 Review Article
Review Article
Role of Gene Therapy in Treating Heart Failure
Sloan E Almehmi1, Masa Abaza2 and Ammar Almehmi3*
1Department of Biology, University of Alabama at Birmingham, USA
2Department of Biology, University of Alaska at Anchorage, USA
3Department of Medicine, University of Alabama at Birmingham, USA
Ammar Almehmi, Department of Medicine, University of Alabama at Birmingham, USA.
Received Date: August 02, 2021; Published Date: November 23, 2021
Abstract
Heart failure (HF) is a global public health problem that affects 1-2% of the western countries. Further, it is associated with high morbidity,
mortality and health expenditures. Despite the advancements in the pharmacological treatment of HF, only 50% of patients survive at 5 years.
Recent advances in the pathophysiologic mechanisms of HF have created a new opportunity in developing non-pharmacological approaches
for treatment. Physiologic studies showed that cardiac sarco-endoplasmic reticulum Ca2+ATPase (SERCA2a) plays a major role in regulating the
calcium levels in cardiomyocytes. Alteration in calcium handling within the cardiomyocytes is one of the main pathognomonic processes that occurs
in the failing heart caused by the reduction in SERCA2a activity, which adversely affects both systolic and diastolic functions.
Gene transfer therapy is a novel approach that is used to target the calcium dysregulation seen in the failing cardiomyocytes. New vectors, namely
adeno-associated viruses (AAVs), were re-engineered to become more cardiotropic resulting in sustained transgene expression within the affected
cardiac tissue. The above advancements have been coupled with the development of new delivery methods that include endovascular, surgical, and
direct myocardial approaches. As a promising target for treating HF, AAV1-SERCA2a is being used in several clinical trials with promising clinical
outcomes.
Despite the significant progress of the cardiac gene therapy field, several challenges remain to be overcome including host neutralizing antibodies
of AAV, improving AAV vector cardiac-transduction affinity, and optimizing the dose, route and method of delivery.
Keywords: Heart failure; Gene therapy; Ca2+ATPase; Vector; Cardiomyocytes
Introduction
Heart failure (HF) is a global public health problem with high prevalence in the world. HF is the common final clinical presentation of a variety of cardiovascular diseases. Further, with aging of world population, it is expected that the number of HF patients will continue to increase [1]. Over the last 50 years, many effective pharmacologic therapies have been developed. However, HF morbidity and mortality remain high with only 50% of patients survive at 5 years [1,2]. Several mechanisms have been implicated in the pathogenesis of HF including adrenergic signaling abnormalities, alterations in calcium handling, disturbances cardiomyocyte relaxation, and activation of apoptosis pathways [2]. It is within this context that new approaches, such as gene transfer therapy, have been developed for treating HF [3]. The main focus of the current work is the calcium handling dysregulation seen in HF, with special attention to the role of sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) in the health and disease state of the cardiomyocytes [1-3]. Accordingly, the overarching objective of this paper is to review the role of SERCA2a gene transfer in treating HF and its clinical implications.
Materials and Methods
The information about HF and GT were collected by conducting literature search using PubMed search engine (the MEDLINE library), American Heart Association website and google search engine. The main words that were used in this search included heart failure, pathogenesis, cardiomyocytes, target of HF treatment and GT. The search was restricted to review articles, English language and time limit of 10 years. Basic physiologic background of the heart contractility was reviewed. Despite the multiple targets of GT, the main focus of this paper was the calcium regulation within the cardiomyocytes as the main target for gene therapy [4,5].
Outcomes
Heart failure is an epidemic of the developed world that is associated with increased morbidity and mortality. Despite the improved outcomes in HF population, the current pharmacological approaches are limited by the side effect profiles of medications requiring the search for new therapeutic approaches [1,2]. Hence, advances in bench research have elucidated much about the cellular and molecular pathways that govern the physiology and pathophysiology of HF. In the following paragraphs we will discuss the physiology of heart contractility, major pathologic pathways of HF, and role of calcium dysregulation in cardiomyocytes.
