 Research Article
Research Article
The Role of Polyploidy and Soil Nutrient Levels on Aphid-Predators Interaction
Peter A.Y. Ampim1*, Eric Obeng1, Ernesto Olvera Gonzalez2, Aruna Weerasooriya1, Godson O. Osuji1 and Deland J. Myers Sr.1
1College of Agriculture and Human Sciences, Prairie View A&M University, USA
2Technological Institute of Pavilion of Arteaga, Aguascalientes, Mexico
Peter A.Y. Ampim, College of Agriculture and Human Sciences, Prairie View A&M University, Texas, USA.
Received Date: September 02, 2020; Published Date: October 30, 2020
Abstract
Understanding the consequences of intra specific genetic variation within same plant communities represents a critical research direction to explore. Polyploidy plants create intra specific interactions within same species which leads to complex associations within communities of organism. We investigated the effect of polyploidy (cytotypes) and soil nutrient levels on U. nigrotuberculatum–predator interaction. We used S. altissimato investigate the interactions between U. nigrotuberculatum, ant and Hippodamia parenthesis. Plants were grown individually in 4L pots in a Metro Mix 360 soil medium combined with 50% sand. There were 32 plants in the garden; 16 diploid and 16-hexaploid plants. Eight of the plants from each cytotype received a N.P.K (18-6-12) nutrient addition (Osmocote 4g) and eight received no nutrients (control). We found that plant size play an important role in aphid choice. Plant size (biomass) is greatly influence by soil nutrient levels. Ants are in a mutual association with U. nigrotuberculatum. The presence of large population of ants prevented or reduced the activities of lady beetles.
Keywords: Predator; Aphid; Polyploidy; Plant Biomass; Soil nutrients
Introduction
Herbivores are faced with complex interactions such as interactions with host-plant, predators and the environment. Polyploidy plants create intra specific interactions within same species of plant, and thereby influence the composition and diversity of insects [1]. There are two general ways in which polyploidy occur: multiplication of one chromosome set and merger of structurally different chromosome set [2]. Polyploidy can also occur through polyploidization, and such, results in genomic change and production of new complex gene [3]. When genome reorganized polyploidy plant is formed [2]. There are two major types of polyploidy: Autopolyploid and Allopolyploid (Avraham et al., 2004). Environmental condition, herbivore, water deficit and poor nutrients enhance the rate of autoploid formation [3].
Insect herbivores have been reported to move amongst plants as a result of genetic variation [4]. Polyploidy cause an array of phenotypic changes (increase in flower size, leaf and stem) in plants and influence interactions with other species [5], and alters interactions, rapid genetic and changes in flowering plants [6]. Soil nutrients have been documented to have direct effect on plants biomass or indirectly influence plant secondary chemistry [7-9]. Nutrients such as Nitrogen, potassium and phosphorus are essential for plant growths and development [10]. Soil nutrient levels can either positively or negatively influence insect abundance.
Prior to this study, it was largely unknown the possible impact of polyploidy (cytotype) and soil nutrient levels may play on aphidpredators interactions. This study attempts to answer a critical ecological question: how does polyploidy and soil nutrient levels affect the complex interactions between aphid and predators? To provide answers to this question, this study had three main objectives: (1) Polyploidy effects on biomass, (2) Soil nutrients effects on biomass and (3) Insects abundance differences among cytotypes and soil treatment. Our hypotheses were: (1) Cytotypes of S. altissima with more chromosome number would have bigger biomass such that 6n>2n. (2) Soil nutrients level would be a major deciding factor in aphids choices (plants with high nutrients would have more insect abundance), and predators would be more on plants with more aphids.
