 Research Article
Research Article
Antityphoid Activity and Phytochemical Screening ofAzadirachta Indica Leaf Extracts
Muhammad Ali1*, Muhammad S. Abdallah2, Rabiu M. Kutama3 and Lurwanu Muazu4
1Department of Microbiology, Federal University Gusau, Nigeria
2Desert Research Monitoring and Control Centre, Yobe State University Damaturu, Nigeria
3Department of Biology, Saadatu Rimi College of Education, Nigeria
4Department of Biological Sciences, Federal University Gusau
Muhammad Ali, Department of Microbiology, FederalUniversity Gusau, Nigeria.
Received Date:March 25, 2020; Published Date: April 03, 2020
Abstract
Herbal medicines have been known to human for centuries and practitioners of traditional medicine have described therapeutic efficacy ofmany indigenous plants for several disorders. The study was aimed to determine the anti-typhoid activity of Azadirachta indica leaf extracts againstSalmonella typhi and Salmonella paratyphi isolated from typhoid fever patients. A clinical isolates of S.typhi and S. paratyphi were obtained frompatients attending Murtala Muhammad Specialist Hospital, Kanowere tested against aqueous and methanol extracts of A. Indica leavesusing agarwell diffusion method. The phytochemical screening of the extract was conducted using conventional laboratory methods. The result showed thatthe leaf extract of A. Indica contain alkaloids, tannin, anthraquinone, flavonoids, phenols and steroids. The result of antibacterial activity of theextracts against the test isolated indicated that the extracts were active against the isolate with higher activity shown by methanol extract (16.0 mm)when compared to aqueous extract (13.5 mm). Salmonella typhi was found to be the most sensitive isolate (15.8 mm) than Salmonella typhi (13.3 mm).It is concluded that the leaf extracts of A. indica were active against bacterial isolates associated with typhoid fever.
Keywords: Antibacterial activity, Azadirachta indica, phytochemicals, Salmonella typhi.
Introduction
Herbal medicines have been known to human for centuries.Practitioners of traditional medicine have described therapeuticefficacy of many indigenous plants for several disorders. This is dueto the fact that plants contain many biologically active compoundswhich have potential for development as medicinal agents [1].Herbal medicines already form the basis of therapeutic use in thedeveloping countries, but of recent, there has been an increase inthe use of herbal medicines in the developed world too [2]. It is likelythat plants will continue to be a valuable source of new moleculeswhich may, after possible chemical manipulation and providenew and improved drugs [3]. Bacterial resistance to antibioticsrepresents a serious problem for clinicians and the pharmaceuticalindustry and great efforts are being made to reverse this trend,and one of them is the widespread screening of medicinal plantsfrom the traditional system of medicine hoping to get some newer,safer, and more effective agents that can be used to fight infectiousdiseases [4].
Enteric fever is a systemic bacterial infection caused by theGram-negative Salmonella enterica serovar typhi (S. typhi) and theparatyphi serovars A, B and C (S. paratyphi A, B and C) of whichS. paratyphi A is most common [5]. Enteric fever is a generic termfor infections caused by both S. typhi and S. paratyphi. Typhoid andparatyphoid fever refers to the infections caused by the individualserovars [6]. Throughout this work enteric fever will be mostlyused, but in cases focusing on S. typhi infections typhoid fever willalso be used. S. typhi has historically been the most common causeof enteric fever but recently there have been several reports on theemergence of enteric fever caused by S. paratyphi A especially inAsia [7,8].
Neem plants (Azadirachta indica) are mostly trees and rarelyshrubs that belong to family Maliacea [9]. It is naturalized in mosttropical and subtropical countries. It is broad-leaved evergreenthat grows up to 30 m tall. The plant has been used for a longtime in agriculture and medicine [4]. Neem is the most versatile,multifarious trees of tropics, with immense potential. All partsof the neem tree-leaves, flowers, seeds, fruits, roots and barkhave been used traditionally for the treatment of inflammation,infections, fever, skin diseases and dental disorders [10]. The medicinal utilities have been described especially for neem leaf.Neem leaf and its constituents have been demonstrated to exhibitimmunomodulatory, anti-inflammatory, anti-hyperglycaemic,antiulcer, antimalarial, antifungal, antibacterial, antiviral,antioxidant, antimutagenic and anticarcinogenic properties [11].
