the application dose of glyphosate (RS groups, R= −0.289, P<0.05; TS groups, R= −0.506, P<0.01). Besides, influences among various enzymes should also receive attention.
Conclusion
In summary, our present results supported that longterm planting of transgenic glyphosate-resistant soybeans and different doses of glyphosate application confirmed complex effects on interactions between physicochemical components and enzyme activities of soil quality rather than recipient soybeans. After spraying glyphosate for 28 d, the use of glyphosate under recommended dose (1.2 kga.i./hm2) were not favorable to, whereas transgenic glyphosate-resistant soybeans clearly attributed to looser soil and higher SOM content. In addition, recommended glyphosate suggested to promoting the soil AK, on the contrary, was not conducive to soil AN and AP. The transgenic glyphosateresistant soybeans planting history showed a positive correlation with soil cellulase, urease and phosphatase activity, while followed by inhibiting the soil catalase activity. The attempting established linkage model between application time, dosage, soil properties, nutrients and enzyme activities demonstrated that soil phosphatase and catalase were negatively correlated with glyphosate dose. Further long-term comprehensive studies, considering the environmental safety assessment of transgenic glyphosate-resistant soybeans on microbial community and influence mechanism, are required to facilitate elucidate in-depth discussion about the safe release possibility of genetically modified crops. On the other hand, in view of the potential risks on soil health generated by the recommended dose glyphosate, the application standard of glyphosate needs further improvement.
Figure 3:
Acknowledgement
This work was supported by the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (Grant number UNPYSCT-2018159).
Conflict of Interest
No conflict of interest.
References
- Li S, Jin Z, Hu D, W Yang, Y Yan, et al. (2020) Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT-Food Sci Technol 125: 109264.
- Ma M, Zhang H, Xie Y, Yang M, Tang J, et al. (2020) Response of nutritional and functional composition, anti-nutritional factors and antioxidant activity in germinated soybean under UV-B radiation. LWT-Food Sci Technol 118: 108709.
- Guo B, Hong H, Han J, Zhang L, Liu Z, et al. (2020) Development and identification of glyphosate-tolerant transgenic soybean via direct selection with glyphosate. J Integr Agr 19: 1186-1196.
- Xiao PY, Liu Y, Cao YP (2019) Overexpression of G10-EPSPS in soybean provides high glyphosate tolerance. J Integr Agr 18: 1851-1858.
- Sun M, Li H, Jaisi DP (2019) Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water Res 163: 114840.
- Clapp J (2021) Explaining Growing Glyphosate Use: The Political Economy of Herbicide-Dependent Agriculture. Global Environ Change 67: 102239.
- Tzanetou E, Karasali H (2020) Glyphosate Residues in Soil and Air: An Integrated Review pp. 1-32.
- Duke SO (2020) Glyphosate: environmental fate and impact. Weed Sci 68: 201-207.
- Meftaul IM, Venkateswarlu K, Dharmarajan R, Prasath Annamalai, Asaduzzaman, et al. (2020) Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ Pollut 263: 114372.
- Kronberg MF, Rossen A, Munarriz ER (2021) Chapter 9 - Glyphosate-based herbicides and oxidative stress. Toxicology-Oxidative Stress and Dietary Antioxidants pp 79-90.
- Zhao J, Pacenka S, Wu J, Brian K Richards, Tammo Steenhuis, et al. (2018) Detection of glyphosate residues in companion animal feeds. Environ Pollut 243: 1113-1118.
- Okada E, Allinson M, Barral MP, Bradley Clarke, Graeme Allinson, et al. (2020) Glyphosate and aminomethylphosphonic acid (AMPA) are commonly found in urban streams and wetlands of Melbourne, Australia. Water Res 168: 115139.
- Battaglin WA, Meyer M, Kuivila K, J E Dietze, et al. (2014) Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. J Am Water Resour As 50: 275-290.
- Vazquez MB, Moreno MV, Amodeo MR, M V Bianchinotti (2021) Effects of glyphosate on soil fungal communities: A field study. Rev Argent Microbiol 53(4): 349-358.
- Correia FV, Moreira JC (2010) Effects of glyphosate and 2,4-D on earthworms (Eisenia foetida) in laboratory tests. B Environ Contam Tox 85(3): 264-268.
