 Research Article
Research Article
The Response of Egyptian Spinach and Vegetable Amaranth Microgreens to Different Light Regimes
Peter A.Y. Ampim1*, Eric Obeng1, Ernesto Olvera Gonzalez2, Aruna Weerasooriya1, Godson O. Osuji1 and Deland J. Myers Sr.1
1College of Agriculture and Human Sciences, Prairie View A&M University, USA
2Technological Institute of Pavilion of Arteaga, Aguascalientes, Mexico
Peter A.Y. Ampim, College of Agriculture and Human Sciences, Prairie View A&M University, Texas, USA.
Received Date: February 11, 2020; Published Date: March 24, 2021
Abstract
Production of specialty microgreens under controlled environments can help increase their supply in Texas. This experiment was carried out to evaluate the effects of light regimes on the growth of Egyptian spinach and vegetable amaranth microgreens in a grow tent. Seeds were planted in 10.2 × 10.2 cm trays containing promix potting soil. Egyptian spinach and vegetable amaranth received 6 light treatments which includes red, yellow, green, blue, white, and natural light. Light properties of all the light treatments were 1.66 mol m-2 d-1 for daily light integral (DLI), 40 μmol m-2 d-1 for photosynthetic photon flux density (PPFD) and 11.5 hours for photoperiod. Each tray received 40 ml of water at 48 hours intervals. Environmental factors such as CO2, temperature, and relative humidity were adjusted to simulate greenhouse conditions. This study was set up in a completely randomized design with three replications. Microgreens were harvested at three weeks after planting when the plants reached their first true leaf stage. The data collected including plant height, SPAD reading, and microgreens yield was analyzed using the JMP software at P< 0.05. Plant height of Egyptian spinach microgreens increased under blue and natural light. However, plant height for vegetable amaranth microgreens increased for all the light treatments except for yellow light. Increases in SPAD readings were observed for Egyptian spinach microgreens growing under yellow, green, blue, white light, and natural light. Vegetable amaranth did not show any differences in SPAD reading for the light treatments. A decrease in yield was observed for vegetable amaranth microgreens when it grew under blue light, but it increased under natural and green light. Light treatment did not affect yield of Egyptian spinach microgreens.
Keywords: Microgreens; LED lights; Specialty vegetables; Controlled environment production; Grow tent
Introduction
Increased interest in better nutrition and healthy living coupled with the need to increase food production to meet the dietary needs of the world’s growing population has result in enhanced interest in nutrient rich functional foods like microgreens. Microgreens are tender immature greens usually harvested at 7-14 days after germination [1]. Microgreens contain high levels of vitamins, minerals and antioxidants in comparison to mature leaves [2,3]. Their rich nutritional contents coupled with the health benefits associated with their consumption is making microgreens very popular [4,5]. Consequently, they are increasingly becoming components of popular recipes in many upscale markets and restaurants [6,7]. Microgreens are often grown indoors under controlled environmental conditions with the help of artificial lights such as light emitting diodes (LEDs). The use of LEDs can be advantageous because it allows for adjustment of light intensity and wavelength to meet requirements for different plant species [8-11]. In addition, studies have shown that LED light can improve the shelf life and nutritional quality of horticultural produce at decreased cost [10]. The flexibility in LED settings has permitted researchers to investigate and document the effects of light quality on the concentration of phytonutrients and yield of horticultural produce under controlled environments [11-15]. One of such studies [16] showed that though artificial light had a similar effect on photosynthesis of single leaves, species or cultivar morphology caused differences in light response and plant yield was affected by both photosynthetic and morphological responses [16]. Artificial light regimes can modify photosynthetic processes in plants since they do not provide the same light intensity or photoperiod as sunlight [11]. Studies suggest that red light is more effective in improving photosynthesis compared to blue or green light [16,17]. For example, leaf area of grape (Vitis vinifera cv. ‘Jingxiu’) as well as the dry mass distribution ratio increased when they were provided with supplementary red light in a greenhouse [18]. In another study, photosynthetic rate of green and red perilla (Perilla frutescens) a popular herb in East Asia increased with red light for both varieties [19]. Similarly, red perilla produced more and bigger leaves, and greater dry weight when grown under redenriched light treatments (red light, red and blue light mix, and red and green light mix) than under blue light, blue and green light mix, or green light [20]. Though the effects of LED lighting on microgreens has been investigated for several vegetables especially the Brassicaceae [5], much work has not been done on their effect on specialty vegetables like amaranth (Amaranthus spp.) and Egyptian spinach (Corchorus olitorius L.). These vegetables are very nutritious and have good niche markets in the large metropolitan areas of Texas including Houston, Dallas-Fort Worth, Austin and San Antonio because of the diverse nature of their residents and increasing population of minorities [21,22]. Therefore, exploring controlled environment cultivation of these vegetables can add to their production options and provide consumers with more buying choices. This study evaluated effects of red, yellow, green, blue, white, and natural light regimes on the growth of Egyptian spinach and vegetable amaranth microgreens in a controlled environment.
