 Research Article
Research Article
Carbon Sequestration Potential of The Main Tree Species and Vegetation Formations in The Ecological Zone of Sudan in Mali
Siriki Fané1*, Maharazu Yusuf1, Moussa Karembé2, Fadiala Dembélé2 and Aruwajoye Dami3
1&2Institut Polytechnique Rural de Formation et recherche Appliquée (IPR/IFRA) de Katibougou, Mali (Rural Polytechnic Institute of Training and Applied Research (IPR/IFRA) of Katibougou, Mali)
1Department of Geography, Bayero University, Kano State, Nigeria
2Faculté des Sciences et Techniques de l’USTTB, Mali (Faculty of Science and Technology of USTTB, Mali)
3Department of Forestry, Federal College of Agriculture, Akure, Ondo State, Nigeria
Siriki Fané, Professor/Teacher-Researcher at the Institute Polytechnique Rural de Formation et de Recherche Appliqué (IPR/ IFRA) in Katibougou, Mali.
Received Date: November 22, 2022; Published Date: December 13, 2022
Abstract
Savannah plant formations have enormous carbon sequestration potential, which contributes to the reduction of climate change effects. However, countries in savannah areas do not receive sufficient funds to ensure the conservation and management of forests, however, this potential is poorly exploited. This study aims to contribute to the knowledge of building and estimating the carbon sequestration capacity of tree species in different vegetation formations of the Sudan ecological zone of Mali. This research was conducted in Dioïla district located in Koulikoro region. Both primary and secondary data were used. Primary data were generated from the use of forest inventory questionnaires while the secondary data were generated from the Technical Service of Water and Forests of Mali. The stratified two-stage sampling method was used to collect data. Tree measurements (dendrometical inventory) were performed in the four vegetation formations encountered. Different statistical software was used for data analysis. The amount of carbon sequestered varies according to the vegetation formations area. The agroforestry parks and fallow are the formation that have the largest amount of carbon (12,091,159.49 tons). The carbon sequestration capacity is higher in woody savanna and arborous savannah than in other vegetation formations in the study area. The species Vitellaria paradoxa G. Don, have the higher carbon sequestration capacity in vegetation formations of Park agroforestry and Fallow (11. 087 t C ha-1) and of arborous savannah (9.5 t C ha-1). While in the vegetation formation of the shrubby savannah Daniellia oliveri (Rolfe) Hutch. & Dalziel has the higher carbon sequestration capacity (4. 04 t C ha-1). The amount of carbon sequestered, and the number of tree species are significatively correlated to trees circumference. It will be interesting to conduct a longterm experiment to study and a better understanding of the carbon sequestration capacity of tree species of Sudan ecological zone of Mali.
Keywords: Carbon sequestration; Tree species; Vegetation formation; Sudanian zone; Mali
Introduction
Biological resources, including vegetation, occupy a very important place in people’s lives and survival. In Africa, populations get most of their subsistence needs (food, energy, medicines etc.) from these resources. In Mali about 90% of populations’ needs are met by vegetation exploitation [1]. This exploitation affects the production and productivity of the different formations and therefore their carbon stock tanks. Forests, which are important reservoirs of biomass including carbon, are continuously burnt. The repeated burning and anthropogenic disturbances can cause vegetation to lose a significant portion of the carbon stored for centuries [2].
The ecosystem of Mali, a Sahelian country, is rich and hold diverse tree species, which are heritage to preserve. These ecosys tems are subject to significant deterioration, due to combined effects of climatic and anthropogenic disturbances [3]. Most of the ecosystems are fragile and the soils are generally poor. Also, the natural vegetation production is low and gradually decreases because of human exploitation. So, carbon sequestration is an important way to attenuate the effects of climate change on plant production and productivity [4].
The vegetation of the Sudan zone is mainly savannah ecosystems which are grassland, shrubland, woodland, gallery forest, open forest. The grassland cover is dominated by annual and perennial grasses [5]. The structure and composition of the vegetation present is thus mainly dictated by plant accessibility to water resources. The presence of different trees species is therefore governed by the capacity of the soil to preserve enough level of water and nutrient availability. However, this water dependent organization is altered by human activities that exert substantial and constant pressure on the vegetation formation [6-8].
In the Sudanian zone of Mali, There are four vegetation formations, and the potential product is reducing under human pressure for farming and fuel-wood collection [5,9,10]. These activities affect carbon sequestration potential of the formations. Also, this scenario has often been investigated in the context of fuel-wood energy policy of Mali [11-13]. The carbon sequestration of different formations has been widely studied [14-20]. However, carbon sequestration by local trees species has been less frequently addressed [21-24].
This paper focuses on the carbon sequestration by the different formations and in each formation by tree species. The vegetation formations types in Koulikoro region (Dioïla districk), in the southern part of the Sudan ecological zone of Mali, were the subject of tree species inventoried. And sought for advance knowledge on the carbon sequestration in the vegetation formations of Sudanian ecological zone and improve our knowledge and understanding of the amount of carbon sequestered by tree species of vegetation formations in the Sudan ecological zones of Mali.