Materials And Methods
Study Species
Solidago altissima L., commonly known as tall goldenrod, is a rhizomatous perennial plant species found in North America [11]. Its habitats include old field, forest openings roadsides, and other disturbed or succession areas. There are three well known cytotypes S. altissima; diploid (2n=18), tetraploid (4n=36), and hexaploid (6n=54) [1]. Diploid is mostly found in the western part of its range [11], hexaploid mostly found in the eastern part, while tetraploids are also found in the Midwest. S. altissima serves as a perfect midget to investigate the effects of cytotype (polyploidy) on aphid-predator associations due to previous investigations showing the importance of intra specific genetic variation on associated insects [12,13]. The two aphids we investigate were Uroleucon Nigrotuberculatum U. nigrotuberculatumbelong to order Hemisphere; Aphides). U. nigrotuberculatum is native to North American [14]. S.altissimaserve as primary host (most especially for U. nigrotuberculatum), and their population are regulated by different factors, such as temperature, predators and fungal disease [15,16]. The predator we observed was lady beetle. Lady beetles (Hippomania parenthesis), ladybugs, or ladybird beetles one of the most visible and best known beneficial predatory insects. Some are native to North America, and are beneficial as both adults and larvae, feeding primarily on aphids. We also documented ant-aphid interaction. Ants and aphids have a symbiotic relationship. Both species of the insects benefit from each other. The ants provide protection and prevent any predators from feeding on aphids, while the aphid, in return, provide food (honeydew) for the ants.
Experimental Design
