 Research Article
Research Article
Silver and Gold-Silver Nanoparticles Can Serve as Potential Protease Inhibitors for Staphylococcus aureus and Serratia marcescens Hydrolytic Enzymes
Jayden Vanterpool1, Erwin Stuffle2, and Elaine Vanterpool3*
1Department of Biological Sciences, Oakwood University, USA
2Department of Basic Sciences, Division of Microbiology, Loma Linda University, USA
3Department of Biological Sciences, Oakwood University, USA
Elaine Vanterpool, Oakwood University, 7000 Adventist Blvd Huntsville, AL 35896, USA.
Received Date: December 28, 2020; Published Date: February 16, 2021
Abstract
Staphylococcus aureus and Serratia marcescens are bacterial opportunistic pathogens. Their infections can range from mild and local to systemic and life-threatening. It is imperative to identify ways of reducing the virulence potential of these pathogens. Proteases of S. aureus and S. marcescens can destroy host tissues and evade host immune defenses. Reducing the function of the toxins and/or proteolytic enzymes of these pathogens may be a potential strategy of reducing pathogenicity. Silver and other ions have been used as an antimicrobial agent for years, but its effect on S. aureus and S. marcescens proteolytic enzyme function has not been evaluated. The hypothesis of this study is that silver-based monometallic and/or bimetallic silver-gold nanoparticles can inhibit the proteolytic enzyme function of S. aureus and S. marcescens through interference with enzymatic structure. To test the hypothesis, experimental procedures were used including synthesis of silver nanoparticles (AgNP) and gold-silver nanoparticles (Ag- AuNP), bacterial culture fractionation, spectrometry, and protease/peptidase assays. Results show that both monometallic AgNP and bimetallic Ag-AuNP can reduce bacterial proteolytic activities in S. aureus and S. marcescens. In conclusion, the findings of this study support the hypothesis that monometallic AgNP and bimetallic nanoparticles Au-AgNP can inhibit bacterial enzyme function. Silver and silver-gold nanoparticles may be a potential protease inhibitor treatment option that can help treat and prevent complications caused by these pathogens.
Keywords: S. aureus; S. marcescens; Virulence factors; Proteases; Bacterial pathogenesis; Nanoparticles
Introduction
Bacterial pathogens S. aureus (gram-positive cocci) and S. marcescens (gram-negative rods) are both opportunistic pathogens which invoke damage in susceptible and immunocompromised hosts [1-5]. These pathogens, and others, produce weapons called virulence factors that can cause various physiological complications in the host. Such host-damaging virulence factors include proteases or hydrolytic enzymes [6-10]. The proteases are enzymes capable of degrading or cleaving susceptible substrates present on host tissues and immune components. S. aureus produces many proteases including metalloproteases, serine proteases, and cysteine proteas es. Some specific proteases include staphopain B (SspB), staphopain A (ScpA), aureolysin, and V8 protease (among others) [6-10]. The staphopains can modulate the immune system and destroy tissues. Aureolysin and V8 protease can also evade the immune defenses [4]. Non-protease virulence factors produced by S. aureus include hemolysins, toxic shock syndrome toxin 1, enterotoxins, exotoxins and coagulase (and others). S. marcescens also produce several proteases including serralysin and metalloproteases which too can damage host tissues and evade host immune defenses [8- 10]. It is important that we find ways to modulate or regulate the activity and damaging impacts of these microbial proteases. In this study, we focused on evaluating the potential role of ion-based nanoparticles as bacterial protease inhibitors. Ions like silver (Ag+), have been evaluated for antimicrobial properties and have been found to be effective at killing microbes [11,12]. However, the role of silver to regulate virulence factor function has not been studied. Silver (Ag) can interact and possibly disrupt the structure enzyme catalytic sites. This study evaluated the role of ion-based nanoparticles on modulating protease activity in S. aureus and S. marcescens.
Material and Methods
Bacterial growth conditions and growth assessment
S. aureus and S. marcescens were grown in Brain Heart Infusion (BHI) broth at 37°C for 20-24 hours. Bacterial growth was determined by using a spectrophotometer at visible wavelength of OD600.
Nanoparticle Synthesis
Silver nanoparticles (AgNP) and silver-gold nanoparticles (Ag- AuNP) were synthesized similarly to that described by Holden et al with some modifications. Briefly, for AgNP synthesis, 0.01M AgNO3 was placed in a round bottom flask and heated (13). 1% trisodium citrate was added and continued heating with stirring until the solution was an amber yellow color. The sample was removed from the heat immediately with continued stirring until it cooled to room temperature. A sample of the solution was evaluated in the UV-vis spec to confirm identity. Characteristic absorbance at 410nm was recorded and particles had an average diameter of 32nm as determined by Dynamic Light Scattering (DLS) spectrophotometry. For Ag-AuNP synthesis, a maltose solution was prepared. NH4OH was added and pH was adjusted to 10-11 using 1M NaOH. 0.02M AgNO3 was then added followed by HAuCL4 and 0.1M glutathione (GSH). Pluronic F127 triblock copolymer was further added and heated to dissolution. Samples were cooled, centrifuged and then resuspended in a solution containing 0.1% F127 and 0.001M GSH with pH adjusted to about 10. UV-Vis spectrophotometry showed characteristic absorbance at 410nm for Ag and 520nm for Au and the particles had an average diameter of 58nm.
