 Research Article
Research Article
Typing of Candida Strains Straightened from Immunesupressed Patients and Investigation of Their Sensitivity to Antifungal Drugs by E-Test Method
Feray Ferda Senol1*, Mehmet Ozcan1, Zulal ASCI Toraman2, Yasemin Bulut2 and Yusuf Yakupogullari3
1Department of Medical Microbiology, Fethi Sekin City Hospital, Turkey
2Department of Medical Microbiology, Firat University, Faculty of Medicine, Turkey
3Department of Medical Microbiology, Inonu University, Faculty of Medicine, Turkey
Feray Ferda Senol, Department of Medical Microbiology, Fethi Sekin City Hospital, Turkey.
Received Date: July 07, 2021; Published Date: August 22, 2022
Abstract
Objective: Investigation of the Antifungal Susceptibility of Candida Strains Isolated from Immune Suppressed Patients, with E test Method It is well-known that Candida causes a considerable mortality via leading invasive and disseminated-systemic infections in the patients whose immune suppressed or in poor clinic condition.
Materials and Methods: In this study, it was aimed to investigate typing of Candida species that were isolated from various clinic samples from inpatients whose immune-suppressed or in poor clinic condition and also to determine the antifungal susceptibility of these isolates. During the study, a total 46 Candida were isolated from urine (6), sputum (6), blood (11), wound (4), endotracheal aspirate (7), nasopharynx (3), catheter (7) and stool (2) samples of 39 febrile neutropenic patients and 7 patients with poor clinic condition in intensive care units. Typing of the Candida isolates were carried out with API ID 32 C (bio Mérieux) kits. Antifungal susceptibility was determined with the E test (AB Biodisk, Sweden).
Result: Of the strains included into the study, 24 (52.1%) were identified as Candida albicans, five (10.8%) were Candida krusei, seven (15.2 %) were Candida tropicalis, four (8.6 %) were Candida glabrata, three (6.5 %) were Candida parapsilosis and three (6.5%) were Candida guillermondii. In the susceptibility assay with E test, 2.1% of the isolates were found resistant to amphotericin B, 21.7 % to ketoconazole and fluconazole, 17.3 % to voriconazole, 23.9% to itraconazole and 6.5 % to flucytosine. The MIC50 and MIC90 values of the most active antifungal, amphotericin B, were determined as 0.019 and 0.75 μg/ml, respectively; and the MIC50 and MIC90 values of the least active antifungal, itraconazole, were determined as 0.032 and 16 μg/ml, orderly.
Conclusion: Due to development of the medicine, the target patient population of Candida, which is an opportunistic pathogen, is progressively augmented, and by the time, it is observed that the prevalence of the invasive candida infections are being increased. Therefore, to successfully treat the serious infections caused by Candida in the patients at high risk, typing of the Candida should be routinely performed and antifungal susceptibility of these strains should be investigated with a reliable method, regularly.
Keywords: Candida strains; Amphotericin B; Voriconazole; Ketoconazole; Itraconazole; Fluconazole; Flucytosine; Antifungal susceptibility
Introduction
The increase in fungal infections in recent years is directly proportional to the widespread use of broad-spectrum antibiotics and immunosuppressive drugs, increasing invasive procedures and the widespread use of major surgical interventions [1]. Candida species often cause opportunistic infections in patients with poor general condition or immunocompromised. Candida species, which are responsible for 86% of fungal infections in such patients, are the fourth most frequently isolated microorganisms from blood cultures [2]. Candida species are also responsible for 8-12% of nosocomial sepsis [3].
candidemia caused by candida; It is a serious clinical picture with a high mortality rate and difficult to diagnose and treat [4,5]. In mycological diagnosis, molecular methods are applied together with conventional diagnostic methods. [5-9] It causes prolonged hospital stay and has a higher mortality compared to sepsis developing with bacterial pathogens [4].
Although there are more than 150 candida species in nature, only some of them have been determined to cause disease in humans. These species are; Candida albicans (C. albicans), Candida tropicalis (C. tropicalis), Candida krusei (C. krusei), Candida glabrata (C. glabrata), Candida guiiliermondii (C. guiiliermondii), Candida stellotoidea (C.stellotoidea), Candida kefir (C.kefyr), Candida lusitania (C. lusitania) and Candida dubliniensis (C. dubliniensis) [5]. Although C. albicans is the most frequently isolated species, the number of infections caused by non-albicans species is increasing [3,4,10].
Antifungal drugs are effective against local or systemic infections of the skin, mucous membranes and organs; are agents that can be administered topically, orally or parenterally. Antifungal drug therapy was limited to potassium iodide and methylene blue until the 1950s due to its toxic effects. Later, amphotericin B was discovered and it has maintained its importance in the treatment of systemic fungal infections since its introduction in the 1950s [11]. Systemic-acting antifungal drugs are few in number. The discovery of azole derivatives such as miconazole, ketoconazole, itraconazole, and fluconazole after amphotericin B led to significant improvements in treatment [12].