Materials and Methods
Growth chamber set up and light treatment
This study was conducted in a Gorilla grow tent at the Research Farm of Prairie View A&M University located in Prairie View, TX. The grow tent was compartmentalized using a storage rack and an opaque material. Each compartment was equipped with multispectral LED-based lighting system (Philips Hue White and Color Ambiance A19 Smart LED Bulb – Multicolor, 60 W, Somerset, NJ, USA). The lighting system for each compartment comprised of 18 selected bulbs attached to a wooden board placed 40 cm above the microgreens. The LED lights were adjusted to the following specifications: photoperiod, 11.5 hours; daily light integral (DLI), 1.66 mol m-2d-1; and photosynthetic photon flux density (PPFD), 40 μmol m-2s-1. The light treatments namely red, yellow, green, blue, white, and natural light (i.e. light under greenhouse conditions; control) were randomly assigned to the compartments in a completely randomized design. The LED bulbs were configured according to light treatment and each treatment was replicated three times.
Microgreens production and environment
Egyptian spinach and vegetable amaranth (var. Red Leaf) seeds were broadcast at 2.5 g and 1 g respectively in 10.2 × 10.2 cm trays containing promix potting soil. The production of microgreens for each vegetable was considered a separate experiment and had 3 replications per light treatment. Each tray received 40 ml of water at two-day intervals. Carbon dioxide, temperature, and relative humidity in the grow tent were adjusted to simulate greenhouse growing conditions. Average carbon dioxide, temperature, and relative humidity in the growth tent were 529 ppm, 22.2oC, and 61.9% respectively, whereas average carbon dioxide, temperature, and relative humidity in the greenhouse were 524 ppm, 24.5oC, and 69.0% respectively.
Microgreens harvesting, data collection and analysis
The Egyptian spinach and vegetable amaranth microgreens were harvested 3 weeks after planting at the first true leaf stage by cutting the microgreens from the potting soil level, and weighed for yield determination. Prior to harvesting, plant height was recorded using a meter stick by measuring from the potting soil level to the tip of the top-most leaf. SPAD meter reading was taken from three randomly selected leaves from each treatment using a Minolta SPAD 502 Plus chlorophyll meter (Spectrum Technologies Inc., Aurora, IL, USA). All the data collected including plant height, SPAD reading, and microgreens yield, were subjected to analysis of variance using the JMP software and conclusions drawn at P< 0.05. Plant height, SPAD reading and yield data for each vegetable were also correlated using the same software. Significance of the correlation was also determined at the 5% significance level.