Bacterial Fraction Preparation and Peptidase/Protease Assay
Whole culture samples of S. aureus and S. marcescens grown in BHI to stationary phase were prepared for protease activity assessment. Cell-free (extracellular) and cellular fractions were also prepared after centrifugation to separate the secreted and cell-associated proteases. Harvested cell fractions were treated with lysozyme and/or sonication for lysis. The cellular envelope fraction was then separated and collected using centrifugation. Extracellular fractions were harvested by using Vivaspin columns or protein salting methods. 100μg of extracellular fractions or cellular fractions were used for protease activity assessment in the presence or absence of AgNP or Ag-AuNP using the EnzChek Protease/Peptidase Assay Kit. Protease assays were done according to the EnzChek Protease/ Peptidase Assay manufacturers recommendations (Life Technologies). All solutions, substrates, and directions were prepared and followed as instructed. The protease levels were determined by using a microplate reader. For background interference, nanoparticles were also assessed to eliminate background values caused by the nanoparticle solution itself. All experiments were done in five independent trials, with each trial done in triplicate. Data were plotted showing standard error.
Results and Discussion
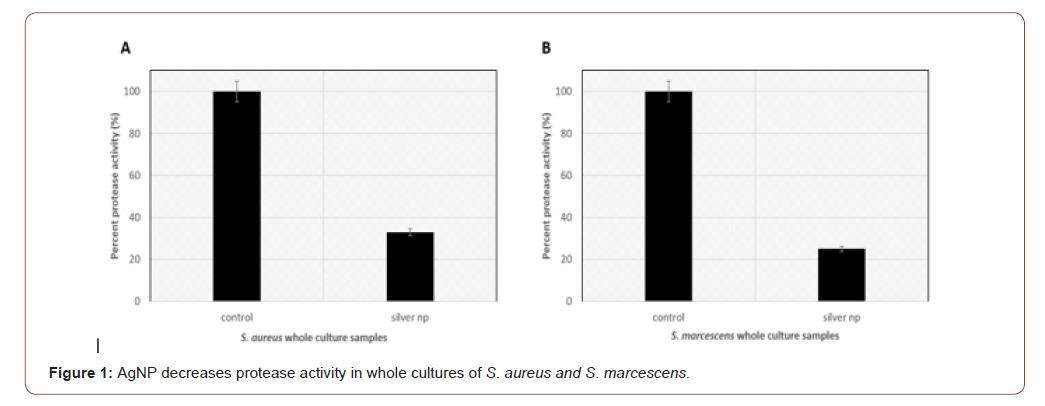
AgNP on protease activities of whole cultures of S. aureus and S. marcescens
Synthesized AgNP were incubated with whole bacterial cultures grown to stationary phase. Protease assays show that S. aureus has 32.9% protease activity in the presence of AgNPs compared to the untreated cultures control (Figure 1A). S. marcescens show 25% protease activity in the presence of AgNPs compared to the untreated control (Figure 1B). This supports the hypothesis that ions in the AgNP may be interacting with the catalytic active site or inducing a conformational change in the enzyme resulting in reduction of activity. This will have to be further evaluated.
AgNP and Ag-AuNP impact on extracellular and cellular fractions of S. aureus
Synthesized AgNP and Ag-AuNP were incubated with extracellular or cellular bacterial fractions from cultures grown to stationary phase. Protease assay experiments show that AgNP reduces S. aureus extracellular activity to 11.1% and Ag-AuNP to 5.6% in comparison to the untreated control (Figure 2A). Regarding S. aureus cellular fractions, AgNP decreases protease activity to 9.1% and Ag- AuNPs to 4% compared to the untreated control (Figure 2B). AgNP causes a great reduction in protease activity in the cellular and extracellular S. aureus fractions, however, the bimetallic Au-AgNP has a greater impact on bacterial protease activity suggesting the dual pairing of gold and silver ions can act as a more potent protease inhibitor of both the cellular and extracellular fractions. The mechanism of interactions are currently being evaluated.