Diploid and hexaploid (2n and 6n) were used for this study. Plants were grown individually in 4L pots in a Metro Mix 360 soil medium combined with 50% sand. There were 32 plants in the garden; 16 diploid and 16-hexaploid plants. Eight of the plants from each cytotype received a N.P.K (18-6-12) nutrient addition (Osmocote™- 4g) and eight received no nutrients (control). The distance between each plant was 45cm. The initial height and diameter of each plant was measured following Williams and Megan, 2015 protocol.
Insect Abundance
Quantification of U. nigrotuberculatum, ants and lady beetles abundance started on May 13, 2020, and ended on July 28, 2020. We visually observed and monitor three times a week (Monday, Wednesday and Friday) in order to accurately estimate the time of peak abundance [17]. We estimated the peak abundance of the insects, and take the final biomass measurement [18]. We measured the above-ground biomass by measuring plant stem diameter (D) and height (H) according to this formula: Biomass (g) =D2H*0.0022) +6.3668 (R=0.70, P<0.0001).Using the nondestructive estimate of plant biomass, aphid’s abundance, ant and lady beetles could be expressed as the actual number or aphids/g, ant/g, and lady beetle/g to account for treatment effects on plant biomass.
Results
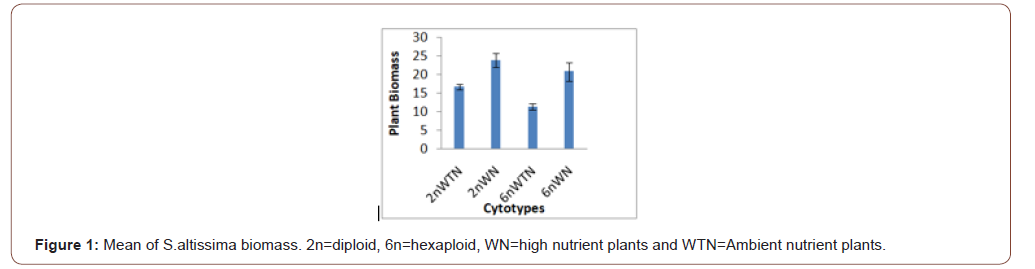
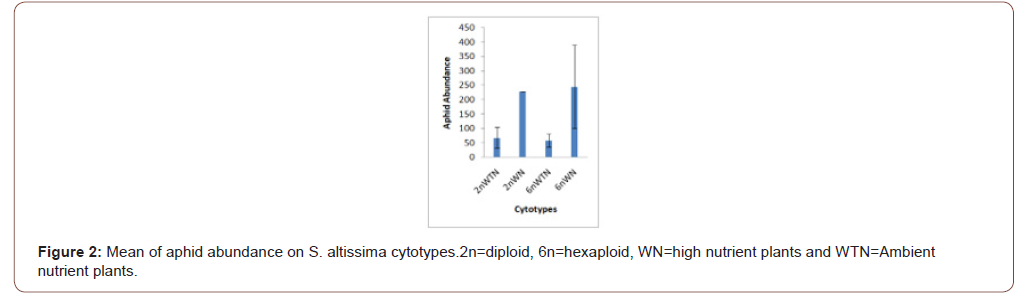
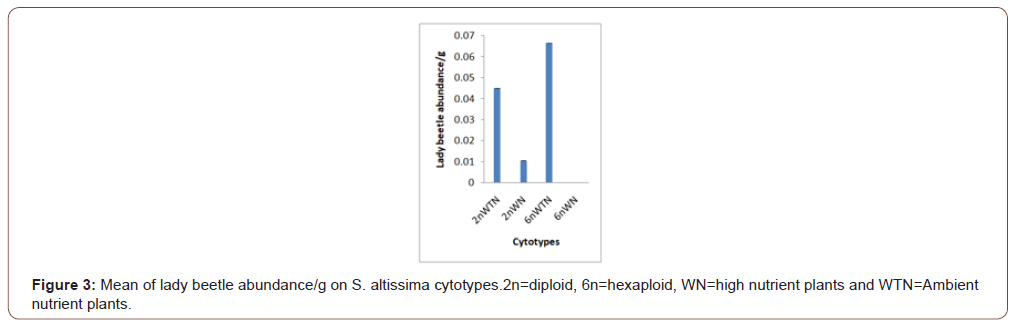
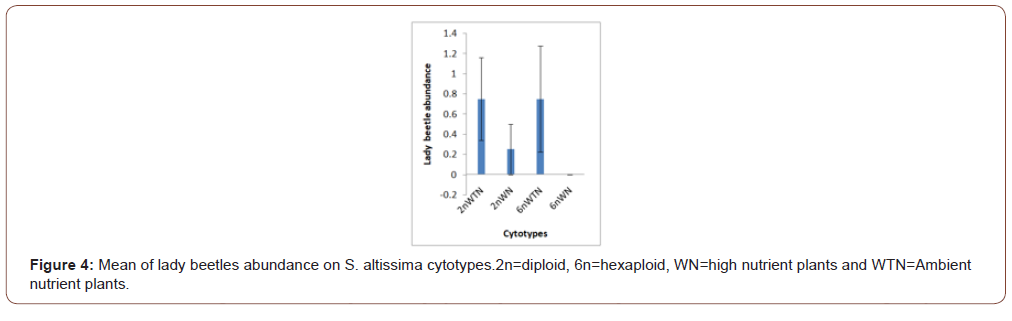
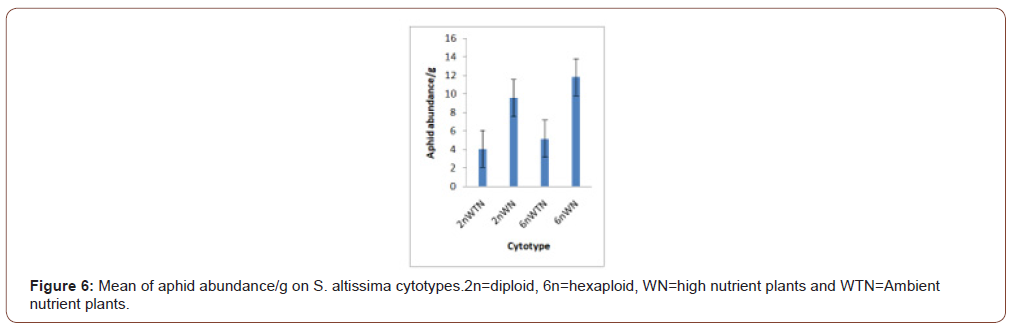
Soil nutrients had a significant effect plant biomass (Figure 1) and aphid abundance (Figure 2) Plants with nutrient addition had more aphids irrespective of the cytolyses. Aphid population was much higher in both 2n and 6n plants with nutrients addition compared to those with no nutrient addition. Aphid abundance/g shows no significant difference (Figure 3). Predator abundance (ladybeetle) show no significant difference in Figure 4, though predator was absent on 6Nwn plants (Figure 4). Figure 5 Show that ant associated with all the cytotypes that had aphids. The association was more on 6nwn plants (Figure 5). (Figures 6&7) shows a significant difference in ant associations with each plant.