5-flucytosine, which is an alternative to amphotericin B, came into use in 1964. In the late 1960s, imidazole group antifungal drugs, which formed the first group of synthetically obtained azoles; It consists of clotrimazole, miconazole, econazole, isoconazole, ketoconazole and fenticonazole. After 1980, terconazole, itraconazole and fluconazole from triazoles, which are the second azole derivatives, came into clinical use. Itraconazole and fluconazole, which are called second generation azoles, have become alternatives to amphotericin B in the treatment of invasive infections caused by many different fungal species, due to their clinical effectiveness and safety. In particular, the success of fluconazole has been encouraging for the development of new third generation azoles (second generation triazoles). Voriconazole, pseoconazole and ravuconazole are second generation triazoles, and although they have been used in our country in recent years, there are few studies on their clinical effectiveness [1,11-14].
While resistance to the antifungal drugs used today was very rare until 10 years ago, in the 1990s, resistance, especially in immunocompromised patients, started to become an important problem. For example, it has been reported that resistance to antifungal drugs often occurs in oropharyngeal candida infections in patients with AIDS [15].
In this study, we aimed to differentiate the species of candida isolated from various clinical samples taken from patients with poor general condition or immunocompromised patients, who are considered as high-risk patients for candida infections, and to determine these strains by using the E test (AB Biodisk, Sweden) method with amphotericin B, flucytosine, fluconazole. It was aimed to determine the susceptibility of antifungal drugs such as ketoconazole, voriconazole and itraconazole.
Materials and Methods
In this study, 46 candida strains isolated from various clinical samples sent to the Microbiology Laboratory from patients who were hospitalized in different clinics of Fırat University Fırat Medical Center between January 1st and August 31st, 2005 and whose general condition was impaired due to various diseases or whose neutrophil count fell below the critical level; It was examined in terms of species identification and detection of antifungal susceptibility. Some epidemiological information of the patients, such as age, gender, major disease, hospital admission, risk factors for fungal infection, were recorded. Clinical samples sent to our laboratory for microbiological examination were cultivated in blood culture bottles (Organon or Becton Dickenson blood culture bottles), blood agar, EMB and/or Sabouraud Dextrose Agar medium (SDA) according to the type of sample. Gram staining was done first from cultures that produced pure yeast colonies. Samples with Gram (+) yeast cells in Gram staining were passaged into SDA medium and incubated at 30° C for 24-48 hours. SDA growing yeasts; Colony structures, typical yeast cells appearance on Gram stain, germ tube formation, presence of true or false hyphae in Tween 80 Corn Meal Agar, chlamidospore structures were examined. API ID 32 C (Bio- Merieux, France) strips were used for the biochemical evaluation of yeasts.
Colonies of yeasts taken from fresh cultures (24 hours) in SDA medium with the help of sterile wipes were mixed in 0.85% suspension medium. This suspension was spread on RPMI 1640 medium prepared in 90 mm petri dishes with the help of cotton-tipped non-adsorbent sterile wipes. Two E-test antifungal strips were placed in the middle of the medium. The plates were covered with paraffin to retain moisture. The prepared plates were incubated for 24-48 hours at 350° C. When the inhibition ellipses formed at the end of the incubation were evaluated, the value at which growth was completely inhibited (100% inhibition) for amphotericin B and flucytosine in accordance with the manufacturer’s and NCCLS recommendations; For azoles, the value at which growth was inhibited by 80% was accepted as the MIC value for that drug. (11th). According to the obtained MIC values, the susceptibility or resistance status of the strains to the tested antifungal was interpreted in accordance with NCCLS M27A recommendations. The strains were classified as susceptible, less susceptible or resistant according to their MIC values [11].