Physiology of heart contractility
The heart is made of cardiomyocytes, which are composed of contractile proteins (actin and myosin) and mitochondria. The contraction of the heart depends mainly on the coordinated action of individual cardiomyocytes. The beat-to-beat cycling of cardiomyocytes is dependent on intracellular Ca2+ cycling [6]. Calcium is released into the cytosol from the sarcoplasmic reticulum (SR) during systole resulting in the activation of actin and myosin coupling leading to contraction. Diastole is dependent on efflux of Ca2+ to the sarcoplasmic reticulum via sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) system. In other words, the contraction and relaxation of cardiomyocytes are dependent on the binding and dissociation process of Ca2+ from troponin protein [6] (Figure 1).
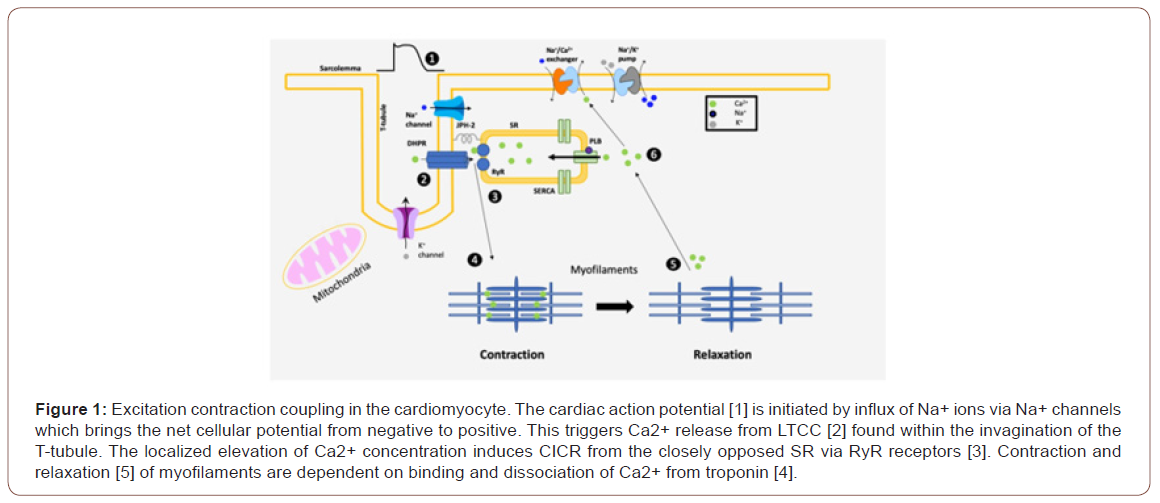
Major Pathophysiologic Pathways of HF
Over the past several decades, major advances in cellular and molecular biology have offered insights into pathogenesis of HF. The major pathognomonic processes of HF include adrenergic signaling abnormalities, alterations in calcium handling, disturbances cardiomyocyte relaxation, and activation of pathways that result in cell death [2,7]. The main focus of this paper is to explore the changes in the cardiomyocyte calcium dysregulation as targets for GT.
Role of SERCA2a in HF pathophysiology
The SERCA family is an intramembrane calcium channel pump that is composed of multiple domains. SERCA2a is the predominant isoforms that is found in the heart tissue and slow-twitch muscles. In order to demonstrate the role of SERCA2a in HF, several complete and partial knockout animal models were developed. While total knockout studies of SERCA2a were lethal to the mice embryos, the partial knockouts of SERCA2a predisposed these animals to HF. Other studies have shown that cardiomyocytes downregulate the expression of SERCA2a in the chronic HF model [6,8]. Importantly, Ca2+ cycling has been found to be dysregulated in HF with increased cytosol Ca2+ and decreased SERCA2a activity leading to a decrease in systolic function and abnormal diastolic functions [6,7].