Results
Plant height response to light treatment
Plant height for Egyptian spinach microgreens ranged from 3.3 (red light) to 5.6 cm (natural light) (Figure 1). Egyptian spinach plant height was greater for microgreens grown under natural light and was 18%, 24%, 29%, and 41% higher than white, green, yellow and red light respectively (Figure 1). However, plant height for Egyptian spinach microgreens was not different between blue and natural light. Height of plants growing under red light was 23% and 28% shorter than plants growing under green and white light respectively, but it was not different from yellow light (Figure 1). Plant height for vegetable amaranth microgreens ranged from 3.3 (yellow light) to 4.16 cm (natural light) (Figure 2). Vegetable amaranth microgreens growing under natural light was 20% taller than yellow light (Figure 2). Nonetheless, there was no difference in plant height between red, yellow, green, blue and white light (Figure 2).
SPAD reading response to light treatment
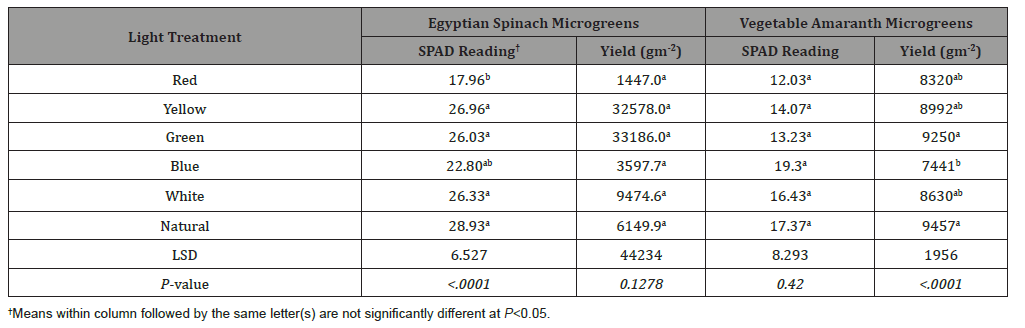
SPAD readings for Egyptian spinach microgreens ranged from 17.9 (red light) to 28.9 (natural light) (Table 1). SPAD reading for Egyptian spinach microgreens for red light was 31%, 32%, 33% and, 38% less than green, white, yellow, and natural light respectively (Table 1). However, there was no difference in SPAD reading for Egyptian spinach produced under red and blue light (Table 1). SPAD readings from vegetable amaranth microgreens ranged from 12.0 (red light) to 19.3 (blue light) but did not show any difference among the light treatments (Table 1).
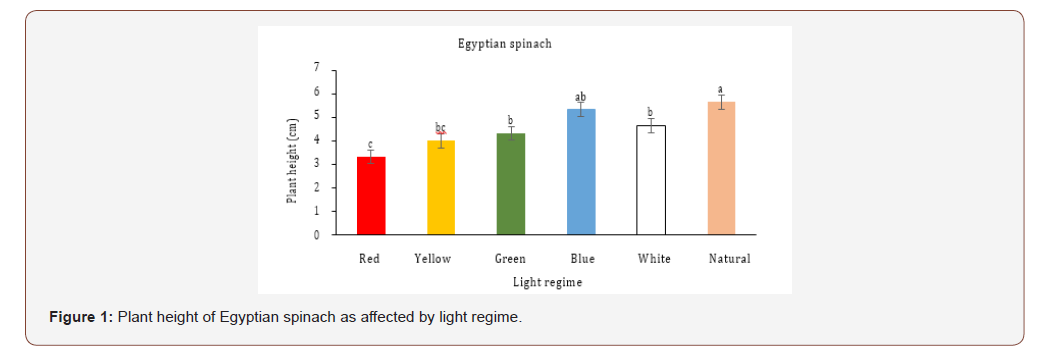
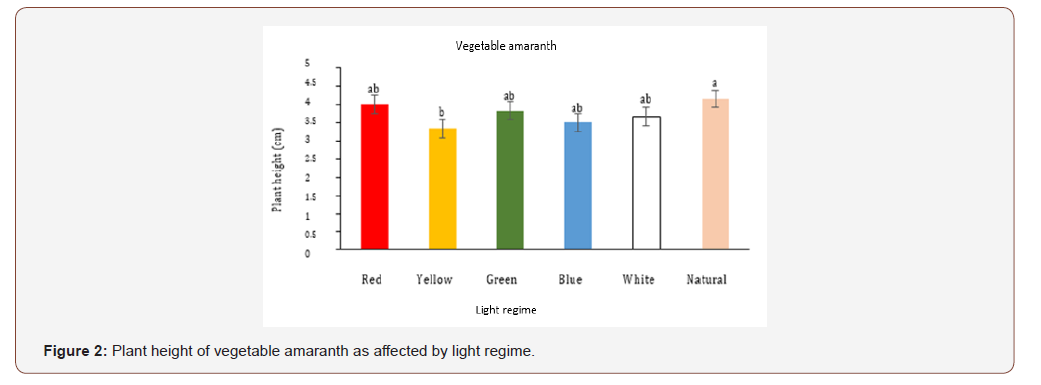
Table 1: Effect of light treatment on SPAD reading and yield of vegetable amaranth and Egyptian spinach microgreens..