Results
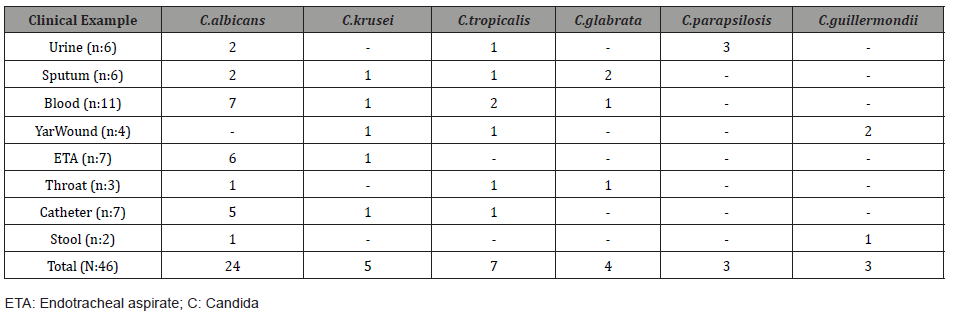
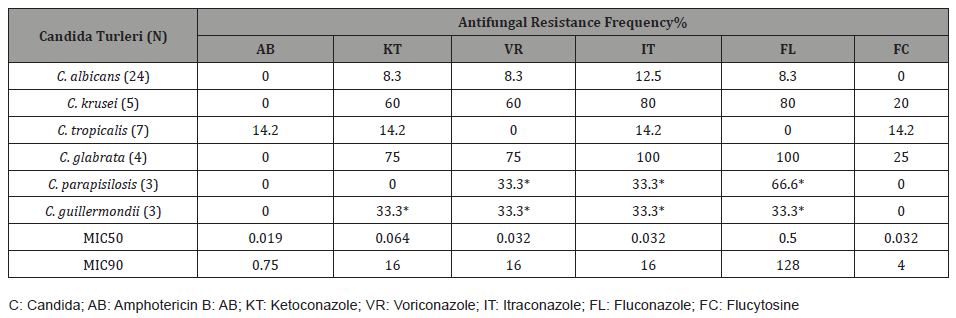
Distribution of Candida Species 39 of the 46 candida strains included in the study were isolated from febrile neutropenic patients. The remaining 7 strains were isolated from comatose patients in the surgical intensive care unit. of the studied candida, 24 (52.1%) C. albicans, five (10.8%) C. krusei, seven (15.2%) C. tropicalis, four (8.6%) C. glabrata, three (6.5%) C. parapsilosis and three (6.5%) were identified as C. guillermondii. The distribution of Candida species according to the clinical samples from which they were isolated is shown in Table 1.
Table 1: Distribution of Candida Species According to the Clinical Samples from which They were Isolated.
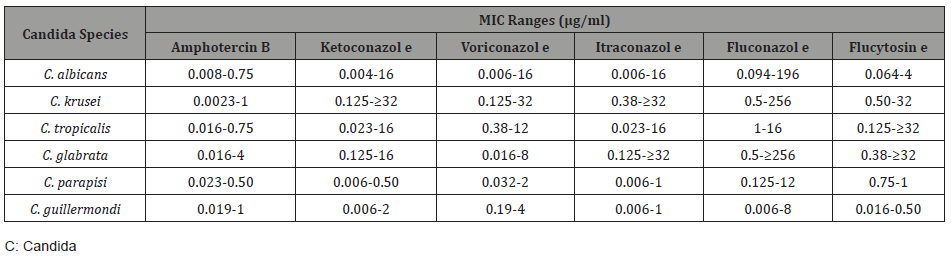
The MIC ranges of the drugs tested against candida species in the antifungal 35 susceptibility experiment performed with the E test method for the candida strains included in the study are shown in Table 2.
Table 2: MIC Ranges of Antifungal Drugs Detected by Candida Species.
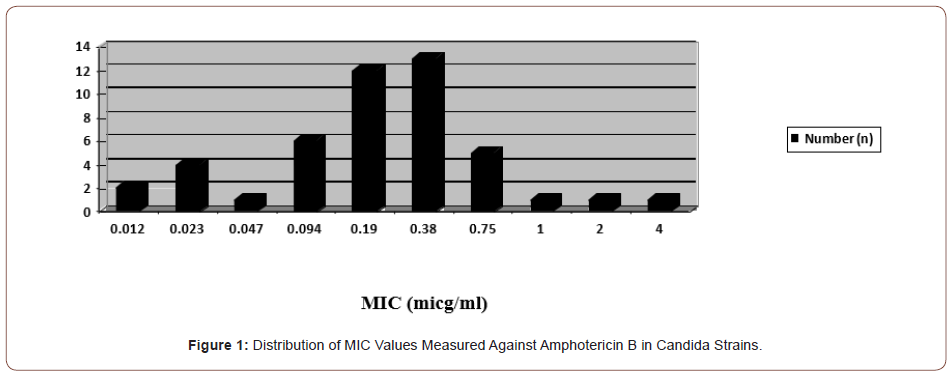
Resistance to amphotericin B was detected in only one (2.1%) C. glabrata strain out of a total of 46 candida strains (4 μg/ml). The MIC distribution of amphotericin B against the studied candida strains is shown in Figure 1.