The Ca2+ homeostasis and the tight control of SR Ca2+ levels are considered the key factors for the cardiomyocytes contractile function. Therefore, it is plausible that correction of SERCA2a activity levels could improve the contractility of the failing cardiomyocytes leading to clinical improvement and potentially better outcomes. As such, GT evolved as a promising therapeutic alternative that targets the cardiomyocyte calcium regulation. In animal models of HF, the treatment with gene transfer therapy using a viral vector to deliver the SERCA2a gene to the heart improved the symptoms of these animals [7,8].
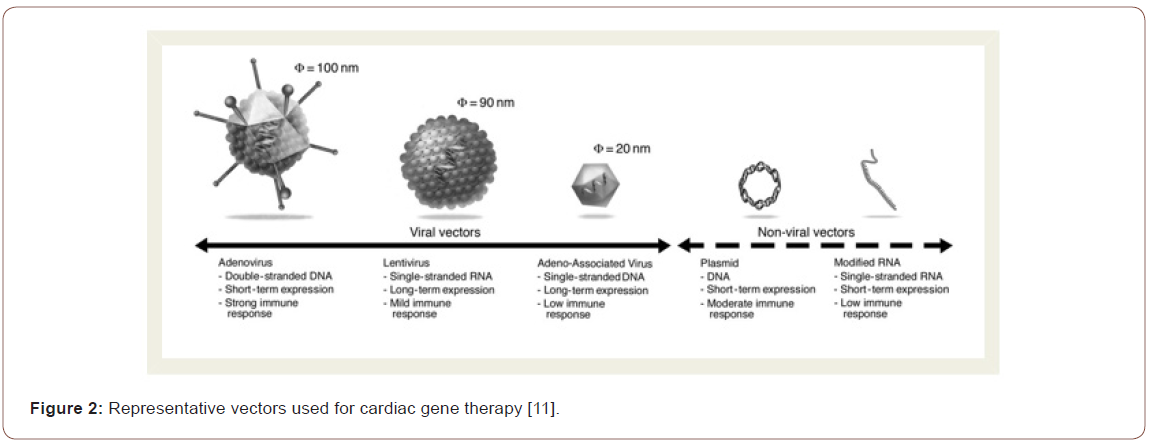
Methods of gene transfer
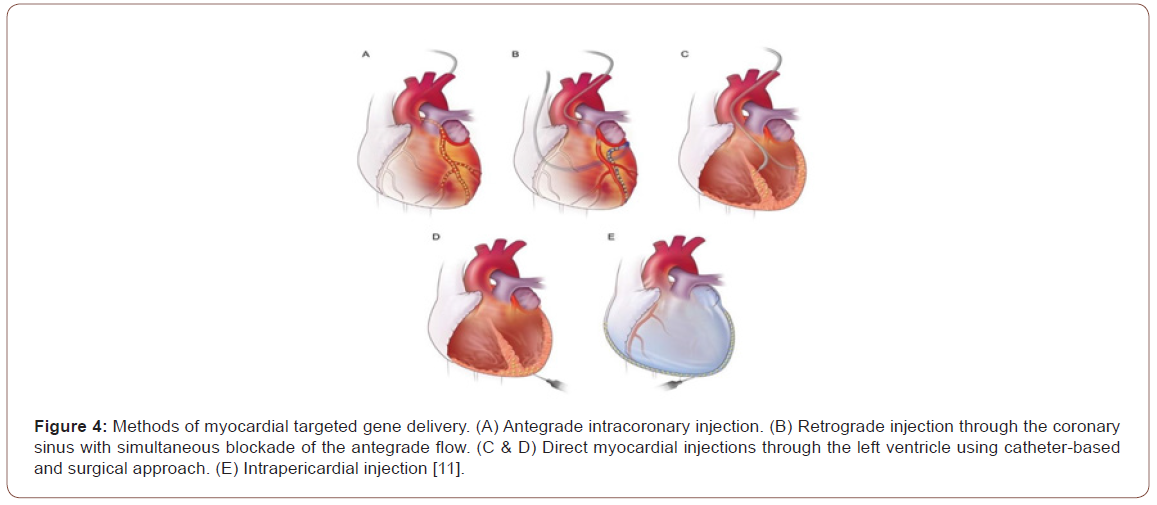
Gene delivery determines the efficacy of GT. Because the current available technologies using intravenous route do not sufficiently transduce myocardium, direct cardiac delivery is necessary. Several methods of myocardial targeted gene delivery have been developed over the years as shown in Figure 4.
