 Research Article
Research Article
The Effect of Fabric Covering on the Emergence and Yield of Container Grown Egyptian Spinach (Corchorus olitorius L.)
Eric Obeng, Peter AY Ampim*, Andrew C Nwachukwu, Faith Isabelle, Aruna Weerasooriya and Godson Osuji
College of Agriculture and Human Sciences, Prairie View A&M University, USA
AY Ampim, College of Agriculture and Human Sciences, Prairie View A&M University, P.O. Box 519, MS 2008, Prairie View, TX 77446, USA.
Received Date: January 04, 2021; Published Date: March 11, 2021
Abstract
Egyptian spinach is a nutritious leafy vegetable consumed in many parts of the world. Seed dormancy is a common problem associated with the germination of Egyptian spinach seeds. As a result, this study investigated the effect of fabric covering on the emergence and yield of Egyptian spinach. The treatments comprised pots covered with Dewitt ultra-web 3000 landscape fabric and, pots without covering. Each treatment was replicated three times with twenty Egyptian spinach seeds planted in each pot. Each pot received 500 ml of water every other day, from planting to harvesting. The covering was removed 10 days after planting. Data collected included stand count, SPAD reading, total number of leaves per pot, number of leaves per plant, leaf fresh weight per pot, total harvest weight per pot, and harvest weight per plant. The results showed significant increase in total number of leaves per pot and number of leaves per plant, when Egyptian spinach was covered compared to uncovered (P<0.1). Though insignificant, harvest weight was greater for the uncovered treatment compared to the covered.
Keywords: Egyptian spinach; Germination; Fabric covering; Dormancy; Yield
Introduction
Egyptian spinach (Corchorus olitorius L.) is a leafy vegetable widely grown and consumed in the tropical regions of Africa and Asia [1-3]. It is also commonly found in the open bushland, grassland, and cultivated lands in African countries such as Bostwana, Ghana, Kenya, Nigeria, South Africa, Zambia [4], and Asia (e.g. India, and Bangladesh). Other common names of Egyptian spinach include jute leaf [3], jute mallow, saluyot, jute, Jew’s mallow, and bush okra [5]. Egyptian spinach is a good source of protein, vitamins (A, B, C, E), lipids, carbohydrates, mineral nutrients including iron and calcium [3,6-8], and essential amino acids [9,8]. Egyptian spinach also has medicinal properties and is reported to be used for the treatment of fever, waist pain, stomach problem, and loss of appetite [8,10]. Egyptian spinach leaves are typically eaten fresh or dried [11,12].
Egyptian spinach seeds are known to have dormancy [13] and this is attributable to their nature [14]. They have hard seed coats which serve as a barrier that interferes with imbibition, oxygen uptake, gaseous exchange, and radicle emergence [4,15]. Though seed dormancy may aid seeds to survive environmentally stressful condition [16], dormancy typically leads to non-uniform germination and seedling emergence that is problematic for vegetable growers [17]. Poor germination of Egyptian spinach seeds fraught attempts made to promote its cultivation in Bostwana [4]. Physical dormancy is usually mitigated by scarification of plant seeds through a mechanical, thermal or chemical means to make hard seed coats permeable to water or gas [12,18]. For Egyptian spinach several different strategies have been used to break seed dormancy. These include seed steeping in 80-97°C water at timeframes ranging from five seconds to 15 minutes [12,19-21], and treating the seeds with dry heat at 80°C and 100°C and concentrated sulfuric acid at room temperature [12]. However, Velempini et al. [4] described soaking Egyptian spinach seeds in 80°C water for 15 as the most effective method as it resulted in greater than 90% germination. In contrast, Emongor et al. [14] found that treating Corchorus tridens seeds with sulphuric acid (98%) significantly broke seed dormancy and enhanced germination capacity of the seeds. Other researchers found that seed pre-chilling accompanied by exposure to greater than 30°C enhanced the germination of C. olitorius seeds [22]. In the wild, Corchorus olitorius grows in grassland areas, fallows or abandoned fields, often close to marshes, rivers and lakes and at altitudes of up to 1259 m [13]. It is likely that the acidic conditions of marsh lands and the other locations plays a role in their successful establishment in those environments.