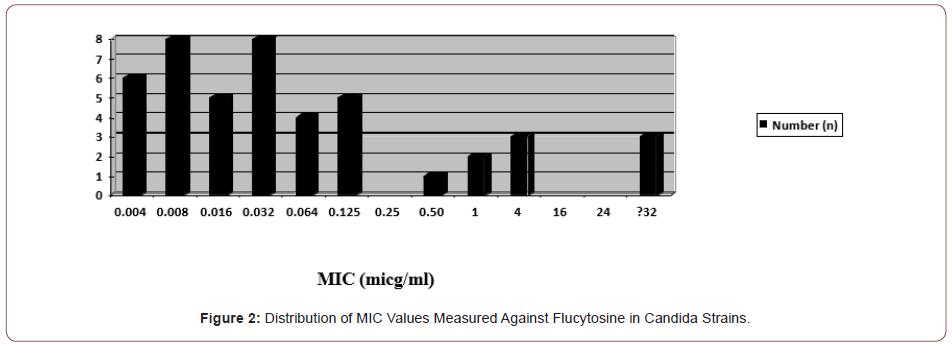
In the study, resistance to flucytosine was found in a total of three (6.5%) candida species, including one C. krusei, one C.tropicalis, and one C. glabrata strain. The MIC distributions of flucytosine against the strains tested are shown in Figure 2. No strains less susceptible to flucytosine were detected.
In the study, fluconazole resistance was observed in a total of 10 (21.7%) candida species, including two C. albicans, four C. krusei and four C. glabrata. Less sensitivity was detected in two C. parapsilosis and one C. tropicalis strains (16-32 μg/ml) (Figure 3).
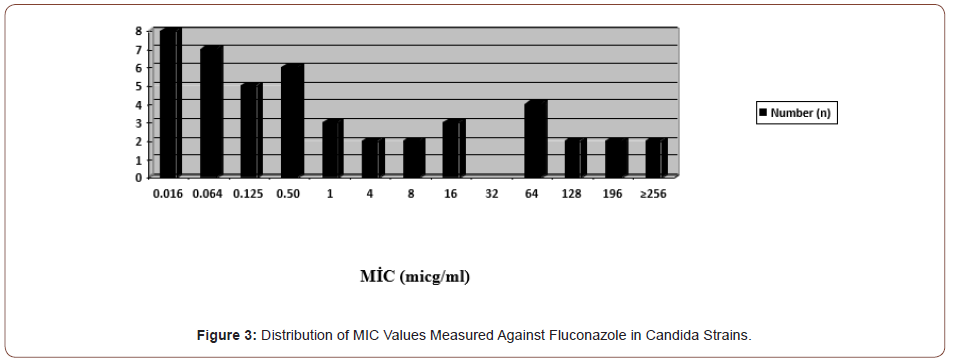
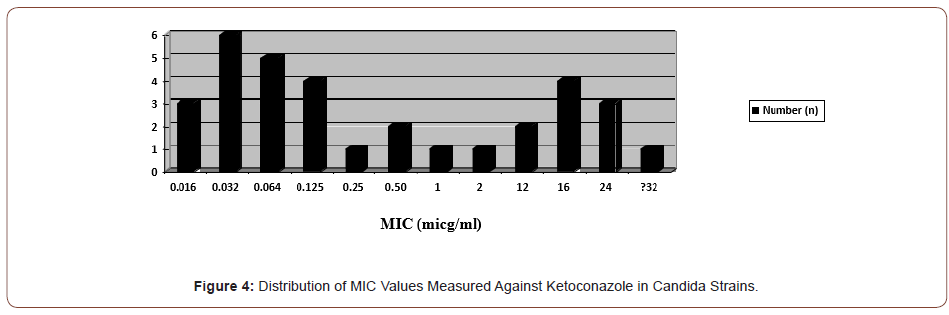
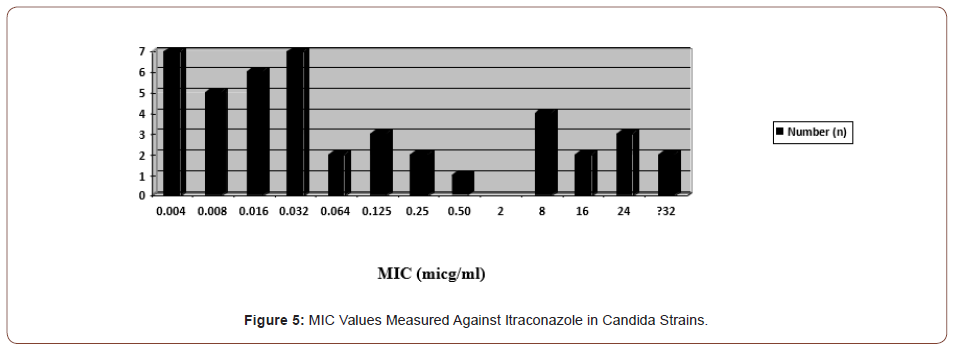
In the study, itraconazole resistance was observed in 11 (23.9%) candida species, three of which were C. albicans, four C. krusei, four C. glabrata and one C. tropicalis. Less susceptibility was detected in one C. parapsilosis and one C. guillermondii strain (1 μg/ ml) (Figures 4&5).