Yield response of microgreens to light treatment
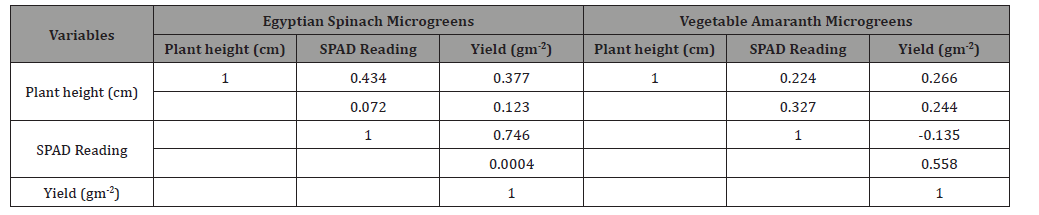
Yield of Egyptian spinach microgreens ranged from 1447 (red light) to 9474 gm-2 (white light) but did not show any differences among the light treatments (Table 1). Vegetable amaranth microgreens yield ranged from 7441 (red light) to 9250 gm-2 (green light) (Table 1). Yield of vegetable amaranth microgreens growing under blue light was 19% and 21% less than green and natural light respectively (Table 1). Notwithstanding there was no difference in yield between blue, red, yellow, and white light for vegetable amaranth microgreens (Table 1). As presented in Table 2 only the SPAD reading for Egyptian spinach had a significant correlation with microgreens yield (r2=0.76; p= 0.0004).
Discussion
Light is essential for photosynthesis, but light provided by the LEDs can modify the process of photosynthesis as artificial light does not provide the same light spectrum, light intensity or photoperiod like natural light [11]. Our results show that SPAD reading increased for Egyptian spinach microgreens when it was grown under yellow, green, blue, white, and natural light, however SPAD reading reduced under red light. Vegetable amaranth microgreens on the other hand did not show differences in SPAD reading in response to light treatment. Studies [23-25] found that blue light increased the rate of chlorophyll a/b and promoted stomatal opening in plants. SPAD reading is a proxy for measuring chlorophyll content in leaves, hence yellow, green, blue, white, and natural light may have increased the rate of chlorophyll a/b and promoted stomatal opening of Egyptian spinach microgreens. SPAD reading is positively correlated to leaf chlorophyll concentration [26, 27], leaf nitrogen [26, 27], photosynthesis [26, 28], and crop yield [29-31]. Yield for vegetable amaranth microgreens increased when it was grown under green and natural light, notwithstanding yield decreased under blue light. This is similar to reported findings that biomas production in red leaf lettuce increases under exposure to green light [32]. In contrast, most plant species increase yield under combined wavelengths with large proportion of red light supplemented with blue light [10]. The SPAD reading for Egyptian spinach microgreens had a significant positive correlation with yield (Table 2). This indicates that red, yellow, green, blue, white and natural lights increased SPAD reading with a corresponding increase in Egyptian spinach microgreens yield.