- Liu JY, Sheng ZW, Hu YQ, Qi Liu, Sheng Qiang, et al. (2021) Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean. Transgenic Res 30(1): 105-119.
- Guan X, Chen X, Qiu C, Yinfei Qian, Caihong Shao, et al. (2020) Effects of long-term herbicide application on the crops in soybean-peanut rotations in the red soil upland of Southern China. Field Crops Research 248: 107723.
- Mandal A, Sarkar B, Owens G, J K Thakur, M C Manna, et al. (2020) Impact of genetically modified crops on rhizosphere microorganisms and processes: A review focusing on Bt cotton. Appl Soil Ecol 148: 103492.
- Gao X, Xiao Y, Deng L, Qi-quan LI, Chang-quan W, et al. (2019) Spatial variability of soil total nitrogen, phosphorus and potassium in Renshou County of Sichuan Basin, China. Journal of Integrative Agriculture 18: 279-289.
- He L, Lu S, Wang C, Jun Mu, Yulin Zhang, et al. (2021) Changes in soil organic carbon fractions and enzyme activities in response to tillage practices in the Loess Plateau of China. Soil and Tillage Research 209: 104940.
- Li X, Wang Y, Zhang Y, Yujie Wang, Chengmin Pei, et al. (2021) Response of soil chemical properties and enzyme activity of four species in the Three Gorges Reservoir area to simulated acid rain. Ecotox Environ Safe 208: 111457.
- Agarwal AV, Singh RP (2021) Assessment of the Environmental and Health Impacts of Genetically Modified Crops. Policy Issues in Genetically Modified Crops pp. 335-354.
- Guan SY (1986) Soil Enzymes and its Methodology. Agricultural Press, Beijing pp. 274-340.
- Feyzi H, Chorom M, Bagheri G (2020) Urease activity and microbial biomass of carbon in hydrocarbon contaminated soils. A case study of cheshmeh-khosh oil field, Iran. Ecotox Environ Safe 199: 110664.
- Bento CPM, Yang X, Gort G, Sha Xue, Ruud van Dam, et al. (2016) Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci Total Environ 572: 301-311.
- Villarreal R, Lozano LA, Polich NG, Maria Paz S, Guido Lautaro B, et al. (2020) Influence of soil water holding and transport capacity on glyphosate dynamics in two agricultural soils from Pampas Region. Geoderma 376: 114566.
- Frimpong JO, Ofori ESK, Yeboah S, D Marri, BK Offei, et al. (2018) Evaluating the impact of synthetic herbicides on soil dwelling macrobes and the physical state of soil in an agro-ecosystem. Ecotox Environ Safe 156: 205-215.
- Muskus AM, Miltner A, Hamer U, Karolina M N, et al. (2022) Microbial community composition and glyphosate degraders of two soils under the influence of temperature, total organic carbon and pH. Environ Pollut 297: 118790.
- Muskus AM, Krauss M, Miltner A, Ute Hamer, Karolina M N, et al. (2020) Degradation of glyphosate in a Colombian soil is influenced by temperature, total organic carbon content and pH. Environ Pollut 259: 113767.
- Meftaul IM, Venkateswarlu K, Annamalai P, Aney Parven, Mallavarapu M, et al. (2021) Glyphosate use in urban landscape soils: Fate, distribution, and potential human and environmental health risks. J Environ Manage 292: 112786.
- Bottrill D, Ogbourne SM, Citerne N, Tanzi Smith, Michael BF, et al. (2020) Short-term application of mulch, roundup and organic herbicides did not affect soil microbial biomass or bacterial and fungal diversity. Chemosphere 244: 125436.
- Rodriguez AM, Jacobo EJ, Golluscio RA (2018) Glyphosate Alters Aboveground Net Primary Production, Soil Organic Carbon, and Nutrients in Pampean Grasslands (Argentina). Rangeland Ecol Manag 71:119-125.
- Sebiomo A, Ogundero VW, Bankole SA (2011) Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afri J Biotechnol 10(5): 770-778.
- Virginia A, Zamora M, Barbera A, Mauricio C F, Marisa Domenech, et al. (2018) Industrial agriculture and agroecological transition systems: A comparative analysis of productivity results, organic matter and glyphosate in soil. Agr Syst 167:103-112.