The use of plant material as cover to promote seed germination is common among small-scale farmers in some parts of Africa. However not much research efforts have been dedicated to investigating effectiveness of the practice or the use of covers in general. Mayor et al. [23] investigated the effect of shrub cover on the germination, dormancy and seed viability of Piptochaetium napostaense, a common perennial grass in central semi-arid Argentina and found that seeds buried under shrub cover had very low germination rate. Since most of the research on dormancy breaking and germination of Egyptian spinach are heat and acid based which farmers may not feel comfortable working with, it is necessary to explore other approaches including the use of covers which are relatively less sophisticated to use. Since temperature and humidity are known to play crucial roles in the germination of seeds buried [24-26], readily available materials such as landscape fabric which can conserve soil moisture and moderate temperature around sown seeds may aid in breaking dormancy and promote germination. Besides, landscape fabrics or their analogues can be used several times. Hence, the objective of this study was to determine the effect of fabric covering on the emergence and yield of Egyptian spinach.
Material and Methods
This study was conducted as a pot experiment on the research farm of the College of Agriculture and Human Sciences on the campus of Prairie View A&M University, Prairie View, Texas (Longitude 30.080400, Latitude -95.990930), from June to October in 2018 and 2019. Prairie View is located in the northwestern edge of the Houston-The Woodlands-Sugar Land metropolitan area. Weather conditions during the study period is summarized in Table 1.
Table 1: Mean air temperature, and precipitation in Prairie View, Texas in 2018 and 2019.
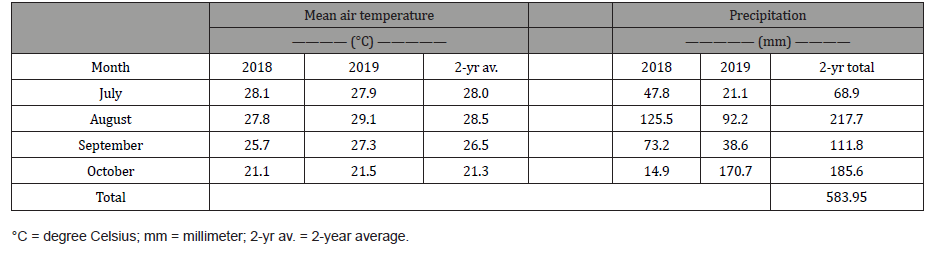
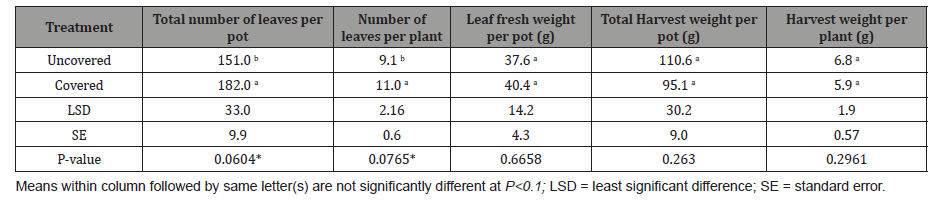
Table 2: Effect of fabric covering on the total number of leaves per pot, number of leaves per plant, leaf fresh weight, total harvest weight, and harvest weight per plant.
The experiment was arranged in a completely randomized design with three replications. A Ghanaian cultivar of Egyptian Spinach was used to conduct the study. Planting was done in 15-cm diameter pots filled to the rim with Pro-mix Bx Mycorrhizae (Premiere Tech Horticulture, Quakertown, PA, USA) potting soil. The potting soil in each pot was thoroughly wetted until drainage from the bottom and planted with 20 seeds and placed on a bench in the open. The pots were randomly assigned to the two treatments namely Dewitt ultraweb 3000 landscape fabric (DeWitt Company, Sikeston, MO, U.S.A.) cover and the control (no cover). The covering lasted for only 10 days for the cover treatment and was removed. Subsequently, each pot received 500 ml of water every other day. Watering was not done if a significant amount of rain fell before or on the day scheduled for doing so. Nitrogen fertilizer was applied at 20 kg N/ha at 6 weeks after planting using Peters Professional® fertilizer (20-20-20). Data collected included stand count (i.e. the total number of plants per pot), SPAD reading, total number of leaves per pot, number of leaves per plant, leaf fresh weight, total harvest weight, and harvest weight per plant. SPAD reading was taken using a Minolta Chlorophyll Meter SPAD-502DL (Spectrum Technologies Inc., Aurora, IL, USA) from three randomly selected plants from each pot. One-time harvesting was done at 4 months after planting by cutting the stems at the ground level. Total harvest weight was determined by weighing the whole harvest including the stem and leaves on a weighing scale. Harvest weight per plant was assessed by dividing the total harvest weight by the total number of plants per pot. The total number of leaves per pot were determined by counting. Leaves were detached from the stems and branches and weighed for leaf fresh weight. Leaf weight per plant was determined by dividing the total leaf weight by the total number of plants per pot. The data collected was subjected to analysis of variance (ANOVA) using the Statistical Analysis Software (SAS) version 9.3 and conclusions were drawn at the 10% significance level.