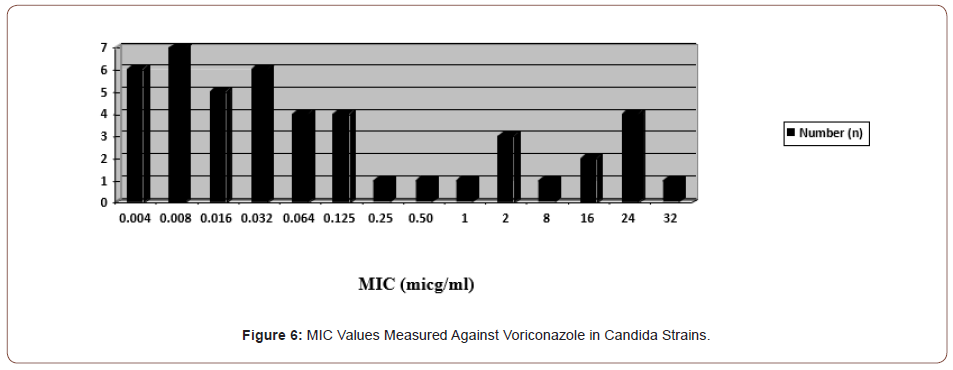
In the study, voriconazole resistance was observed in a total of eight (17.3%) candida species, including two C. albicans, three C. krusei, and three C. glabrata. Less sensitivity was detected in one C. guillermondii and one C. parapsilosis strain (2 and 4 μg/ml) (Figure 6).
The MIC50, MIC90 values of the antifungal drugs tested against the Candida species included in the study and the antifungal resistance rates according to the species are calculated and shown in Table 3.
Table 3: MIC50 and MIC90 Values of Antifungal Drugs and Resistance Rates by Strains.
Discussion
Antifungal drug therapy was limited to methylene blue and potassium iodide until amphotericin B came into clinical use [6]. Flucytosine after amphotericin B; and then azoles were started to be used [12]. Since the 1990s, resistance has started to become an important problem especially in immunosuppressed patients [15]. Candidas are yeasts that live as hosts in many body flora such as the gastrointestinal and uro-genital system, skin, and upper respiratory tract, and there are more than 200 taxonomic species [17]. Only 10% of candida are pathogenic for humans [17]. Five species have been identified in more than 95% of the Candida infections detected in humans. These agents are C. albicans, C. tropicalis, C. parapsilosis, C. krusei and C. glabrata [18,19].
In healthy people, they mostly cause self-limiting, easy to diagnose, superficial and mucosal infections. Such infections can generally be cured with simple hygienic measures and local drug treatments [20,21]. Although life-threatening invasive and systemic infections due to candida can be observed rarely, most of them develop in individuals whose immune system is suppressed or whose general condition is severely impaired [22]. Invasive candidiasis is a common term for two different clinical conditions. One of them is candidemia, and the other is systemic or diffuse candidiasis. Candidemia is the isolation of a candida species from blood accompanied by positive infective findings. Sometimes clinical findings may not be detected in neutropenic patients, graft recipients or patients receiving steroids. Systemic or diffuse candidiasis is the culture or histopathological detection of the presence of a candida species in a tissue (in sterile areas) that should not be. However, it is generally used for invasive candidiasis, mostly hematogenous candida infections. On the other hand, although it has been reported that invasive candidiasis is seen approximately 2 times more in cancer or transplantation centers compared to other hospitals, common candida infections in hospitals providing tertiary health care with intensive care units are increasing their importance. In the light of this information, it is understood that invasive infections due to candida are no longer rare clinical conditions and are not limited to neutropenic and immunosuppressed patients. Intensive care patients with poor general condition and dependent on life support units have been an important target population for serious systemic candida infections [23]. Mortality in candidemia varies between 20-30%. Opportunistic mycoses also cause prolonged hospitalization and increased cost [24,25].