In 2012, the antimicrobial activity of leaf extract of neem wasconducted against Pseudomonas aeruginosa, Staphylococcus aureus,Salmonella typhi and Bacillus pumillas and concluded that ethanoland methanol extract show maximum inhibition on Bacillus pumillas,Pseudomonas aeruginosa and Staphylococcus aureus in ascendingorder [12]. Neem leaf contains several valuable components suchasisoprenoids that include terpenoids containing limonoids,azadirone and its derivatives [13]. The medicinal properties of theplant were studied by several workers, the antimalaria effect [14]antidiabetic effect [15] and anti-fertility effect [16], effect on thecentral nervous system[17], cardiovascular effect [18] and woundhealing [19]. A indica has been shown to possess anti-microbialproperties by several studies. Rao et al. [20] reported the antimicrobialactivity of the seed oil against a variety of pathogens.Oils from the leaves, seeds and bark possess antibacterial actionagainst certain bacteria [21]. Extracts of neem leaf, neem oil andseed kernels are effective against certain human fungi [11]. Thestudy was aimed to determine the antityphoid activity of A. indicaleaf extracts against S.typhi and S. paratyphi isolated from typhoidfever patients.
Materials and Methods
Ethical approval
Ethical approval (with reference no. HMB/GEN/492/VOL.1) forthis research was obtained from the Hospital Service ManagementBoard (HSMB), Kano based on the consent of Murtala MuhammadSpecialist Hospital ethical committees
Test isolates
Two (2) bacterial strains of S. typhi and S. paratyphi isolatedfrom typhoid fever patients were obtained from pathologylaboratory of Murtala Muhammad Specialist Hospital, Kano. Theisolateswere identified using different laboratory proceduresincluding; Gram’s stain, cultural characterization and Biochemicaltests include (Indole, Methyl red, Voges Proskauer, and Citrateutilization) [22,23]. The isolates were maintained on Nutrient agarslants for further use.
Collection of plant leaves and identification
The leaves of A. indica were collected at Karfi village, Kura LocalGovernment Area in Kano State, Nigeria. The identification andauthentication of the plant materials was done at the Herbariumin the Department of Biological Science, Bayero University Kanowith the following voucher number BUKHAN 0312, and voucherspecimens were deposited there for future reference. The leaveswere washed thoroughly with distilled water and shade dry for2 weeks. The dry leaves were grinded into powder using a sterilepestle and mortar under laboratory condition. The powder waskept in air tight container for future use as described by Ali et al.[24].
Preparation of plant extracts
Methanol and water were used in the extraction process. Fiftygrams (50 g) powder of the plant leaf was soaked in 500 mL each ofdistilled water and methanol respectively. The flasks were kept atroom temperature for 3 days with intermittent shaking after whichfiltration was done using Whatman filter paper. The methanolextracts was evaporated at 50°C using rotary evaporator while theaqueous extract was evaporated at 40°C in water bath until driedextract samples were obtained. All the dried extract samples weredissolved in 10% DMSO separately to the final concentration of 200mg/mL as a stock concentration. The stock solutions were stored inrefrigerator at 40C for further use [24].
Qualitative phytochemical screening
The qualitative phytochemical screening of the leaf extractof A. indica was conducted to determine the presence of variousphytochemical components such as terpenoids, flavonoids,alkaloids, steroid, phenol, anthraquinone, saponin and tannin usingstandard methods as described by Sofowora [25] and Trease andEvans [26].
Antibacterial activity of the extracts
The antibacterial activity of the extracts against the isolateswas determined using agar well diffusion method as describedby Ali et al. [24] with slight modifications. The prepared bacterialsuspension equivalent to 0.5 McFarland Standard (equivalent to 1.5x 106 CFU) was inoculated into sterile Mueller- Hinton agar mediumin a sterile Petri-dish. A sterile 6 mm diameter sterile cork-borerwas used to bore 5 wells into the agar medium at equidistance. Thewells were then filled up with approximately 0.1mL of the extractsolution at a concentration of 50, 100, 150 and 200 mg/L takingcare to prevent spillage onto the surface of the agar medium. Theplates were allowed to stand on the laboratory bench for 1 hour toallow proper diffusion of the extract into the medium after whichthe plates were incubated at 370C for 24 hours, and thereafterthe plates were observed for zones of inhibition and measured.Amoxicillin 100 mg/mL (Pal Pharmacy) was used as a positivecontrol in the experiment. The experiment was conducted intriplicate and the average zone of inhibition was calculated.