Materials and Methods
Description of the study area
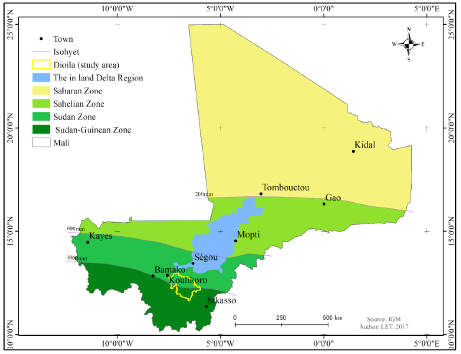
Mali is a landlocked country in West Africa, located between 100 and 250 North latitude and between 40 East and 120 West, with land mass of 1,241,238 km² (Figure 1). The prevailing wind is the North-East trade wind. Apart from the republic of Niger, Mali is the largest state in West Africa. It extends for considerable distances 1500 km from north to south and 1,800 km from east to west [25]. Two-thirds of its area (about 61%) is occupied by dry areas [3], where most of the territory is desert. On the rest of the country, almost 30% is affected by desertification.
The Sahelian nature of Mali justifies its present in the Inter-State Committee for the Fight against Drought in the Sahel (ICFDS) [5]. There are essentially five major ecosystems types that are: Sahara, Sahel, Central Niger Delta, Sudanian Zone and North-Guinean Zone. These zones, including the Mandingo Plateau, Upper Bani Niger, Central Niger Delta, Gourma and the Adrar of Ifoghas, [3] are of great interest because of the potential of biological importance they harbor.
This study was carried out in the Sudanian zone specifically within the Dioïla district in Koulikoro region at the extreme south of the Sudan zone of Mali (Figure 1). This choice is not only because of its strategic position in the Sudan Zone of Mali, but also, of the importance of its vegetation and multiple anthropogenic disturbances. It is located in the Southeast part of Koulikoro region with an area of 12,794 km², an estimated population of 387,565 and a density of 30.58 in habitants’ km². The dominant ethnic groups are the Bambara, the Somono, Bozo, Fulani and Marka [3].
Figure 1: Map of Eco-climatic zones of Mali (IER, 2000) and the study area (yellow border) covering the Sudanian and Sudanon-Gunean zone.
The climate is hot and humid Sudanian type, with high average annual rainfall which can reach up to 800 mm year-1. The average annual temperature is 26.9 °C with a maximum of 31.9 °C in March-April. Soils are mainly of five types: gravelly, sandy loam, clay loam, sandy and clay in the depression areas. Abundant vegetation is mainly savannah ecosystems (grassland, shrubland, savannah, woodland, gallery forest, open forest).
Agriculture is the main activity of the populations in this area, with major crop types being sorghum, cotton, maize and rice (Figure 1).
Field survey
Research design for this work on the carbon sequestration capacity of tree species in different vegetation formations of Sudan ecological zone of Mali, was basically a field work based on field survey in which several data equipment’s such as: GPS, tailor tape, pole of six meters, data sheets etc. were deployed for data acquisition handling and management.
The quantitative data related to the diameter of breast height, height, and composition of different tree species in the different vegetation were used. Two sources of data namely primary and secondary have been used. Primary data were generated from the use of forest inventory questionnaire sheets and phyto-ecological questionnaire sheet while, the secondary data were generated from the Technical Service of Water and Forests in Mali. Also, from any others information sources related on the Sudan vegetation zone in Mali.
Sampling frame
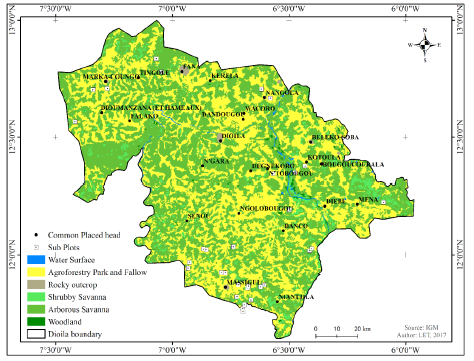
For data collection, a stratified two-stage samplings method was adopted due to the unavailability of time and resources. However, it was identified that the different types of vegetation had already been carved out from the vegetation formation on vegetation map by Observatory of Saharan and Sahelian (OSS) [4]. We extracted the area of interest from that map to identify the different vegetation formation.
The map was produced as a way to minimize the variability of variables as much as possible to estimate stem density per hectare and to measure the volume of timber. Each different type of vegetation is represented as a group of strata. In which six vegetation formations were identified. According to the reality in the study zone, the study area was limited to four vegetation formations with 1602 strata on an area of 1,247,917 hectares (Figure 2).
Figure 2:Map showing different forms of vegetation in the district name. Samples plots are indicated by white squares.