Most of the candida infections in humans are caused by C. albicans. This is thought to be due to the fact that C. albicans is the most common species in the flora [1,2,4,23]. In our study, the most frequently isolated species from clinical specimens was C. albicans (52%). This was followed by C. tropicalis (15%) with the second frequency, and the least defined species were C. parapsilosis and C. guillermondii (7%). Gultekin B, et al. [26], In a study they conducted and used different identification methods, 56% of 94 Candida species were C. albicans, 20% C. glabrata, 10% C. tropicalis, 5% C. kefyr and 1% C. dubliniensis. They defined it as C. dupliniensis [26]. In a study reported by Ozcelik et al. [27], in 2004, using ID 32 C kits, C. albicans was the most frequently identified species (77%) and the least frequently isolated species was C. sake (0.9%) out of a total of 104 clinical isolates [27]. Tumturk A, et al. [28], of the candida isolated from the blood cultures of the patients hospitalized in the intensive care unit, 75 (49.1%) of the candida were C. albicans and 78 (50.9%) were non-albicans candida (NAC). NACs; 32 (21%) C. parapsilosis, 21 C. glabrata (13.7%), 7 (4.6%) C. tropicalis, 6 (3.9%) C. lusitaniae, 5 (3.2%) consisted of C. lipolytica and 7 (4.6%) other candida growths [28]. Togay A, et al. [29], AuxaColor evaluated 71 candida strains isolated from various clinical specimens of patients hospitalized in the intensive care unit, and 28 (39%) C. albicans, 13 (18.3%) C. parapsilosis, 11 (15.5%) C. glabrata, Ten (14.1%) were identified as C. tropicalis, four (5.6%) as C. krusei, three (4.2%) as C. lusitaniae, and one (1.4%) as C. kefyr and C. dubliniensis [29]. Beder D, et al. [30], in their study, they identified C. albicans with 41.3% and C. parapsilosis with 38% [30]. Altin N, et al. [31], of 51 Candida strains isolated from various clinical specimens of patients hospitalized in the intensive care unit, 26 (51%) C. albicans, 7 (14%) C. tropicalis, 5 (10%) C. parapsilosis, 4 (8%) ) C. glabrata, 3 (6%) C. krusei, 3 (6%) C. lusitaniae, 2 (4%) C. famata and 1 (2%) C. kefyr [31]. Muderris T, et al. [32], in the study in which they investigated deaths due to candidemia, C. parapsilosis was the most common 49.1%, followed by C. albicans 26.9% and C. tropicalis 7.9% [32]. Species distribution of candida varies according to the center of study, the patient population studied and the characteristics of the geographical regions [23].
In our study, when the clinical samples from which candida were isolated, 11 of 46 strains were obtained from blood, 7 from endotracheal aspirate and catheter, 6 from sputum and urine, 4 from wound, 3 from throat and 2 from feces. C. albicans was the most isolated candida species from almost all clinical specimens. Among the antifungals tested in our study, the most effective drug was found to be amphotericin B. Moderate (4 μg/ml) amphotericin B resistance was observed in only one C. glabrata strain (2%) out of a total of 46 strains studied. Both MIC50 and MIC90 values for amphotericin B were within the sensitive limits, respectively, as 0.019 μg/ml and 0.75 μg/ml. The second most effective drug among the antifungals whose efficacy was measured in our study was found to be flucytosine (93.5%). Among all strains, one C. krusei, one C. tropicalis and one C. glabarata strain were found to be resistant to flucytosine at MICs of 32 μg/ml, ≥32 μg/ml and ≥32 μg/ml, respectively. Both MIC50 and MIC90 values for flucytosine were within the sensitive limits, measuring 0.032 μg/ml and 4 μg/ml, respectively. In our study, the most effective antifungal from the azole group was voriconazole (82.7%); the least sensitive antifungal was found to be itraconazole (76.1%). Ketoconazole and fluconazole were observed as moderately active antifungals among azoles and their sensitivity was found to be 78.3%.
In the study conducted by Ozcelik B, et al. [27], 100% of 104 candida strains were found to be sensitive to amphotericin B and voriconazole, and 93% to flucytosine and fluconazole [27]. In another study by Kaya S, et al. [33], the antifungal susceptibility of 97 candida, of which 70% of the strains were hospital-acquired, was measured with a commercial kit (Candifast/Italy) and the sensitivity of these strains to amphotericin B 47 100%, flucytosine sensitivity 98.9%, ketoconazole sensitivity was %. 90 and fluconazole sensitivity has been reported to be 89% [33]. Yilmaz B, et al. [34], the efficacy of amphotericin B, fluconazole, and itraconazole in 100 candida strains was measured by the E test method, and their sensitivities were 98%, 58%, and 56%, respectively, in C. albicans strains; efficacy in non-albican strains was 96%, 44% and 64%, respectively. The measured MIC50 and MIC90 values for amphotericin B, fluconazole and itraconazole were 0.125-0.75, respectively; It was determined as 0.50->256 and 0.125->32 μg/ml [34].