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration of the extract wasdetermined using broth dilution technique. Two fold serialdilutions of the extracts were prepared by adding 2mL of 200mg/mL of the extract into a test tube containing 2mL of Nutrient broth,thus producing solution containing 100mg/mL of the extract. Theprocess continues serially up to test tube No. 5, hence producingthe following concentrations; 100, 50, 25, 12.5, 6.25 mg/mL. Testtube Number 6 does not contain extracts and serve as negative control. Exactly 0.5 mL of 0.5 McFarland equivalent standards oftest organisms were introduced into the test tubes and incubatedat 370 C for 24 hours. After incubation the test tubes were observedfor growth by checking for turbidity [10].
Minimum bactericidal concentration (MBC)
Minimum Bactericidal Concentration of the extracts wasdetermined using procedure of Ahmed and Beg [27].From each tubethat did not show visible growth in the MIC, 0.1mL was asepticallytransferred into extract free Mueller Hilton agar plates. The plateswere incubated at 37°C for 24 hours. The MBC was recorded as thelowest concentration of the extract that had less than 99% growthon the agar plates.
Result
Phytochemical screening of the extracts
The phytochemical constituents of aqueous and methanolleaf extracts of A. indica are presented in the table below Table 1.The result showed that both aqueous and methanol leaf extractscontain alkaloids, tannin, anthraquinone, flavonoids, phenols andsteroids. Terpenoids are present in aqueous leaf extracts but absentin methanol extract. On the other hand, glycoside and saponin wereabsent.
Table 1:Phytochemical Screening of Aqueous and Methanol LeafExtracts of A. indica.

Antibacterial activity of the extracts
The antibacterial activity of aqueous and methanol extracts ofA. Indica against S. typhi and S. paratyphi is presented in Table 2. Theresult shows that highest antibacterial activity was demonstratedagainst S. Typhi with zone of inhibition of 21.70 mm at 200 mg/mLof methanol leaf extract. Zones of inhibition shown by control (100mg/mL Ciprofloxacin) were 25.30 mm and 23.70 mm for S. typhiand S. paratyphi respectively.
MIC and MBC of the extracts
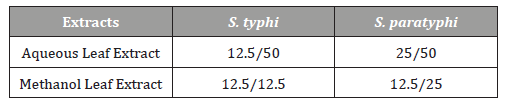
Minimum inhibitory concentration (MIC) and minimumbactericidal concentration (MBC) of aqueous and methanolextracts of A. indica against S. typhi and S. paratyphi is presentedin presented in Table 3. The result shows that dilutions of variousconcentrations of the plant leaf extract are active against testisolates. The MIC ranges from 6.25 – 25 mg/mL while MBC rangesfrom 12.5 – 50 mg/mL.
Table 2:Antibacterial Activity of the Extracts against S. typhi and S.paratyphi.
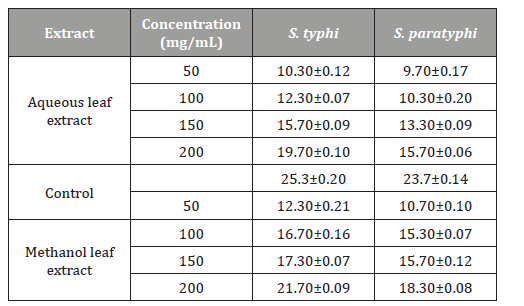
Table 3:MIC and MBC of the Extracts.
Discussion
The presence of secondary metabolites in plant lead toproduction of some biological activity of the plant in man and itis responsible for their use as herbs [10]. These compounds alsoserve to protect the plant against infection by microorganisms,predation by insects and herbivores, while some give plants theirodors and /or flavors and some still are responsible for theirpigments [2]. Salmonellosis and enteric fever are always a publichealth concern in most developing countries, which are mostlylow or middle-income countries with inadequate sanitation andhygiene, particularly regarding food, water and disposal of humanexcreta [28].
The result of the present study revealed the present ofphytochemical compound in the leaf extracts of A. indica. Thisresult was in conformity with the result of several findingsconducted by many researchers [29]. A. indica leaf contains severalvaluable components such as alkaloid, flavonoid and isoprenoidsthat include terpenoids containing limonoids, azadirone and itsderivatives [13].Many plants have been investigated scientificallyfor antimicrobial activity and a large number of plant productshave been shown to inhibit growth of pathogenic bacteria [30].Some of these metabolites particularly the flavonoids, tannin andalkaloid were reported to be responsible for antimicrobial activityassociated with some ethno-medicinal plant [31].