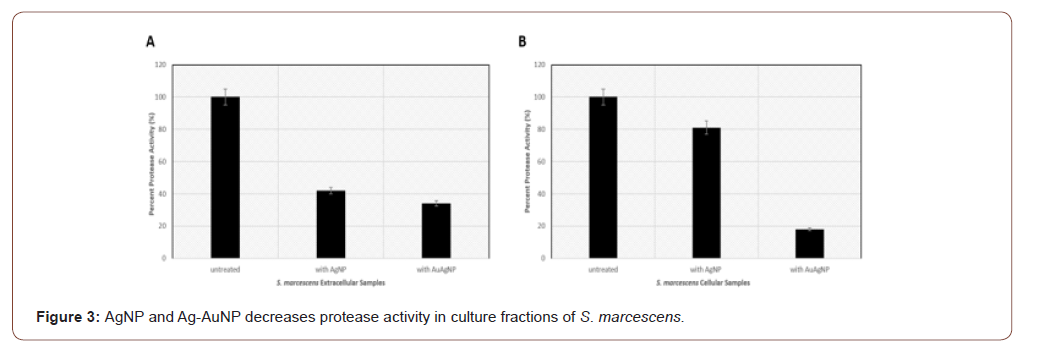
AgNP and Ag-AuNP impact on cell-free and cellular fractions of S. marcescens
Synthesized AgNP and Ag-AuNP were incubated with extracellular or cellular bacterial fractions from cultures grown to stationary phase. Protease assay data show that AgNP can reduce S. marcescens extracellular activity to 42% and Aug-AuNP decrease protease activity to 34% in comparison to the untreated control (Figure 3A). Regarding S. marcescens cellular fractions, AgNP decreases protease activity to 81% and Ag-AuNP to 18% compared to the untreated control (Figure 3B). Similar to S. aureus protease assay studies, the bimetallic Au-AgNP has the greatest impact on protease activities in S. marcescens. It appears that both monometallic AgNP and bimetallic Au-AgNP have the greatest inhibitory effects on the extracellular/secreted protease compared to the cell-associated proteases. This may be explained by potential differences in posttranslational modifications (i.e. glycosylation) of the cell-associated proteases in S. marcescens in comparison to the secreted proteases. This will be further examined by our lab.

Conclusion
The findings of the study support the hypothesis that silver ionbased nanoparticles can inhibit the protease activity of S. aureus and S. marcescens hydrolytic enzymes. This can potentially prevent the tissue-damaging effects caused by these virulence factors and lead to a reduction in the complications associated with S. aureus and S. marcescens infection.
Acknowledgements
We would like to acknowledge Oakwood University, Department of Biological Sciences, Chemistry Department, and the UNCF Henry C. McBay Fellowship awarded to Dr. Elaine Vanterpool.
Conflict of Interest
The authors have no conflict of interest.
References
- Acar JF (1986) Serratia marcescens Infect Control. 7(5): 273-278.
- Hejazi A, Falkiner FR (1997) Serratia marcescens. J Med Microbiol 46(11): 903-912.
- Lowy FD (1998) Staphylococcus aureus N Engl J Med 339(8): 520-532.
- Pietrocola G, Nobile G, Rindi S, Speziale P (2017) Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases. Front Cell Infect Microbiol 7: 166.
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG, Jr (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28(3): 603-661.
- Singh V, Phukan UJ (2019) Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med Microbiol Immunol 208(5): 585-607.
- Kantyka T, Shaw LN, Potempa J (2011) Papain-like proteases of Staphylococcus aureus. Adv Exp Med Biol 712: 1-14.
- Fredy J Romero, Luis A Garcı́a, José A Salas, Mario Dı́az, Luis M Quirós (2001) Production, purification and partial characterization of two extracellular proteases from Serratia marcescens grown in whey. Process Biochemistry. 36(6): 507-515.
- Salamone PR, Wodzinski RJ (1997) Production, purification and characterization of a 50-kDa extracellular metalloprotease from Serratia marcescens. Appl Microbiol Biotechnol 48(3): 317-324.
- Zhang L, Morrison AJ, Thibodeau PH (2015) Interdomain contacts and the stability of serralysin protease from Serratia marcescens. PloS One 10(9): e0138419.
- Tang S, Zheng J (2018) Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater (13): e1701503.
- Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, et al. (2020) The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomedicine 15: 2555-2562.
- Holden MS, Nick KE, Hall M, Milligan JR, Chen Q, et al. (2014) Synthesis and catalytic activity of pluronic stabilized silver-gold bimetallic nanoparticles. RSC Adv 4(94): 52279-52288.
-
Jayden Vanterpool, Erwin Stuffle, Elaine Vanterpool. Silver and Gold-Silver Nanoparticles Can Serve as Potential Protease Inhibitors for Staphylococcus aureus and Serratia marcescens Hydrolytic Enzymes. Sci J Biol & Life Sci. 1(4): 2020. SJBLS.MS.ID.000517.
-
S. aureus, S. marcescens, Virulence factors, Proteases, Bacterial pathogenesis, Nanoparticles
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