Survey technique
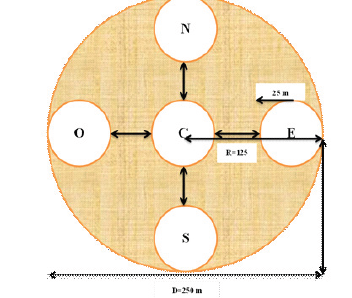
The circular plots and subplots were chosen according to the ecological conditions in the study area and the type of inventory. The diameter of the plot was set at 250 m and that of subplots at 50 m with a radius of 25 m. The area of the subplots is 0.20 ha corresponding to 1962.5 m² well, above the minimum of the area 1000 m² [26]. Each plot consists of five subplots with an area of 0.98 ± 1 ha (Figure 3).
On the demarcation of the major round of sub plot for inventory the center was located by using GPS. The field assistant was placed in the center and another took one end of the cord whose length was adjusted to the radius of the plot or subplot. The other field assistant took the other end of the cord and went around making out a line round the boundaries of the plot or subplot. Once the plots and subplots were made, data collection was performed (Figure 3).
Figure 3:Forms and dimensions of sampling plot. NB: N=North; E= East; S= South; O= West.
Data collection technique
To measure the various trees parameters, two methods of data collection were used; the Phyto ecological inventory and forest inventory/ dendrometry.
Phyto-ecological inventories:The method of Phyto-ecological inventories [27] was adopted to determine the composition and diversity of vegetation formation in the area. Indeed, on the survey of each plot to investigate a census was made to cover all the plot surface. Different plots of tree species were systematically identified and counted while considering the environment of the communities living species (health status of species, logging, climate, soil.
Dendrometry inventory:For the evaluation of woody plant biomass, a dendrometry inventory in each plot for each plant formation was undertaken. In each unit plot inventoried, each stem having a basal circumference greater than or equal to 10 cm the circumference at the base (C0, 0), the circumference to height (C1, 30) and the total height (H); were measured. Choosing this basal circumference greater than or equal to 10 cm, were explained by the fact that the stems having this size and larger are used as wood fuel. In contrast, all stems having a basal circumference of less than 10 cm were rejected [24,26]. These measurements have been made using the tailor’s tapes.
Height measurement:The height measurement was done using a graduated pole of 6 meters for each individual tree. Individual species that exceed 6 meters of heights were estimated electronically with the measuring apparatus (SUUNTO PM-5/360PC, CITY, COUNTRY).
a. Calculation of tree species carbon sequestration:Tree species carbon sequestration was calculated in two stages:
Calculation of tree species biomass:
a. Aboveground:According to the inventory, the volume of wood or aboveground biomass of each species was determined based on the formula developed by Morel in 1987 in Mali [25] with Excel software.
V=10xGxP (1)
Where V is the volume of wood in m3, 10 a constant, G the basal area in m² and P the average annual precipitation on the site or the nearest station in (cm).
b. Calculation of below ground biomass:Ground biomass of each species was calculated using the estimating guide to the amount of sequestration in Mali ILWAC project [4]. The underground biomass of trees is the volume or weight of the root system. In Mali it has been estimated at 50% of the total volume of wood on feet. Thus, this formula has been used:
C (root) = (V*1/2) *0.27) (2)
Estimation of tree species carbon sequestration
To change the volume of timber to the quantities of carbon sequestered by different trees species, the following equation [28] was used with Excel software:
1m3 of woody exploited=1 ton CO2=0.27 tons of carbon (3)
Determination of tree species carbon sequestration:To determine tree species carbon sequestration for each vegetation formation, we made the ratio between the individual tree species carbon sequestered and the subplot number of this vegetation formation.
Determination of the amount of carbon sequestered by vegetation formations
To determine the amount of carbon sequestered by each vegetation formation of the study area, we calculated the sum of the quantity of carbon sequestered by each tree species in this vegetation formation.
Data analysis
The different amounts of carbon sequestered by tree species and vegetation formations were calculated using SAS software. Also, the Dynamic cross table was established using Excel software.
To compare carbon sequestration capacity of different tree species and vegetation formations, ANOVA was performed. Indeed, to evaluate the correlation between some parameters of trees species and the amount of carbon sequestered, Pearson correlation test was performed with the package “SAS” software. These tests have been conducted at the level of the general population of our study environment. Also, for data homogenization, we used logarithm function (log(x+1)).
Results
The results of this study are related to the amount of carbon sequestered in different vegetation formations via their trees species. Four vegetation formations were identified in the study area for inventory: Park Agro forestry/Fallow (2-5 years), woody savannah, Shrubby savannah and Arborous savannah.
Quantity of carbon sequestered by vegetation formations
Table 1:Carbon sequestration capacity of tree species in different vegetation formations in the study area.

NB: *Significant at 10% level of significance (p < 0.10), **Significant at 5% level of significance (p < 0.05), ***Significant at 1% level of significance (p < 0.01).