The antibacterial property of the extracts against the testisolates revealed that S. Typhi showed the highest zones ofinhibition (23 mm) than S. paratyphi. This result justifies thefinding of Al-Akel et al. [28]. The leaf extracts of the plant is known to possess antibacterial activity against pathogenic bacteria such asPseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhiand Bacillus pumillas [12]. The antimicrobial activity of the leafextracts is attributed to the presence of phytochemical constituentsin the plant’s leaves. The methanol extract was found more activethan aqueous extract and this justified several studies conductedinvolving aqueous and organic extract [10,24,32] since moststudies have reported that organic solvents were better chemicalreagents for consistent extraction of antimicrobial substancesfrom medicinal plants. The test organisms had the same minimuminhibitory concentration (MIC) value ranges of 6.25 to 25 mg/mLand minimum bactericidal concentration (MBC) value ranges of12.5 to 50 mg/mL. This indicated that the leaves A. indica possessedantibacterial property against S. typhi and S. paratyphi. This issimilar to the findings of the National Library of Medicine at theNational Institutes of Health (www.pubmed.com) who reportedthat in test tubes, A. indica has been shown to have significant effectson both Gram-positive and Gram-negative organisms and otherbacteria that cause a wide array of human and animal diseases.
Conclusion
It may be concluded from this study that A. indica leaf extractshave antibacterial activity against S. typhi and S. paratyphi isolatedfrom typhoid fever patients. The antibacterial activity is probablydue to the presence of phytochemical constituents such as alkaloids,tannin, anthraquinone, flavonoids, phenols and steroids. The wideuse of A. indica is attributable to the presence of these bioactivecompounds, which may explain its many traditional uses againsttyphoid fever.
Acknowledgement
The authors wish to acknowledge the technical staff ofPathology Department of Murtala Muhammad Specialist Hospitalfor samples provision. Thanks to Kano State Government throughMinistry of Health for the ethical approval.
Conflict of Interest
No conflict of interest.
References
- De N, Ifeoma E (2002) Antimicrobial effects of components of the bark extracts of neem (Azadirachta indica Juss). J. Technol. Dev 8: 2328.
- El-Mahmood AM, Ogbonna OB, Raji M (2010) The antibacterial activity of Azadarichta indica (neem) seed extracts against bacterial pathogens associated with eye and ear infections, Journal of Medicinal Plants Research 4(14): 1414-1421.
- Shah JS, Shah MB, Goswami SS, Santani DD (2006) Mechanism of action of antiulcer activity of bark extracts of Manikarahex andra against experimentally induced gastric ulcers in rats. Phcog. Mag 2: 40-45.
- Natarajan V, Veugopal PV, Menon T (2003) Effect of Azadirachta indica (neem) on the growth pattern of Dermatophytes, Indian J Med Microbiol 21(2): 98-101.
- Bhan MK, Bahl R, Bhatnagar S (2005) Typhoid and paratyphoid fever. The Lancet 366(9487): 749-762.
- Woods CW,Murdoch DR, Zimmerman MD, Glover WA, Basnyat B, et al. (2006) Emergence of Salmonella enterica serotype Paratyphi A as a major cause of enteric fever in Kathmandu, Nepal. R. Soc. Trop. Med. Hyg 100(11): 1063-1067.
- Ochiai RL, Wang X, von Seidlein L, Yang J, Bhutta ZA, et al. (2005) Salmonella Paratyphi A Rates, Asia. Infect. Dis 11(11), 1764-1766.
- Sahastrabuddhe S, Carbis R, Wierzba TF, Ochiai RL (2013) Increasing rates of Salmonella Paratyphi A and the current status of its vaccine development. Expert Rev. Vaccines 12(9): 1021-1031.
- Margaret SN (1965) Introduction to the flowering plants of West Africa. (2nd edition).University of London Press Limited, London 169.
- Nas FS, Ali M (2017) Phytochemical screening and Antibacterial Activity of Azadirachta indica Stem and Leaf Extracts on Escherichia coli and Staphylococcus aureus Isolated from Spoiled Cabbage. Journal of Pharmaceutical and Allied Sciences 14(4): 2616-2624.
- Biswas K, Chattopadhyay I, Ranajit KB, Bandyopadhya U (2002) Biological activities and medical properties of neem (Azadirachta indica). Current Sciences 82 (11): 1336 -1345.
- Brindha MS, Kariyarasi S, Annadurai NS, Gangwar SK (2012) Antimicrobial activity in leaf extractof Neem ( Azadirachta indica ). International journal of science and Natural 3: 110.