Intra-myocardium and endovascular delivery are the methods of choice in clinical applications. Accordingly, GT dose is delivered invasively using surgical methods or less invasively using endovascular catheters (Figure 4). These methods are limited by the systemic leak of the vectors through the venous drainage, lymph system, and from the injection needle holes [10].
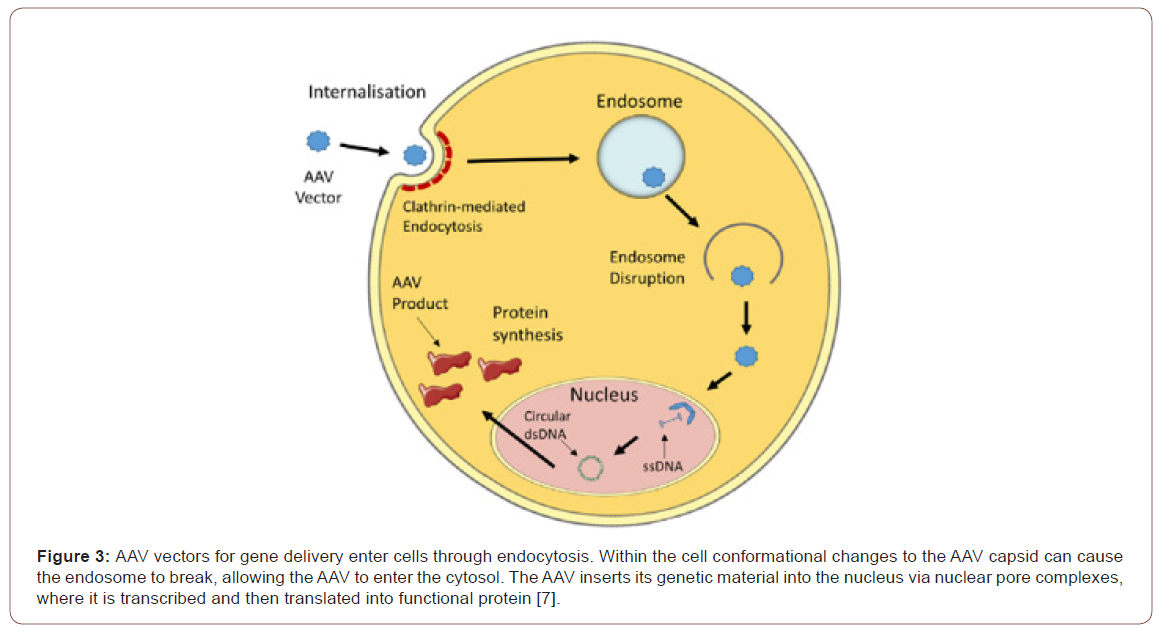
When delivered into the circulation, the AAV particles bind to surface receptors and then incorporated into cardiomyocytes by endocytosis. Subsequently, the particles (SERCA2a gene) get released into the cytoplasm where they are transported to the nucleus. At the nuclear level, the vector is disassembled, and its genetic material is released (Figure 3). After double-stranded synthesis of the gene of interest is complete, transcription and translation begin leading to the production of the molecule of interest. The SERCA2a gene transfer enhances the contractility of the injected area, restore the energetic state of the heart both in terms of energy supply and utilization, decrease ventricular arrhythmias, and enhance coronary flow [10-12].
Clinical trials using SERCA2a gene transfer
Several clinical trials have been initiated using AAV1-SERCA2a in HF population. The Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) trial was the first attempt to use AAV gene therapy to treat HF in human subjects. The CUPID trial involved a single intracoronary infusion of varying doses of the AAV1-SERCA2a vector to investigate the potential therapeutic benefit of increasing expression of SERCA2a in nine individuals with advanced HF [12-14].