A C. parapsilosis strain moderately susceptible to Amphotericin B and a resistant C. tropicalis of 47 strains evaluated by Fungitest were examined by the Etest method, and the minimum inhibitory concentration (MIC) was 1.5 μg/ml. Three C. albicans strains moderately susceptible to fluconazole (FLU) were evaluated with the E test method, and MIC values of two were found as 256 μg/ml and one as 32 μg/ml. The MIC value of one t strain resistant to FLU was found to be 256 μg/ml by the E test method. The MIC value of the resistant C. tropicalis strain was found to be 0.38 μg/ml [24]. Altın N et al. [31], while flucytosine was susceptible in 47 strains (95.9%) in candida isolated from intensive care patients, moderate in 2 strains of C. krusei, fluconazole was susceptible in 42 strains (85.7%), and resistant in 6 strains (3 of them C. krusei), 3 C.glabrata ), 1 C. glabrata strain was moderately susceptible, while voriconazole was sensitive in 48 (98%) strains, 1 C. glabrata strain was resistant. While amphotericin B was sensitive in 44 (90%) strains, 4 strains (2 C. krusei, 2 C. glabrata) were found to be moderately susceptible [31].
In our study, the antifungal susceptibility status of candida, mostly isolated from immunocompromised patients with invasive candidiasis, was investigated using the E test method; amphotericin B and flucytosine were found to be the most effective drugs. The efficacy of the azole antifungals included in the study was found to be quite low, especially against non-albicans species. Therefore, when starting empirical treatment in candida infections, at least making the species distinction of the pathogen will be a factor that can increase the success of the treatment. With the rapid spread of AIDS, the development of organ or tissue transplantation, more frequent invasive interventions and major surgeries, the increase in the number of elderly and debilitated patients with the prolongation of life expectancy, the more frequent use of chemotherapeutics that are toxic to the bone marrow due to malignant diseases, and the widespread use of intensive care units, life support units are required. As a result of the increase in the number of patients with diabetes mellitus, the patient population targeted by candida is increasing.
Conclusion
The importance of candida, which is at least as a cause of mortality and morbidity as much as bacterial pathogens, has increased. For the treatment of invasive and widespread candida infections, especially in risky individuals, it will be beneficial to start routine identification of species and antifungal susceptibility tests, and to establish antifungal use protocols that will minimize the development of resistance.
Ethics Committee Approval
Approval was obtained from the Ethics Committee of the Republic of Turkey, Fırat University (Decision No: 14/6 Date: 30.12.2004).
Acknowledgement
None.
Conflict of Interest
No conflicts of interest related to this article were declared by the authors.
References
- Bruun B, Westh H, Stenderup J (1995) Fungemia: An increasing problem in a Danish university hospital from 1989 to 1994. Clin Microbiol Infect 1(2): 124-129.
- Beck CM, Jarvis WR (1993) Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. National Nosocomial Infections Surveillance System. J Infect Dis 167(5): 1247-1251.
- Kao AS, Brandt ME, Pmitt WR, Conn LA, Perkins BA (1999) The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. J Clin Infect Dis 29(5): 1164-1170.
- Sumru C, Berrin O, Salih C, Ufuk A (2000) In vitro susceptibility of Candida species isolated from blood cultures to antifungal agents. Jpn J Infect Dis 58(1): 44-46.
- Ascı Toraman Z (2021) Staining methods and media used in mycology. In: Medical Mycology Book (Edn) Tumbay E, Meta Press, Izmir, Turkey, Pp. 609-656.
- Ozdarendeli A, Toroman ZA, Bulut Y (2002) Type classification of Candida species obtained from blood samples by PCR and DNA sequence anlysis. The 8th Congress of the European Confederation of Medical Mycology-ECMM, Budapest, Hungary, Pp. 46.
- Toroman ZA, Bulut Y, Ozdarendeli A, Unlu G, Seyrek A (2002) Detection of Candida albicans and other yeasts by PCR in the clinic samples. The 8thCongress of the European Confederation of Medical Mycology-ECMM, Budapest, Hungary, pp. 63.
- Bulut Y, Toroman ZA, Ozdarendeli A, Kizirgil A (2002) Molecular identification of Candida dubliniensis detected in clinical samples. The 8thCongress of the European Confederation of Medical Mycology ECMM, Budapest, Hungary. 10: 25-27.
- Toraman ZA, Bulut Y, Yilmaz M, Ozdarendeli A (2005) Molecular identification of Candida albicans and Candida dubliniensis strains isolated from clinical samples. Mikrobiyol Bul 39(2): 199-204.
- Edwards JE, Candida Species. In: Mandell GL, Bennett JE, Dolin R (eds) Principles and Practice of Infectious Diseases, (5th edn). Philadelphia, Churchill Livingstone, pp. 2656-2674.
- Stevens DA, Bennett JE. Antifungal agents (2000) In: Mandell GL, Bennett JE, Dolin R.(eds) Principles and Practice of infectious Diseases, 5th edn. Philadeiphia, Churchill Livingstone, pp. 448-459.
- Tumbay E, İnci R (1996) Antifungal drugs, Infectious Diseases. Topcu AW, Soyletir G, Doganay M (eds). Ankara, Nobel Medicine Bookstore, pp. 195-202.