- Xu J, Du YH, Yin ZQ, Li XT, Jia RY, et al. (2010) The preparation of neem oil microemulsion (Azadirachta indica) and the comparison acaricidal time between neem oil microemulsion and other formulation in vitro. Veterinary Parasitology 169(3-4): 399-403.
- Rochankij S, Thebraranonth Y, Yenjal C (1985) Nimbolide a constituent of indica inhibits Plasmodium falciparum in culture. South East Asian Journal Tropical Medicine and Public Health 16(1): 66-72.
- Shukla R, Singh S, Bhandari CR (1973) Preliminary clinical trail on antidiabetic actions of Azadirachta indica. Medicine and Surgery 13: 11-12.
- Sinha KC, Riar SS, Tiwarry RS, Dhawan AK, Bhadhan J, et al. (1984) Neem oil as a vaginal contraceptive. Indian Journal of medicinal Research 79: 131-136.
- Phillai NR, Shanthakumari G (1984) Effect of nimbidin as acute and chronic grastroduodenal ulcer models in experimental animals. Planta Medica 50(2): 143-146.
- Thompson EB, Anderson CC (1978) Cardio vascular effects of Azadirachta indica Journal of Pharmaceutical Sciences 67(10): 1476-1478.
- Jayaprakasan MV, Viswanathan K, Rajesh M, Pradymnan PP (2014) Ayurvedic preparation from Azadirachta indica, Terminaliachebula, Hemigraphiscolorataextracts and its antimicrobial investigation, IOSR Journal of Pharmacy and Biological Sciences 9(2): 01-06.
- Rao DVK, Sing K, Chopra R, Chatra PC, Ramanujalu G (1986) In vitro antibactericidal activity of neem oil. Indian Journal of Medicinal Research 84: 314 -316.
- Khan M, Wassilar SW (1987) In natural pesticides from the Neem tree and other Tropical plants. Schmutterer H, Asher KRS Eds. Eschborn, Germany, pp. 650.
- Holt JG, Krieg NR, Sneath PA, Stanley JT, Williams ST (1994) Bergey’s manual of systematic bacteriology. Williams (Edt.), (9th edn), Baltimore, Maryland, USA, pp.786.
- Cheesbrough M (2006) District Laboratory Practice in Tropical Countries, (2nd edn), Part Two, Cambridge University Press, USA, pp. 80-85.
- Ali M, Yahaya A, Zage AU, Yusuf ZM (2017) In vitro Antibacterial Activity and Phytochemical Screening of Psidium guajava on Some Enteric Bacterial Isolates of Public Health Importance. Journal of Advances in Medical and Pharmaceutical Sciences 12(3): 1-7.
- Sofowora A (1993) Medicinal Plants and Traditional Medicine in Africa. John Wiley and Sons Ltd. New York, London, pp. 143-145
- Trease GE, Evans WC (1983) (12th edn), English Language Book Society, Bailliere Tindall, pp. 374-404.
- Ahmed I, Beg AZ (2001) Antimicrobial and phytochemical studies on 45 Indian Medicinal plants against multi-drug resistance human pathogens. J Ethnopharmacol 74(2): 113-123.
- Al Akeel R, Ayesha Mateen A, Janardhan K, Gupta VC (2017) Analysis of anti-bacterial and anti-oxidative activity of Azadirachta indica bark using various solvents extracts. Saudi Journal of Biological Sciences 24(1): 11-14.
- Timothy SY, Adamu S, Nyandaiti WY, Sugun MY, Bukbuk DN (2011) Phytochemical and Antimicrobial Activity of Aqueous leaf extract of Sennasiamea(Lam.) on Enterobacteriaceae. Nig J. of Exp. and Applied Biol 9(2): 159-163.
- Naima S, Muhammad RK, Maria S (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilisleptophylla L. BMC Complement. Altern. Med 12: 221.
- Atto V, Koffi DP, Monteomo GF, Adeoti MF (2016) Phytochemical Screening of Sclerocarya birrea (Anacardiaceae) and Khaya senegalensis (Meliaceae), antidiabetic plants. Int J Pharm Chem 2(1): 1-5.
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY (2011) Extraction, isolation and characterization of bioactive compounds from plants extracts. Afr J Tradit Complement Altern Med 8(1): 1-10.
-
M Ali, MS. Abdallah, RM. Kutama L Muazu. Antityphoid Activity and Phytochemical Screening of Azadirachta Indica LeafExtracts. Sci J Research & Rev. 2(4): 2020. SJRR.MS.ID.000541.
S. paratyphi, A. Indica, Salmonella enterica, S. paratyphi
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