- Fan L, Feng Y, Weaver DB, Dennis PD, Glenn R Wehtje, et al. (2017) Glyphosate effects on symbiotic nitrogen fixation in glyphosate-resistant soybean. Appl Soil Ecol 121: 11-19.
- Zheng S, Xia Y, Hu Y, Xiangbi Chen, Yichao Rui, et al. (2021) Stoichiometry of carbon, nitrogen, and phosphorus in soil: Effects of agricultural land use and climate at a continental scale. Soil and Tillage Research 209: 104903.
- Obour AK, Stahlman PW, Holman JD (2016) Soil chemical properties as influenced by long-term glyphosate-resistant corn and soybean production in the central Great Plains, USA. Geoderma 277: 1-9.
- PPDB (2022) Glyphosate-isopropylamine.
- Martinez DA, Loening UE, Graham MC (2018) Impacts of glyphosate-based herbicides on disease resistance and health of crops: a review. Environ Sci Eur 30(1): 2.
- Jenkins MB, Locke MA, Reddy KN, Daniel S M, R Wade Steinriede, et al. (2017) Impact of glyphosate-resistant corn, glyphosate applications and tillage on soil nutrient ratios, exoenzyme activities and nutrient acquisition ratios. Pest Manag Sci 73(1): 78-86.
- Li T, Liang J, Chen X, Huoyan Wang, Shirong Zhang, et al. (2021) The interacting roles and relative importance of climate, topography, soil properties and mineralogical composition on soil potassium variations at a national scale in China. Catena 196: 104875.
- Lane M, Lorenz N, Saxena J, Cliff Ramsier, Richard P D, et al. (2012) Microbial activity, community structure and potassium dynamics in rhizosphere soil of soybean plants treated with glyphosate. Pedobiologia 55: 153-159.
- Lane M, Lorenz N, Saxena J, Cliff Ramsier, Richard P D, et al. (2012) The effect of glyphosate on soil microbial activity, microbial community structure, and soil potassium. Pedobiologia 55(6): 335-342.
- Jaskulak M, Grobelak A (2020) Soil enzymes in a changing climate. Climate Change and Soil Interactions pp. 731-749.
- Riah W, Laval K, Laroche-Ajzenberg E (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12: 257-273.
- Nguyen DB, Rose MT, Rose TJ, Lukasvan Zwieten (2018) Effect of glyphosate and a commercial formulation on soil functionality assessed by substrate induced respiration and enzyme activity. Eur J Soil Biol 85: 64-72.
- Nakatani AS, Fernandes MF, de Souza RA, Adriana P S, Fabio B R, et al. (2014) Effects of the glyphosate-resistance gene and of herbicides applied to the soybean crop on soil microbial biomass and enzymes. Field Crop Res 162: 20-29.
- Singh A, Ghoshal N (2013) Impact of herbicide and various soil amendments on soil enzymes activities in a tropical rainfed agroecosystem. Eur J Soil Biol 54: 56-62.
- Du Z, Zhu Y, Zhu L, Ji Zhang, Bing Li, et al. (2018) Effects of the herbicide mesotrione on soil enzyme activity and microbial communities. Ecotox Environ Safe 164: 571-578.
- Nieder R, Benbi DK, Reichl FX (2018) Health risks associated with pesticides in soils. Soil Components and Human Health pp. 503-573.
- Zhu J, Qu B, Li M (2017) Phosphorus mobilization in the Yeyahu Wetland: Phosphatase enzyme activities and organic phosphorus fractions in the rhizosphere soils. Int Biodeter Biodegr 124: 304-313.
- Kulikova NA, Zhelezova AD, Filippova OI (2020) The Degradation of Glyphosate and Its Effect on the Microbial Community of Agro-Sod–Podzolic Soil under Short-Term Model Experiment Conditions. Moscow Univ Soil Sci Bull 75: 138-145.
- Wolejko E, Jablonska-Trypuc A, Wydro U, Andrzej Butarewicz, Bożena Lozowicka, et al. (2020) Soil biological activity as an indicator of soil pollution with pesticides – A review. Appl Soil Ecol 147: 103356.