Results and Discussion
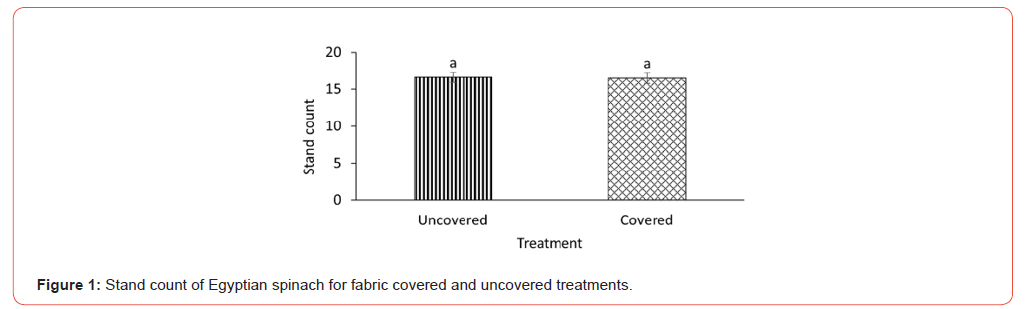
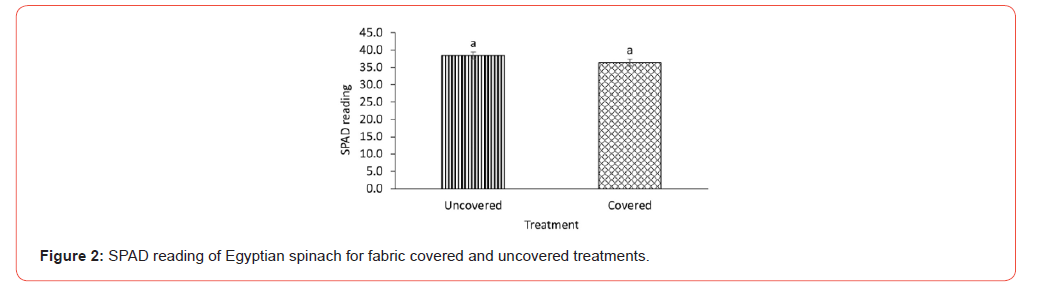
Since the goal of this study was to observe emergence and grow the plants to maturity, stand count is representative of germination rate since none of the plants died after emergence. Each treatment uncovered and fabric covered had an emergence rate of about 83% and therefore were not statistically different (Figure 1). The lack of difference could be because the covering did not necessarily provide an advantage since both treatments received sufficient amounts of water and were exposed to adequate temperature (Table 1). This assertion is made because it has been reported that unfavorable environmental conditions including factors such as moisture, light and temperature can impede emergence even after seed germination [21,27]. Hence dormancy is a survival mechanism to withstand erratic and challenging environmental conditions [14]. The sufficient environmental conditions in both treatments may have enhanced germination and emergence since heat is known to weaken seed coats resulting in dormancy alleviation [28,29]. The stand count results obtained may also be an artifact of the quality of seeds used in the experiment since damaged seeds are known to reduce germination and emergence of field crops [30]. The Egyptian spinach seeds used in this experiment were thoroughly cleaned to remove all debris and broken seeds. The peat moss potting soil used may have also contributed to the lack of difference in emergency since peat moss is widely known to provide excellent environment for germinating seeds [31,32]. The emergence results obtained in this study are significantly higher than the results of Oladiran [21] who investigated the effects of both steeping duration and seed scarification on seedling emergence. Average emergence rate observed for both treatments were 41.9% and 66% respectively. Though a little lower, the emergence rate obtained in this study is comparable to results obtained for reported successful methods such as dry heat [12,33] and combined chilling and heat treatment [22]. SPAD reading was not also different for the covered and uncovered Egyptian spinach treatments (Figure 2) signifying that cover did not affect the leaf chlorophyll content of the covered treatment.