Discussion
This study is the first to investigate the role of Polyploidy and soil nutrient levels on Aphid-Predator interaction. Many studies within the field of community genetics have focused more on the impacts of soil nutrients on insect abundance [19], no study has investigated the impact of soil nutrients and cytolyses of S. altissimo on aphid, ants and predator association. We Quantified U. Nigrotuberculatum abundance, ants abundance and lady beetle activities during the peak abundance of U. Nigrotuberculatum, by observing and monitoring the population three times a week. U. Nigrotuberculatum populations are known to vary across a growing season [17] and we took our mean of abundance during this peak of abundance that provided the best quantification for these associations. This study found with plant biomass considered, aphid abundance was significantly affected by soil nutrients. Plants with nutrients addition had higher aphid population compared to those with no nutrient addition irrespective of the cytotypes (Figures 1&2). This study is in alignment with studies. NPK (nitrogen, phosphorus and potassium) increases plant biomass and alter herbivore composition. Our study documented that aphids prefer to feed on plants with larger biomass. Our investigation found that ant’s associated wherever aphid were found. However, the ant-aphid association was more on plants with nutrient addiction (Figure 3); diploid plants and hexaploid plants with nutrient addition had more ants). The abundance of ants on 6n plants with nutrient addition (Figure 4) possibly prevented lady beetle from feeding on the aphid. This study found no significant association between polyploidy (cytotype) and insects interactions. We did not consider the genotypes of the S. altissimo used for this study. [13] Investigation found that plant genotype influence aphid abundance. Our study may be a foundational study for future directions to answering some complex interactions between plant and insects [20,21].
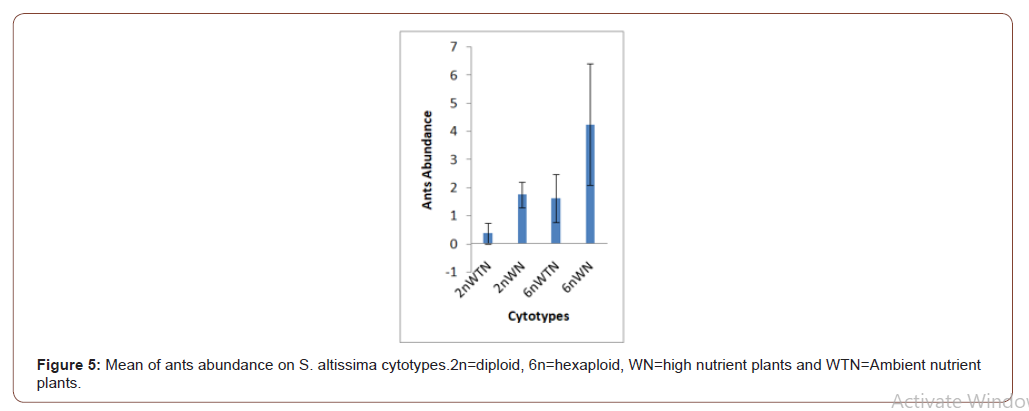
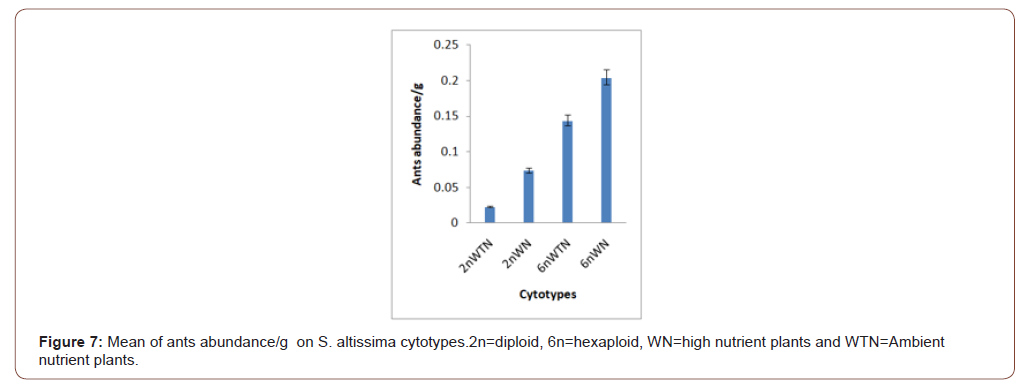
by soil nutrient levels. Ants are in a mutual association with aphids. The presence of large population of ants prevented or reduced the activities of lady beetles.
Acknowledgement
Acknowledgement
Conflicts of Interest
Author declared no conflict of interests.
References
- Halverson K, Stireman JO, Nason JD (2008) Origins, distribution, and local co-occurrence of polyploidy cytotypes in Solidago altissima (Asteraceae). Am J Bot 95(1): 50-58.