- Chabot M, Morales E, Cummings J, Nicholas Rios, Scott Giatpaiboon, et al. (2020) Simple kinetics, assay, and trends for soil microbial catalases. Anal Biochem 610: 113901.
- Li H, Huang WX, Gao MY, Xing Li, Lei Xiang, et al. (2020) AM fungi increase uptake of Cd and BDE-209 and activities of dismutase and catalase in amaranth (Amaranthus hypochondriacus L.) in two contaminants spiked soil. Ecotox Environ Safe 195: 110485.
- Kaczynski P, Lozowicka B, Wolejko E, Piotr Iwaniuk, Rafal Konecki, et al. (2020) Complex study of glyphosate and metabolites influence on enzymatic activity and microorganisms’ association in soil enriched with Pseudomonas fluorescens and sewage sludge. Journal of Hazardous Materials 393: 122443.
- Weinert N, Meincke R, Schloter M, Gabriele Berg, Kornelia Smalla, et al. (2010) Effects of genetically modified plants on soil microorganisms. In: Mitchell, R., Gu, J.-D. (Eds.), Environmental Microbiology, 2nd ed. John Wiley & Sons, Inc., Hoboken pp. 235-258.
- Chen Y, Huang Y, Sun W (2017) Using Organic Matter and pH to Estimate the Bulk Density of Afforested/Reforested Soils in Northwest and Northeast China. Pedosphere 27(5): 890-900.
- Reichert JM, Mentges MI, Rodrigues MF, Jean Pierre C, Gabriel Oladele A, et al. (2018) Compressibility and elasticity of subtropical no-till soils varying in granulometry organic matter, bulk density and moisture. Catena 165: 345-357.
- Simanský V, Jonczak J, Horváthová J, Dusan Igaz, Elena Aydin, et al. (2022) Does long-term application of mineral fertilizers improve physical properties and nutrient regime of sandy soils? Soil and Till Research 215: 105224.
- Prack Mc Cormick B, El Mujtar VA, Cardozo A, Valeria EA, Hernán AR, et al. (2022) Nutrient source, management system and the age of the plantation affect soil biodiversity and chemical properties in raspberry production. European Journal of Soil Biology 111: 103420.
- Bei S, Li X, Kuyper TW, David R C, Junling Zhang, et al. (2022) Nitrogen availability mediates the priming effect of soil organic matter by preferentially altering the straw carbon-assimilating microbial community. Sci Total Environ 815:152882.
- Tan X, Nie Y, Ma X, Zhiming Guo, Yang Liu, et al. (2021) Soil chemical properties rather than the abundance of active and potentially active microorganisms control soil enzyme kinetics. Sci Total Environ 770: 144500.
- Liu B, Xia H, Jiang C, Muhammad Riaz, Li Yang, et al. (2022) 14-year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci Total Environ 841: 156608.
- Sun X, Ye Y, Ma Q, Qingwei Guan, Davey LJ, et al. (2021) Variation in enzyme activities involved in carbon and nitrogen cycling in rhizosphere and bulk soil after organic mulching. Rhizosphere 19: 100376.
- Huang H, Tian D, Zhou L, Haojie Su, Suhui Ma, et al. (2022) Effects of afforestation on soil microbial diversity and enzyme activity: A meta-analysis. Geoderma 423: 115961.
- Steinweg JM, Dukes JS, Wallenstein MD (2012) Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Boil Biochem 55: 85-92.
- Wei X, Hu Y, Razavi BS, Juan Zhou, Jianlin Shen, et al. (2019) Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biology and Biochemistry 131: 62-70.
- Zheng MM, Wang C, Li WX, Long Guo, Ze Jiang Cai, et al. (2021) Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur J Soil Biol 104: 103312.
-
Hui Liu, Kuiyuan Chen and Wei Ding*. Effects Of Glyphosate Application on Soil Ecological Health After Continuous Planting of Transgenic Glyphosate-Resistant Soybeans in Harbin, Northeast China. World J Agri & Soil Sci. 8(2): 2022. WJASS.MS.ID.000681.
-
Transgenic glyphosate-resistant soybeans, Glyphosate, Soil, Properties and nutrients, Enzyme activities, Correlation analysis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