Table 2: Correlation between plant height, SPAD reading and yield for Egyptian spinach and vegetable amaranth microgreens..
Conclusions
An increase in plant height was observed when Egyptian spinach microgreens were grown under blue and natural light. However, the height of vegetable amaranth microgreens was statistically the same for all the light regimes except for yellow light. SPAD readings for Egyptian spinach microgreens grown under yellow, green, blue, and white light were comparable to natural light. However, SPAD readings for all the light regimes were similar to natural light for vegetable amaranth microgreens. Microgreens yield for vegetable amaranth was enhanced when grown under red, yellow, green, white, and natural light. Conversely, the light treatments did not produce significant differences in the yield of Egyptian spinach microgreens. Future research will include nutritional content evaluation of Egyptian spinach and vegetable amaranth microgreens grown under these light regimes.
Acknowledgment
This study was supported by a USDA-NIFA 1890 Capacity Building Grant Award No. 2017-38821-26420 and funds from the Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico. We also acknowledge the assistance provided by our student trainees.
Conflicts of Interest
No Conflict of Interest.
References
- Xiao Z, Lester GE, Luo Y, Wang Q (2012) Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J Agr Food Chem 60(31): 7644-7651.
- Lester GE, Hallman GJ, Pérez JA (2010) γ-Irradiation dose: effects on baby-leaf spinach ascorbic acid, carotenoids, folate, α-tocopherol, and phylloquinone concentrations. J Agr Food Chem 58(8): 4901-4906.
- Burlingame B (2014) Grand challenges in nutrition and environmental sustainability. Front Nutr 1: 3.
- Janovská D, Stocková L, Stehno Z (2010) Evaluation of buckwheat sprouts as microgreens. Acta Agric Slov 95(2): 157.
- Lobiuc A, Vasilache V, Oroian M, Stoleru T, Burducea M, et al. (2017) Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens Molecules 22(12): 2111.
- Gerovac JR, Craver JK, Boldt JK, Lopez RG (2016) Light intensity and quality from sole-source light emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. Hort Science 51(5): 497-503.
- Kamal KY, El Tantawy AA, Moneim DA, Salam AA, Qabil N, et al. (2019) Evaluation of 21 Brassica microgreens growth and nutritional profile grown under diffrenet red, blue and green LEDs combination. bioRxiv 705806.
- Brazaitytė A, Samuolienė G, Jankauskienė J, Sakalauskienė S, Sirtautas R, et al. (2016) Light quality: growth and nutritional value of microgreens under indoor and greenhouse conditions. In VIII International Symposium on Light in Horticulture 1134 pp. 277-284.
- Bugbee B (2016) Toward an optimal spectral quality for plant growth and development: the importance of radiation capture. In VIII International Symposium on Light in Horticulture 1134 p. 1-12.
- Amaki W, Yamazaki N, Ichimura M, Watanabe H (2011) Effects of Light Quality on the Growth and Essential Oil Content in Sweet Basil. Acta Hortic 907: 91-94.
- Dou H, Niu G, Gu M, Masabni JG (2017) Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 3(2): 36.
- D Souza C, Yuk HG, Khoo GH, Zhou W (2015) Application of light‐emitting diodes in food production, postharvest preservation, and microbiological food safety. Compr Rev Food Sci F 14(6): 719-740.
- Muneer S, Kim EJ, Park JS, Lee JH (2014) Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int J Mol 15(3): 4657-4670.
- Han T, Vaganov V, Cao S, Li Q, Ling L, et al. (2017) Improving color rendering of LED lighting for the growth of lettuce. Sci Rep 7: 45944.
- Ruangrak E, Khummueng W (2019) Effects of artificial light sources on accumulation of phytochemical contents in hydroponic lettuce. J Hortic Sci Biotech 94(3): 378-388.