Figure 4:Amount of carbon sequestered of different vegetation formations in the study area. NB: for homogenization of data on SAS analysis, we used logarithm function (log(x+1). Park agroforestry/Fallow=ParkAg; Arboreous Savannah=Arboreous; woody Savannah=Woody, QCSA=Quantity of Carbon Sequestered by Area; Shrub Savannah=Shrub,
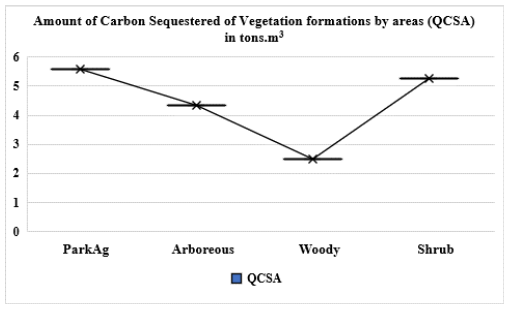
The amount of carbon sequestered by vegetation formations:Figure 4 presents the average amount of carbon sequestered by each vegetation formation, whereas carbon sequestration capacity of each vegetation formation and tree species in the study area are presented in Table 1. This shows that the carbon sequestration capacity and the amount of carbon vary depending on the formation and tree species.
In average, the carbon sequestration capacity of the study area was estimated at 159.75 tons of carbon per hectares. But the carbon sequestration capacity varies depending on the vegetation formations types and the different tree species of formation (Table 1). Indeed, the vegetation formations that are the largest amount of carbon by area are not fiercely the formation that has the higher carbon sequestration capacity. In terms of carbon sequestered per hectare, the woody savannah has the largest carbon sequestration capacity (71.95 t C ha-1). It is followed by the arborous savanna having a capacity of 49.86 t C ha-1 and agroforestry parks/fallow for up to 23.98 t C ha-1. The vegetation formations that have the lowest carbon sequestration capacity per hectare are shrubby savannah with a capacity of 13.97 t C ha-1.
The amount of carbon sequestered varies according to the area. The more the vegetation formations area, the more the amount of carbon sequestered is important (Figure 4). Thus, in terms of total amount of carbon sequestered in the study area, the agrofor estry parks/fallow is the formation that has the largest amount of carbon (12,091,159.49 tons). It is followed by shrubby savannah (9,513,606.304 tons) and arborous savannah (3,079,764.902 tons). The amount of carbon stored is low in the woody savannah vegetation (65,470.17819 tons) compare to others.
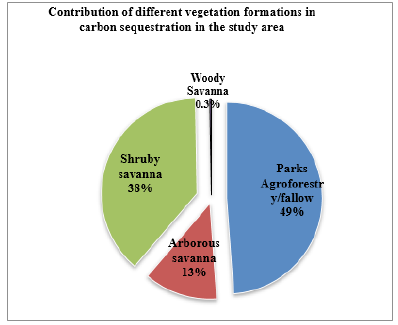
Contribution of the four vegetation formations in carbon sequestration:It appears from the Figure 5 that the contribution of different vegetation formation types in the amount of carbon stored in the area. The amount of carbon available in the study area is estimated to be 24,750,000.88 tons. It appeared that the contribution of different vegetation formation in carbon sequestration is related to the overall amount of carbon stored by all tree species in the area. In this line, in the Sudan ecological zone of Mali, the vegetation formation of parks agroforestry/fallow contributes more to the carbon sequestration (up to 49%) than others vegetation types in the study area. The shrubby savannah is the second vegetation formation to sequester more carbon with 38% followed by arborous savanna (13%), and the woody savannah (0.3%) which is the lowest.
Figure 5:Contribution of different vegetation formations to the carbon sequestration in the studied study area.
It reveals that in Figure 6, the quantity of carbon sequestered by tree species varies from one vegetation formations to the other. Globally, the tree species Parkia biglobosa (Jacq.) R.Br. ex G.Don have the largest capacity in carbon sequestration per hectare (26.8 t C ha-1) in woody savannah, but it is not the tree species that have the higher carbon sequestration capacity in all the vegetation formations in the Sudan ecological zone of Mali.
Figure 6:The best tree species in carbon sequestration in the four’s vegetation formations.
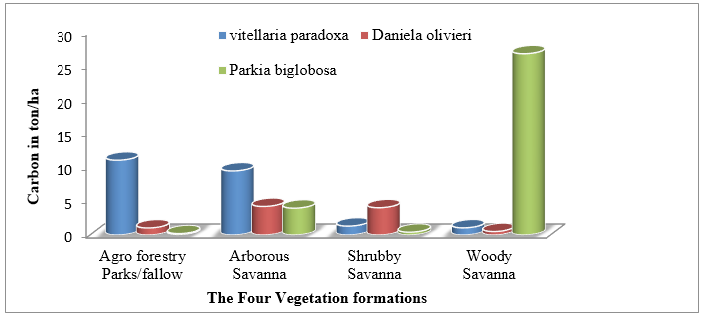
Specifically, Vitellaria paradoxa G. Don is the most dominant carbon sequestration tree in the vegetation formations of Park agroforestry/Fallow (11.087 t C ha-1) and it is followed by arborous savanna (9.5 t C ha-1) while in the vegetation formation of the shrubby savannah Daniellia oliveri (Rolfe) Hutch. & Dalziel is the most dominant (4.04 t C ha-1).