- Soltis PS, Soltis DE, Marchant B, De peer YV (2015) polyploidy and genome evolution in Plants. Current opinion in Genetics and Development 35: 119-125.
- Douglas GM, Gos G, Steige KA, Tanja Slotte, Stephen I Wright et al. (2015) Hybrid origins and the earliest stages of diploidization in the highly successful recent polyploid Capsella bursa-pastoris. Proc Natl Acad Sci USA 112(9): 2806-2811.
- War AR, Taggar GK, Hussain B, Taggar MS, Nair RM, Sharma H (2018) Plant defense against herbivory and insect adaptations. AOB Plants 10(4): ply037.
- Segraves KA, Anneberg TJ (2016) Species interactions and plant polyploidy. Am J Bot 103(7): 1326-1335.
- Song Q, Chen ZF (2015) Epigenetic and developmental regulation in plant polyploids. Curr Opin Plant Biol 24: 101-109.
- Bethke JA, Redak RA, Schuch UK (1998) Melon aphid performance on chrysanthemum as mediated by cultivar, and differential levels of fertilization and irrigation. Entomologia Experimentaliset Applicata 88: 41-47.
- Casey CA, Raupp MJ (1999) Supplemental nitrogen fertilization of containerized azalea does not affect performance of azalea lace bug (Heteroptera: Tingidae). Environmental Entomology 28: 998-1003.
- Throop HL, Lerdau T (2004) Effects of nitrogen deposition on insect herbivory: implications for community and ecosystem processes. Ecosystems 7: 109-133.
- Johnson CD, Decoteau DR (1996) Nitrogen and potassium fertility affects jalapeno pepper plant growth, pod yield, and pungency. Horticultural Science 31(7): 1119-1123.
- Semple JC, Cook RE (2006) Solidago In: Flora North America editorial committee. Canadian Journal of Botany 84(8): 1282-1297.
- Crutsinger MG, Collins MD, James AF, Zachariah G, Chris C Nice, Nathan J Sanders (2006) Plant genotypic diversity predicts community structure. Science 313(5789): 966-968.
- Genung MA, Crutsinger GC, Bailey JK, Schweitzer J, Sanders NJ (2012) Aphid and ladybird beetle abundance depend on the interaction of spatial effects and genotypic diversity. Oecologia 168(1): 167-174.
- Adachi S, Shirahama S, Tokuda M (2016) Seasonal occurrence of Uroleucon nigrotuberculatum (Hemiptera: Aphididae) in Northern Kyushu and mechanisms of its summer disappearance. Environ Entomol 45(1): 16-23.
- Cappuccino N (1987) Comparative population dynamics of two goldenrod aphids: Spatial patterns and temporal constancy. Ecology 68(6): 1634-1646.
- Alyokhin A, Drummond FA, Sewell G, Storch RH (2011) Differential effects of weather and natural enemies on coexisting aphid populations. Environ Entomol 40(3): 570-580.
- Root RB, Cappuccino N (1992) Patterns in population change and the organization of the insect community associated with goldenrod. Ecological Society of America 62(3): 393-420.
- Williams RS, Megan A (2015) Colonization of Solidago altissima by the specialist aphid Uroleuconnigrotuberculatum: Effects of genetic identity and leaf chemistry. J Chem Ecol 41(2): 129-138.
- Segraves KA (2017) The effects of genome duplications in a community context. New Phytol 215(1): 57-69.
- Pilson D, Rausher M (1995) Clumped distribution patterns in goldenrod aphids: Genetic and ecological mechanisms. Ecological Entomology 20:75-83.
- Wimp GM, Martinsen GD, Floate KD, Bangert RK, Whitham GT (2005) Plant genetic determinant of arthropod community structure and diversity. Evolution 59(1): 61-69.
-
Beck Arebamen Akhiwu, Isaac Gadzekpo. The Role of Polyploidy and Soil Nutrient Levels on Aphid-Predators Interaction. Sci J Biol & Life Sci. 1(3): 2020. SJBLS.MS.ID.000515.
-
Hippodamia, Consequences, Predator, Aphid, Polyploidy, Plant Biomass, Soil nutrients, polyploidization, Herbivore, Negatively, Aphid-predators, Nigrotuberculatum, Alignment, Hexaploid, Herbivores
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