- Yıldıran ST (2001) Newly developed azoles. National Fungal Diseases and Clinical Mycology Congress Ankara, Congress book, pp. 141-148.
- Ener B (1996) Studies on new antifungals. XXVII. Turkish Microbiology Congress Antalya, Congress book, p. 123-125.
- White TC, Marr KA, Bowden RA (1998) Clinical, cellular and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11(2): 382-402.
- National Committee for Clinical Laboratory Standards (1997) Reference method for broth dilution susceptibility testing of yeasts: Approved standard. NCCLS Document M27-A, Villanova, Pensylvania.
- Wey SB, Motomi M, Pfaller MA, Woolson RF, Wenzel RP (1998) Hospital_acquirecandidemia. The attributable mortality and excess length of stay. Arch Intern M 148(12): 2642-2645.
- Tsekoura M, Ioannidou M, Pana ZD, Anna-Bettina Haidich, Charalampos Antachopoulos, et al. (2019) Efficacy and safety of echinocandins for the treatment of invasive candidiasis in children: a meta-analysis. Pediatr Infect Dis J 38(1): 42-49.
- Etiz P, Kibar F, Ekenoglu Y, Yaman A (2015) Retrospective evaluation of the distribution and antifungal susceptibility of Candida species isolated from blood cultures. Ankem Journal. 29(3): 105-113.
- Wey SB, Motomi M, Pfaller MA, Woolson RF, Wenzel RP (1998) Hospital_acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med 148(12): 2642-2645.
- Leleu G, Aegerter P, Guidet B (2002) Systemic candidiasis in intensive care units: a multicenter matched-cohort study. J Critical Care 17(3): 168-175.
- Jarvis WR (1995) Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 20(6): 1526-1530.
- Eggimann P, Garbino J, Pittet D (2003) Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 3(11): 685-702.
- Selim G, Melek B, Suleyman SK (2021) Distribution and antifungal susceptibility of Candida species detected in blood cultures. J Pak Med Assoc 71(6): 1601-1604.
- Yesil E, Celebi S, Sezgin Evim M, Arife Ozer, Cansu Turan, et al. (2020) Evaluation of Micafungin use in children. Find Microbiome 54(1): 120-134.
- Gultekin B, Aydın N Identification of Candida species by different methods. XXX. Turkish Microbiology Congress. Congress Summary Book. Pp. 316.
- Ozcelik B, Cesur S, Sipahi B, Sultan N, Abbasoglu U. In vitro activity of some antifungals in Candida species. XXXI. Turkish Microbiology Congress. Congress Summary Book. Pp. 327.
- Tumturk A Species distribution and development of resistance to antifungal drugs in candidemia in intensive care patients: A single center experience.
- Togay A, Banu Bayraktar B, Sevgi D Y (2015) Bulut E Determination of Candida Species Isolated from Inpatients and their Antifungal Susceptibility. Sisli Etfal Hospital Medical Bulletin 49(4).
- Beder D, Tasbent FE,Dogan M (2020) Distribution and Antifungal Susceptibility of Candida Isolates Detected in Blood Cultures. Ankem Journal 34(3): 77-85
- Altın N, Cesur S, Toros GY, Koldas K, Solgun G, Sencan I (2018) Distribution and antifungal susceptibility of candida species isolated from clinical samples of patients hospitalized in the intensive care unit. Middle East Medical Journal 10(2): 130-134.
- Mudderris T, Kaya S, Ormen B, Gokmen A, Akpinar CV, Gul SY (2020) Mortality and risk factor analysis for Candida bloodstream infection: A three-year retrospective study Mycol Med 30(3):101008.
- Kaya S, Avunduk H, Ozyagcı G, Bakızı ZM. Distribution of candida genus yeasts isolated from various clinical specimens and determination of their susceptibility to antifungals. XXXI. Turkish Microbiology Congress. Congress Summary Book. Pp. 328.
- Yılmaz B, Batırel A, Gencer S, Ozer S Typing of candida strains isolated from various clinical specimens and determination of susceptibility to amphotericin B, fluconazole and itraconazole by Etest. XXX. Turkish Microbiology Congress. Congress Summary Book. Pp. 313.
-
Feray Ferda Senol*, Mehmet Ozcan, Zulal ASCI Toraman, Yasemin Bulut and Yusuf Yakupogullari. Typing of Candida Strains Straightened from Immunesupressed Patients and Investigation of Their Sensitivity to Antifungal Drugs by E-Test Method. Sci J Biol & Life Sci. 2(3): 2022. SJBLS.MS.ID.000538. DOI: 10.33552/SJBLS.2022.02.000538
-
Educational centres, Education dogs, Prosocial behaviour, Emotional dimension, Cognitive capacities, Final survey, Classroom environment
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