- McCree KJ (1971) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural Meteorology 9: 191-216.
- Sager JC, Smith WO, Edwards JL, Cyr KL (1988) Photosynthetic efficiency and phytochrome photo-equilibria determination using spectral data. Transactions of the ASAE 31(6): 1882-1889.
- Kong Y, Wang S, Shen H, Ma C, Yao Y (2006) Effects of supplemental lighting with different light quality on the shoot growth of grape growing in greenhouse. J Beijing Agric Coll p. 23-25.
- Goto E, Matsumoto H, Ishigami Y, Hikosaka S, Fujiwara K, et al. (2013) Measurements of the photosynthetic rates in vegetables under various qualities of light from light-emitting diodes. In International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant 1037 pp. 261-268.
- Nishimura T, Ohyama K, Goto E, Inagaki N (2009) Concentrations of perillaldehyde, limonene, and anthocyanin of Perilla plants as affected by light quality under controlled environments. Sci Hortic 122(1): 134-137.
- Emerson MO, Bratter J, Howell J, Jeanty W (2012) Houston Region Grows More Ethnically Diverse, With Small Declines in Segregation. A Joint Report Analyzing Census Data from 1990, 2000 and 2010.
- Frey WH (2011) Melting pot suburbs. Brookings Institution, Washington, DC, USA.
- Senger H (1982) The effect of blue light on plants and microorganisms. Photochem Photobiol 35(6): 911-920.
- Sæbø A, Krekling T, Appelgren M (1995) Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tiss Org 41(2): 177-185.
- Baroli I, Price GD, Badger MR, Von Caemmerer S (2008) The contribution of photosynthesis to the red-light response of stomatal conductance. Plant Physiol 146(2): 737-747.
- Sim CC, Zaharah AR, Tan MS, Goh KJ (2015) Rapid determination of leaf chlorophyll concentration, photosynthetic activity and NK concentration of Elaies guineensis via correlated SPAD-502 chlorophyll index. Asian J Agric Res 9(3): 132-138.
- Torres Netto A, Campostrini E, Oliveira JG, Yamanishi OK (2002) Portable chlorophyll meter for the quantification of photosynthetic pigments, nitrogen and the possible use for assessment of the photochemical process in Carica papaya L. Braz J Plant Physiol 14(3): 203-210.
- Ma BL, Morrison MJ, Voldeng HD (2001) Leaf greenness and photosynthetic rates in soybean. Crop Sci 35(5): 1411-1414.
- Costa C, Dwyer LM, Dutilleul P, Stewart DW, Ma BL, et al. (2001) Inter-relationships of applied nitrogen, SPAD, and yield of leafy and non-leafy maize genotypes. J Plant Nutr 24(8): 1173-1194.
- Islam MR, Haque KS, Akter N, Karim MA (2014) Leaf chlorophyll dynamics in wheat based on SPAD meter reading and its relationship with grain yield. Sci Agric 8(1): 13-18.
- Monostori I, Árendás T, Hoffman B, Galiba G, Gierczik K, et al. (2016) Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 211(1): 103-112.
- Johkan M, Shoji K, Goto F, Hashida SN, Yoshihara T (2010) Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hort Science 45(12): 1809-1814.
-
Peter A.Y. Ampim, Eric Obeng, Ernesto Olvera Gonzalez, Aruna Weerasooriya, Godson O. Osuji, Deland J. Myers Sr. The Response of Egyptian Spinach and Vegetable Amaranth Microgreens to Different Light Regimes. Sci J Biol & Life Sci. 1(3): 2021. SJBLS.MS.ID.000512.
-
Environmental Perception, Transdisciplinarity, Ecological Awareness, Interaction Man-Environment, Organisms, Imbalance, Humankind, Transdisciplinary, Aristotelian, Dichotomy, Aristotelian
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