However, total number of leaves per pot increased significantly (P<0.1) by 17.0% when Egyptian spinach seeds were covered compared to uncovered (Table 2). Similarly, number of leaves per plant increased significantly (P<0.1) by 17.3% when Egyptian spinach seeds were covered compared to the uncovered (Table 2). Though statistically insignificant, leaf fresh weight per pot increased 6.9% for the covered compared to uncovered treatment (Table 2). In contrast, total harvest weight per pot was 14% greater when Egyptian spinach seeds were not covered in comparison to the covered though not statistically different (Table 2). Following the same trend, harvest weight per plant was 13.2% less for the covered treatment compared uncovered treatment (Table 2). Although there was no difference in emergence, the yield results suggest that there is a benefit to covering since the covered treatment produced more leaves which is the portion of the plant eaten. The results also suggest that the 10-day covering period for the covered treatment appears to be suitable since it did not lead to adverse effects on relative chlorophyll which the SPAD measurements represent and leaf yield. Though statistically insignificant, the higher total harvest weight and harvest weight per plant recorded seem to suggest that plants in the uncovered treatment developed more stems than leaves. This could be notable if Egyptian spinach is grown as a fiber instead of vegetable crop for leaves.
This study has demonstrated that fabric covering can be a useful approach to overcoming physical seed dormancy in Egyptian spinach though its effect was not drastically shown in this study probably because of the more controlled experimental approach used. The effects of cover may be more distinct under natural field conditions as result of more severe fluctuations in environmental conditions. Since the emergence results observed in this study is comparable to the more relatively sophisticated methods of breaking seed dormancy, covering provides an additional option for unsophisticated small-scale farmers and urban gardens looking for simple methods to overcome the challenge of dormancy when growing Egyptian spinach. It also has the advantage of being a relatively inexpensive approach since the covers can be used over and over again. More so, there are several cheaper analogues of the fabric type used in this study available that can be used for the same purpose. Further research under natural field conditions will provide additional information to bolster these results.
Acknowledgements
This study was conducted with funds from USDA-NIFA 1890 Capacity Building Grant Award No. 2017-38821-26420.
Conflict of Interest
There is no conflict of interest.
References
- Dansi A, Adjatin A, Adoukonou-Sagbadja H, Falade V, Adomou AC, et al. (2009) Traditional leafy vegetables in Benin: folk nomenclature, species under threat and domestication. Acta Bot Gall 156(2): 183-199.
- Oboh G, Raddatz H, Henle T (2009) Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus olitorius) leaf. Int J Food Sci Nutr 60(Suppl 2): 124-134.
- Adeniyi SA, Ahiagbonare JE, Nwangwu SCO (2012) Nutritional evaluation of some staple leafy vegetables in Southern Nigeria. Int J Agric Food Sci 2: 37-43.
- Velempini P, Riddoch I, Batisani N (2003) Seed treatments for enhancing germination of wild okra (Corchorus olitorius). Exp Agric 39: 441.
- Awad S (2019) Blueprint for cultivation of Jute Mallow (Corchorus olitorius) under Swedish conditions. M.S. Thesis. Swedish University of Agricultural Science.
- Matsufuji H, Sakai S, Chino M, Goda Y, Toyoda M, et al. (2001) Relationship between cardiac glycoside contents and color of Corchorus olitorius J Health Sciences, 47: 89-93.
- Nyadanu D, Lowor ST (2015) Promoting competitiveness of neglected and underutilized crop species: comparative analysis of nutritional composition of indigenous and exotic leafy and fruit vegetables in Ghana. Genet Resour Crop Ev 62: 1-4.
- Nyadanu D, Amoah RA, Kwarteng AO, Akromah R, Aboagye LM, et al. (2017) Domestication of jute mallow (Corchorus olitorius L.): ethnobotany, production constraints and phenomics of local cultivars in Ghana. Genet Resour Crop Ev 64: 1313-1329.
- Tulio AZ Jr, Ose K, Chachin K, Ueda Y (2002) Effects of storage temperatures on the postharvest quality of jute leaves (Corchorus olitorius L.). Postharvest Biol Technol 26(3): 329-338.
- Masarirambi MT, Sibandze N, Wahome PK, Oseni TO (2012) Effects of kraal manure application rates on growth and yield of wild okra (Corchorus olitorius L.) in a sub-tropical environment. Asian J Agric Res 4: 89-95.
- Chweya JA, Eyzaguirre PB (1999) The biodiversity of traditional leafy vegetables. University of Nairobi.
- Velempini P, Riddoch I, Batisani N (2003) Seed treatments for enhancing germination of wild okra (Corchorus olitorius). Exp Agric 39: 441.
- Fondio L, Grubben GJH (2004) Corchorus olitorius L. In: Grubben GJH, Denton OA (eds). Plant Resources of tropical Africa 2. Vegetables. PROTA Foundation, Wageningen, Netherlands/Baack- huys Publishers, Leiden, Netherlands/CTA, Wageningen, Netherlands. pp. 217-221.