Correlation between tree parameters and the amount of carbon sequestered:The tree parameters include the circumference, floristic richness, tree species diversity and size. These are correlated with total carbon sequestered to understand which tree parameters more significance is.
Figure 7:Correlation between trees circumference and the amount of carbon sequestered
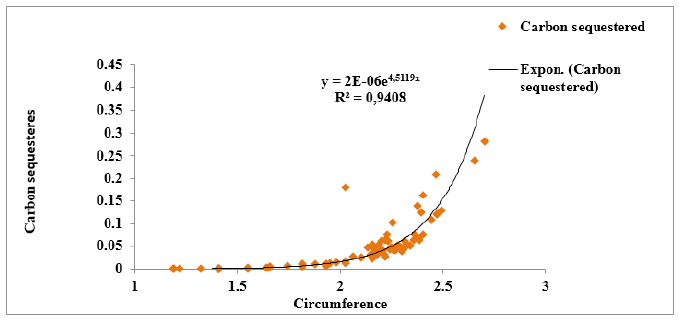
Relationship between the amount of carbon sequestered and the circumference of tree species:The analysis of Figure 7 reveals that there is a positive and exponential correlation between the amount of carbon sequestered by tree species and the circumference class of this tree species. This correlation is very strong with R²=0.94. Which implies that when the tree species circumference is greater the amount of carbon sequestered is important. However, vegetation formations with large circumference classes would have a large amount of carbon sequestered. This is confirmed by the high carbon sequestration in agroforestry parks and fallows.
Figure 8:Correlation between the number of tree species per family and the amount of carbon sequestered in the study area.
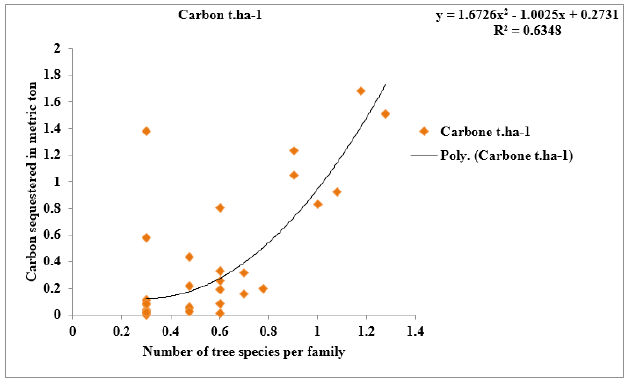
The relationship between floristic richness per family and the amount of carbon sequestered:Floristic richness is the amount of tree vegetation species in the studied area. There is a positive and binomial correlation between the number of tree species per family and the amount of carbon sequestered in the study area. Indeed, it appears from the analysis that the amount of carbon sequestered varies with the number of tree species per family (Figure 8). The statistical correlation analysis between the number of the tree species per family and the amount of carbon sequestered give a high significant correlation.
The correlation coefficient (R²=0.63) shows a positive correlation with significance at 0.05 level. This explains that the more the tree species in a family, the more carbon is sequestered. So, the vegetation formation with higher number of tree species greater have greater potential of carbon sequestration than smaller.
Discussion
The amount of carbon sequestered varies with tree species and the types of vegetation formations. Similar observations were made by researchers [2,16,29]. Besides the area, the amount of carbon sequestered is also depending on the tree species [30-32].
In this study area, Parkiabiglobosa is the species that has the highest (26.8 t C ha-1) capacity in sequestration of carbon per hectare in the woody savanna. Similarly, Ranjan et al. [33], found that the individual tree of Tectona grandisis one species that has great quantity of carbon sequestered (6859.92 pounds of carbon) in Vinoba at Bhave University Campus, Hazaribag. Specifically, the species Vitellaria paradoxa is most dominant in vegetation formations of agro forestry Parks / Fallow (11. 087 t C ha-1) and of arborous savanna (9.5 t C ha-1). The dominance of Parkia biglobosa and Vitellaria paradoxa in these savannas could be explained by the fact that they are spared, not often exploited because of their socio-economic importance and their total protection in forest legislation according to the country law. Similar result was observed by Sanogo et al., [24] about Vitellaria paradoxa species (write the result of this similar study). The tree Daniella oliveri is the best species in carbon sequestration in the shrubby savannah, that can be explained by its rapid growth, but it is also not much used by loggers in the study area due to the lower quality of its wood. Comparatively, Akbari [21], studied the annual sequestration capacity of 12 species and reported that varieties of hybrid poplar (Deltoid) sequester more carbon annually, and at maturity is 14.9 kg/year against the second, blue spruce, with 6.7 kg / year and 29.6kg / year at maturity and against the white pine, the second with 15.2 kg / year.