CUPID 2 was a randomized, multinational, double-blind, placebo-controlled trial involving 250 subjects with advanced HF who received a high dose of AAV1-SERCA2a DNase-resistant particles [13]. Both trials improved the clinical symptoms of patients and exercise tolerance. Several other trials are going where the dose and methods of delivery are being modified [12,14].
Conclusion
HF is a global epidemic that continues to have poor prognosis. In spite of the advances of our understanding of the pathophysiology of HF and ongoing developments of new pharmacologic agents, the prognosis of HF remains poor. This necessitated new approaches and created an opportunity for GT in treating HF. GT appears to be a plausible strategy to approach such a complex pathophysiology seen in HF. Here we addressed the role of AAV1-SERCA2a gene transfer therapy in HF. Despite the disappointing results of CUPID trials, the field of cardiac gene therapy is moving forward. These trials proved the biosafety of AAV gene delivery, its ability to escape immune system and its affinity to heart tissue as demonstrated in the HF population. With improved vectors, broadening the gene therapy targets to include other pathophysiologic pathways, and enhanced gene delivery methods, the therapeutic benefits of gene therapy and its clinical applications will be upcoming in the near future. GT tools are already expanded to other diseases and organs making this field very exciting.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3(1): 7-11.
- Del Monte F, Hajjar RJ (2008) Intracellular devastation in heart failure. Heart Fail Rev 13(2): 151-162.
- Yerevanian A, Yerevanian A, Hajjar RJ (2014) Progress in gene therapy for heart failure. J Cardiovasc Pharmacol 63(2): 95-106.
- Ge Z, Li A, McNamara J, Dos Remedios C, Lal S (2019) Pathogenesis and pathophysiology of heart failure with reduced ejection fraction: translation to human studies. Heart Fail Rev 24(5): 743-758.
- Fish KM, Ishikawa K (2015) Advances in gene therapy for heart failure. Discov Med 19(105): 285-291.
- Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ (2010) Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther 10(1): 29-41.
- Bass-Stringer S, Bernardo BC, May CN, Thomas CJ, Weeks KL, et al. (2018) Adeno-Associated Virus Gene Therapy: Translational Progress and Future Prospects in the Treatment of Heart Failure. Heart Lung Circ 27(11): 1285-1300.
- Greenberg B (2017) Gene therapy for heart failure. Trends Cardiovasc Med 27(3): 216-222.
- Katz MG, Fargnoli AS, Weber T, Hajjar RJ, Bridges CR (2017) Use of Adeno-Associated Virus Vector for Cardiac Gene Delivery in Large-Animal Surgical Models of Heart Failure. Hum Gene Ther Clin Dev 28(3): 157-164.
- Hajjar RJ, Ishikawa K (2017) Introducing Genes to the Heart: All About Delivery. Circ Res 120(1): 33-35.
- Hulot JS, Ishikawa K, Hajjar RJ (2016) Gene therapy for the treatment of heart failure: promise postponed. Eur Heart J 37(21): 1651-1658.
- Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, et al. (2008) Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol 51(11): 1112-1119.
- Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, et al. (2016) Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial Lancet 387(10024): 1178-1186.
- Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, et al. (2014) Long- term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 114(1): 101-108.
-
Sloan E Almehmi, Masa Abaza, Ammar Almehmi. Role of Gene Therapy in Treating Heart Failure. Sci J Biol & Life Sci. 2(2): 2021. SJBLS.MS.ID.000532. DOI: 10.33552/SJBLS.2021.02.000532
-
Heart failure, Gene therapy, Ca2+ATPase, Vector, Cardiomyocytes, Pharmacological treatment, Cardiomyocytes, Sarco-endoplasmic reticulum, Dysregulation, Myocardial approaches, Relaxation, Apoptosis, Physiology
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