- Emongor VE, Mathowa T, Kabelo S (2004) The Effect of Hot Water, Sulphuric Acid, Nitric Acid, Gibberellic Acid and Ethephon on the Germination of Corchorus (Corchorus tridens) Seed. J Agron 3: 196-200.
- Mmolawa OB (1987) Germination and dormancy of meadowfoam seed. M.S. Thesis, Oregon State University, Corvallis, Oregon, USA.
- Ndinya C (2005) Seed production and supply system of three African leafy vegetables in Kakamega District. In: Muriithin AN, Anjichi VE, Ngamau K, Agong SG, Frickle A, Hau B, Stutzel H (eds). Proceedings of the third horticulture workshop on sustainable horticultural production in the tropics. Maseno University, Maseno. pp. 60-67.
- Gilberstone TL, Ferris ML, Brenner AC, Wilkins HF (1981) Effect of storage temperature on endogenous growth substances and shoot emergence in Freesia hybrid corns. J Am Soc Hortic Sci 112(4): 641-644.
- Budy J, Evans R, Young J (1986) Understanding Seed Handling for Germination. Arlington, VA: Volunteers in Technical Assistance.
- Tolorunse KD, Ibrahim H, Aliyu NC, Oladiran JA (2015) The quality of jute mallow seeds exposed to different hot water-steeping and cooling protocols. J Exp Agric Int pp. 107-117.
- Schippers RR (2000) African Indigenous vegetables. An overview of the cultivated species. Chattam, UK: Natural Resources Institute. pp. 214.
- Oladiran JA (1986) Effect of stage of harvesting and seed treatment on germination, seedling emergence and growth in Corchorus olitorius ‘Oniyaya’. Sci Hortic 28(3): 227-233.
- Nkomo M, Kambizi L (2009) Effects of pre-chilling and temperature on seed germination of Corchorus olitorius L. (Tiliaceae) Jew’s Mallow, a wild leafy vegetable. Afr J Biotechnol 8(6): 1078-1081.
- Mayor MD, Boó RM, Peláez DV, Elía OR, Tomás MA (2007) Influence of shrub cover on germination, dormancy and viability of buried and unburied seeds of Piptochaetium napostaense (Speg.) Hackel. J Arid Environ 68(4): 509-521.
- Vegis A (1964) Dormancy in Higher Plants. Annual Review of Plant Physiology 15: 185-225.
- Hilhorst HWM (1998) The regulation of secondary dormancy. The membrane hypothesis revisited. Seed Sci Res 8: 77-90.
- Forcella F, Arnold RLB, Sanchez R, Ghersa CM (2000) Modeling seedling emergence. Field Crops Res 67(2): 123-139.
- Hegarty TW (1979) Factors influencing the emergence of calabrese and carrot seedlings in the field. J Hortic Sci 54: 199-207.
- Dhillion WS, U Singh (1996) Breaking of seed dormancy in different leguminous forage species. Int Rice Res Notes 21: 45-46.
- Ibrahim H, Oladiran JA, Mohammed H (2013) Effects of seed dormancy level and storage container on seed longevity and seedling vigour of jute mallow (Corchorus olitorius). Afri J Agric Res 8: 1370-1374.
- Okabe A (1996) Inheritance of seed coat cracking and effective selection method for resistance in soybean. Jpn Agric Res Q 30: 15-20.
- Çelik H, H Zenginbal, M Özcan (2006) Enhancing germination of kiwifruit seeds with temperature, medium and gibberellic acid. Hortic Sci (Prague) 33(1): 39-45.
- Kitir N, Yildirim E, Şahin Ü, Turan M, Ekinci M, et al. (2018) Peat use in horticulture.
- Denton OA, Oyekale KO, Nwangburuka CC, Daramola DS, Adeyeye JA, et al. (2013) Influence of high dry heat temperature on seed germination, seedling emergence and seedling vigour of three cultivars of Corchorus olitorious seeds. Am J Res Commun 1(5): 98-114.
-
Eric Obeng, Peter AY Ampim, Andrew C Nwachukwu, Faith Isabelle. The Effect of Fabric Covering on the Emergence and Yield of Container Grown Egyptian Spinach (Corchorus olitorius L.). Sci J Biol & Life Sci. 1(4): 2020. SJBLS.MS.ID.000519.
-
S. aureus, S. marcescens, Virulence factors, Proteases, Bacterial pathogenesis, Nanoparticles
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