The amount of carbon sequestered per hectare is higher (71. 95 t C ha-1) in the vegetation formations of woody savanna and of arborous savanna (49. 86 t C ha-1) than in parks agroforestry/fallow vegetation formation (23. 98 t C ha-1) and shrubby savanna (13. 97 t C ha-1). According to Ngomeni et al., [34] The assessed carbon sequestration potential varied significantly between sites, from 67.84±45.41 tC ha-1 in Ayos to 41.94±15.50 t C ha-1 in Nkongsamba. Therefore, this finding is lower than those of M Anobla & N’Dja [35] who observed that the mean estimate of carbon (C) stocks in tree biomass were 80.25 t C ha-1 for and 256.5 t C ha-1 in fallows group respectively (fallows from 4 to 8 years and 14 to 24 years). This could be explained by the fact that the first two vegetation formations are less subject to human disturbances and have various species of large trees [5]. Therefore, park agroforestry and shrubby savannah are under farming and fuel-wood logging, which has long been identified as the cause of reducing vegetation formations potential as observed in other African semi-arid areas [5,9,10]. That can be due to the forest management, climate change mitigation activities, and life cycle assessments in this area [39-41].
Besides, woody savanna has the best amount of carbon sequestered per hectare (71. 95 t C ha-1) in the study area, this amount is less than the one found by Claire [42] who, after the evaluation and comparison of carbon stocks in agroforestry systems in Cameroon Cocoa Centre, a Case of the district of Bokitio says that the fallow has an average capacity of sequestration of 58 tons per hectare. Similar results were observed by Awé et al., [43], while Carbon management for savannah ecosystems in Central Africa: a case study from Cameroon, reported that the in the shrubby savannah (56.09 ± 1.16 t C ha-1) and wooded savannah (60.81 ± 1.42 t C ha-1) of Ngaoundere. This finding is higher than those from Kouassi et al., [44] who state that the carbon sequestered by all the trees in his studied area was 42.92 ton.
The rate of carbon sequestration by vegetation formation and trees species depends on many factors and parameters. The age and stage of growth of tree, growth parameter and density of wood are some important parameters among them [33,45].
The amount of carbon sequestered per hectare depends largely on the circumference of a species, the number of species per family in the studied area. The bigger the tree species, the amount of carbon sequestered or stored is important and more the tree species growth its carbon stored increase also [45,46]. Similar result was found by Leys [47] who noted that the amount of carbon stored depends on the species, growth condition in the environment, age of tree and density of surrounding trees. There is positive and binomial correlation between the number of tree species per family and the amount of carbon sequestered. The linear correlation was observed by Martin and Thomas [48]. The amount of carbon sequestered varies according to the tree’s species number per family [17,49].
Conclusion
The results of this work contributed to the knowledge of the carbon sequestration capacity of tree species at different vegetation formations in the Sudanian ecological zone of Mali. It also enables us to understand the relationship between certain parameters of the vegetation formation and the amount of carbon sequestered. It appears from the results that Sudan ecological zone of Mali have big potentiality in carbon sequestration, of about 159.75 tons of carbon through their tree species and the carbon sequestration capacity. It also reveals that the variation of the amount of carbon depends on the vegetation formation and tree species. It shows that the quantity of carbon sequestered is correlated to certain parameters of trees species, namely circumference and the tree species number per family. In the light of the conclusions, it should be recommended to supply a greater accuracy in assessing the amount of carbon sequestered by tree species in the savannas of Mali; it would be desirable to develop a formula more adapted to the species and the ecological zone and to also consider roots and branches of the tree species.
Acknowledgement
This work was supported by the Center for Dry Land Agriculture of Bayero University, Kano, Nigeria. The authors wish to thank all staff of Bayero University through CDA project and Geography department. We are thankful to the Laboratory of Tropical Ecology (Mali) for their assistance, cooperation and help for the inventory. We are also grateful to the three anonymous reviewers for their very helpful remarks.
Authors’ Contributions
All authors participated in the design and planning of the study, and Fané S, Karembé M, Fadiala D, in the collection and processing of data. Maharazu AY contributed to the correction of the first draft and the supervision of the data collection and processing. Dami A contributed to the corrections.
Competing Interest
The authors declare that they have no competing interests.
References
- Konaté G, Karembé Y (2001) L’Etude prospective du secteur forestier en Afrique (FOSA).
- Djaouga M, Karimou S, Arouna O, Zakari S, Matilo AO, et al. (2021) Cartographie de la biomasse forestière et évaluation du carbone séquestré dans la forêt classée de l ’ Ouémé supérieur au Centre – Bénin Mapping of forest biomass and assessment of sequestered carbon in the forest reserve of upper Ouémé in Central Benin. Int J Biol Chem Sci 15: 2388–2401.
- MEATEU (2000) Stratégie National en Matière de Biodiversité Biologique. Tome 1, Tome 2.
- ILWAC (2013) Gestion intégrée de la Terre et de l’Eau pour l’Adaptation à la Vulnérabilité et au Changement Climatique au Mali (ILWAC) - Sécheresse info.
- Dembélé F, Picard N, Karembé,M, Birnbaum P (2006) Tree vegetation patterns along a gradient of human disturbance in the Sahelian area of Mali. Journal of Arid Environments 64(2): 284–297.
- Dichio B, Romano M, Nuzzo V, Xiloyannis C (2002) Soil water availability and relationship between canopy and roots in young olive trees (cv coratina). Acta Horticulturae 586: 251–254.
- Kitin P, Funada R (2016) Earlywood vessels in ring-porous trees become functional for water transport after bud burst and before the maturation of the current-year leaves. IAWA Journal 37(2): 315–331.
- Li K, Zhou K, Zhu G (2019) Toward understanding the relationship between the microstructure and propagation behavior of water trees. IEEE Transactions on Dielectrics and Electrical Insulation 26(4): 1116–1124.
- Dube OP, Pickup G (2001) Effects of rainfall variability and communal and semi-commercial grazing on land cover in southern African rangelands. Climate Research 17(2): 195–208.
- Sekhwela MBM (2003) Woody vegetation resource changes around selected settlement along aridity gradient in the Kalahari, Botswana. Journal of Arid Environments 54(2): 469–482.
- Gazull L, Gautier D, Montagne P, Bamako M (2019) Household energy transition in Sahelian cities: an analysis of the failure of 30 years of energy policies in Bamako, Mali. an Energy Policy 129: 1080–1089.
- Konare S, Ninomya I, Kobayashi O, Shimamura T, Terashita T (2013) Rural community perception of fuelwood usage by families living in Wassorola, Mali: Interview with women as main fuelwood collectors. Journal of Agricultural and Crop Research 1(5): 76–83.
- Konare S, OSEI OS, Keïta K (2020) Assessing Community Perceptions on Implication of Water Resource Degradation to the Access of Wash Services: Case of Ankobra Basin, Ghana. International Journal of Advanced Life Sciences and Applied Research 2(1): 4–9.
- Baral A, Guha GS (2004) Trees for carbon sequestration or fossil fuel substitution: the issue of cost vs. carbon benefit. Biomass and Bioenergy 27(1): 41–55.
- Barbier EB, Tesfaw AT (2013) Tenure constraints and carbon forestry in Africa. American Journal of Agricultural Economics, 95(4): 964–975.
- Gray AN (2015) The role of old forests and big trees in forest carbon sequestration in the pacific northwest.
- Hu Y, Su Z, Li W, Li J, Ke X (2015) Influence of Tree Species Composition and Community Structure on Carbon Density in a Subtropical Forest. PLOS ONE 10(8): e0136984.
- Jindal R, Swallow B, Kerr J (2008) Forestry-based carbon sequestration projects in Africa: Potential benefits and challenges. Natural Resources Forum 32(2): 116–130.
- Schelhaas MJ, Esch PW van, Groen TA, Jong BHJ de, Kanninen M, et al. (2004) CO2FIX V 3.1 A modelling framework for quantifying carbon sequestration in forest ecosystems.
- Sohngen B, Mendelsohn R (2003) An optimal control model of forest carbon sequestration. American Journal of Agricultural Economics 85(2): 448–457.
- Akbari H (2002) Shade trees reduce building energy use and CO2 emissions from power plants. Environmental Pollution 116(1): S119-S126.
- Pérez-Cruzado C, Mansilla-Salinero P, Rodríguez-Soalleiro R, Merino A (2012) Influence of tree species on carbon sequestration in afforested pastures in a humid temperate region. Plant and Soil 353(1–2): 333–353.
- Pérez-Cruzado C, Merino A, Rodríguez-Soalleiro R (2011) A management tool for estimating bioenergy production and carbon sequestration in Eucalyptus globulus and Eucalyptus nitens grown as short rotation woody crops in north-west Spain. Biomass and Bioenergy 35(7): 2839–2851.
- Sanogo K, Gebrekirstos A, Bayala J, Villamor GB, Kalinganire A, et al. (2016) Potential of dendrochronology in assessing carbon sequestration rates of Vitellaria paradoxa in southern Mali, West Africa. Dendrochronologia 40: 26–35.
- Karembe M, Traore L, Dembele F, Sanogo Y (2014) Influence de la pression humaine sur la diversité et la production ligneuse des galeries de la rivière Baoulé en zone Mali-Sud. Sciences de La Vie, de La Terre et Agronomie 2(1).
- Bayala J, Ayantunde AA, Somda J, Ky-Dembélé C, Bationo BA, et al. (2018) Methodological guide: Community participatory inventory and prioritization of climate-smart crop-livestock agroforestry technologies / practices. CGSpace, ICRAF Tech, Nairobi: World Agroforestry Centre.
- Dohn J, Dembélé F, Karembé M, Moustakas A, Amévor KA, et al. (2012) Tree effects on grass growth in savannas: competition, facilitation and the stress-gradient hypothesis. Journal of Ecology 101(1): 202-209.
- Temmerman M (2009) Le combustible Bio Terre et l’impact environnemental de l’utilisation des résidus agricoles en substitution au charbon de bois. In Centre wallon de Recherches agronomiques Département.
- Ouedraogo WO, Gomgnimbou APK, Santi S, Ilboudo D, Toguyeni A (2020) Quantification de la Biomasse et stockage du carbone du massif forestier de l’Ecole Nationale des Eaux et Forêts de Dindéresso province du Houet au Burkina Faso. Int J Biol Chem Sci 13(7): 3276–3288.
- Balehegn M, Duncan A, Tolera A, Ayantunde AA, Issa S, et al. (2020) Improving adoption of technologies and interventions for increasing supply of quality livestock feed in low- and middle-income countries. Global Food Security, 26.
- Kaul M, Mohren GMJ, Dadhwal VK (2010) Carbon storage and sequestration potential of selected tree species in India. Mitigation and Adaptation Strategies for Global Change 15(5): 489–510.
- Ramachandran Nair PK, Nair VD, Mohan Kumar B, Showalter JM (2010) Carbon Sequestration in Agroforestry Systems. Advances in Agronomy 108(C): 237–307.
- Ranjan A, Khawas S kumar, Mishra PK (2016) Carbon Sequestration Efficacy Of Trees Of Vinoba Bhave University Campus, Hazaribag. Journal of Multidisciplinary Engineering Science and Technology (JMEST) 3(5): 2458–9403.
- Ngomeni AF, Bidzanga ELN, Avana ML, Tchamba MN, Chimi CD, et al. (2022) Potentiel de séquestration du carbone des agroforêts à base de caféier robusta (Coffea canephora var. robusta) dans les bassins de production du Cameroun. Int J Biol Chem Sci 15(6): 2652–2664.
- M Anobla AOM, N Dja JK (2016) Dynamique De La Végétation De Bamo Et Stocks De Carbone Dans La Mosaïque De Végétation. European Scientific Journal, ESJ 12(18): 359.
- Böttcher H, Verkerk PJ, Gusti M, Havlík P, Grassi G (2012) Projection of the future EU forest CO2 sink as affected by recent bioenergy policies using two advanced forest management models. GCB Bioenergy 4(6): 773–783.
- Byrnes RC, Eastburn DJ, Tate KW, Roche LM (2018) A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. Journal of Environmental Quality 47(4): 758–765.
- Lamers P, Nger MJ, Dymond CC, andrefaaij (2014) Damaged forests provide an opportunity to mitigate climate change. GCB Bioenergy 6(1): 44–66.
- Meragiaw M (2017) Role of Agroforestry and Plantation on Climate Change Mitigation and Carbon Sequestration in Ethiopia. Journal of Tree Sciences 36(1): 1–15.
- Nunes LJR, Meireles CIR, Gomes CJP, Ribeiro NMCA (2020) climate Forest Contribution to Climate Change Mitigation: Management Oriented to Carbon Capture and Storage. Climate 8(21): 1–20.
- Smyth CE, Stinson G, Neilson E, Lemprière TC, Hafer M, et al. (2014) Quantifying the biophysical climate change mitigation potential of Canada’s forest sector. Biogeosciences 11(13): 3515–3529.
- Claire D (2013) De la Savane à la Forêt Mémoire de Fin d ’ Etude Evaluation et comparaison des stocks de carbone des systèmes Cas de l ’ arrondissement de Bokito. UMR System Montpellier SupAgro.
- Awé DV, Noiha NV, Zapfack L (2021) Carbon management for savannah ecosystems in Central Africa: a case study from Cameroon. International Journal of Low-Carbon 16: 1290–1298.
- Kouassi JK, Kouassi HK, Kouassi HR (2018) Evaluation de la diversité floristique et estimation du taux de séquestration de carbone des arbres en alignement de voies de la commune de Daloa (Côte d’Ivoire). Int J Biol Chem Sci 12(4): 1876.
- Eneji IS, Obinna O, Azua ET (2014) Sequestration and Carbon Storage Potential of Tropical Forest Reserve and Tree Species Located within Benue State of Nigeria. Journal of Geoscience and Environment Protection 2(2): 157–166.
- Boulmane M, Santa-Regina I, Khia A, Oubrahim H (2014) Éstimation du stock de carbone organique dans l’écosystème des iliçaies du Moyen Atlas marocain. Nature & Technologie 11: 06–16.
- Leys A (2011) Carbon forestry in the New Zealand social landscape: Post introduction of an Emmisions Trading Scheme (ETS).
- Martin AR, Thomas SC (2011) A Reassessment of Carbon Content in Tropical Trees. PLoS ONE 6(8): 23533.
- Hui D, Deng Q, Tian H (2015) Climate Change and Carbon Sequestration in Forest Ecosystems. Springer Science+Business Media New York 37: 1-40.
-
Siriki Fané*, Maharazu Yusuf, Moussa Karembé, Fadiala Dembélé and Aruwajoye Dami. Carbon Sequestration Potential of The Main Tree Species and Vegetation Formations in The Ecological Zone of Sudan in Mali. World J Agri & Soil Sci. 8(3): 2022. WJASS. MS.ID.000690.
-
Carbon sequestration, Tree species, Vegetation formation, Sudanian zone, Mali
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






