 Research Article
Research Article
Parasitic Flora of Megaphrynium Macrostachyum Leaves sold in Popular Markets in Benin City, Nigeria
Nekpen Osarugue Obamwonyi1, Beck A Akhiwu2*, Isaac Gadzekpo3 and Onas Ikele3
1Department of Medical Laboratory Science, University of Benin, Nigeria
2University of Idaho, Kimberly Research and Extension Center, USA
3Department of Biological Science, Youngstown State University, USA
Beck Akhiwu, University of Idaho, Kimberly Research and Extension Center, Kimberly, Idaho, USA.
Received Date: February 27, 2021; Published Date: March 23, 2021
Abstract
Megaphrynium macrostachyum is a perennial leafy vegetable, up to 4m tall, with rhizome up to 6m long, stems bearing an inflorescence and a single subtending leaf and numerous leaves arising directly from the rhizome. The importance of this plant cannot be over emphasized as it leaves are used for different activities in Africa which includes cooking, wrapping, packing, thatching, and serving food to mention but a few. The leaves of M macrostachyum are used primarily for cooking, wrapping, packaging, and serving food in several African countries including Nigeria. In recent years, food-borne illnesses caused by intestinal parasites have been identified as a major source of public health threats. This study was designed to determine the parasitic flora of M macrostachyum leaves sold in popular markets in Benin City, Edo state. A total of 335 samples collected from six selected markets in Benin City; the leaves were washed in physiological saline and examined microscopically using saline and iodine preparations. Our results showed M macrostachyum is not a potential means for the transmission of intestinal parasites contrary to previous studies. However, proper hygiene and sanitation is advocated for to minimize risks illnesses due to food-borne parasites.
Keywords: Intestinal; Parasite; Contamination; Transmission; Infection
Introduction
M macrostachyum are usually collected from the wild, sometimes found in cocoa intercropping system, or cultivated. M macrostachyum reproduce rapidly and abundantly by sprouting from rhizomes or seed. The leaves are large and are found in the rainforest of west and Central Africa. M macrostachyum leaves are harvested and used for wrapping and boiling food as a way of extending the shelf life of the food. The young leaves of M macrostachyum are consumed as vegetable by humans and these can be agents of transmission of protozoa cysts and helminthes eggs and larva. Vegetables can become contaminated with parasitic pathogens throughout the process of planting to consumption which can lead to intestinal parasitic infections. The parasites are not easily detected when they get to the human body, hence, can live in human body for long without being diagnosed. The detrimental effects of parasites have been established to be species specific. The extent of contamination depends on so many factors which may include application of Nitrogen in the soil, animal manure, the use of untreated wastewater, water supplies contaminated with unhygienic practice by farmers during harvest, post-harvest handling by vendors, poor hygienic conditions of preparation in food service or home settings [1]. There are thousands of types of gastrointestinal parasites that live in the human body and occurrence of these parasites have usually caused serious health conditions by weaking body immune system, thereby increasing its vulnerability to viral, fungal and bacteria diseases as well as chemical and metal poisoning worldwide. Globally, 3.5 billion people are affected with intestinal parasitic infections, with 450 million symptomatic and more than 1.2 million deaths being reported annually. In developed countries, an estimated one-third of the population is affected by intestinal parasitic agents each year but the infections are more severe in the tropical regions of the world. In addition, poor sanitary and environmental conditions are known to be relevant in the propagation of these infectious agents. The objective of this study is to determine the parasitic flora of M macrostachyum leaf, and types of parasites found in some commercially sold M macrostachyum leaves in the study area. We also determined the sales outlet with the highest level of contaminated produce and the likely factors responsible for this trend with a view to proffering measures to curb contaminations. Our null hypothesis was that M macrostachyum leaves do not have parasitic flora, while our alternate hypothesis was that M macrostachyum leaves have parasitic flora.
Materials and Method
The study was carried out in various markets in Benin City which is the capital of Edo state and the city is occupied by both indigenous and elite residents. It covers an area of 19,794 Km2 and a provisional population of 2,159,484 million people with that of Benin City estimated at 1,147,188. The M macrostachyum leaves used for this study were purchased from local vendors at Oba, Osa, uselu, Ikpoba hill, and Oka markets all within select local government areas in Edo State viz. Ikoba Okha, Oredo, and Ovia northeast local government. Sample size was determined by the formula; N = Where: Z= 1.96(for 95% confidence level) P= prevalence rate (as obtained from literature review) C= 0.05 (confidence interval or tolerance error) N= required sample size. The prevalence rate from literature review= 31.7%. The minimum sample size when calculated was M macrostachum leaves were bought from the traders in the markets at different locations in the market between 7:00am to 11.00am. A total of 335 leaves were sampled. The leaves were collected into sterile, labelled polythene bags and transported to the laboratory for further processing.
Specimen Analysis
The parasitological analysis of these products was carried out at the Department of Medical Laboratory Science Research Laboratory, Benin City, Edo state. Nigeria. The leaves were analyzed in the laboratory using sedimentation method [2].
Parasitological Examination of the Samples
100g of each batch of the leaves was chopped into small pieces and placed into a clean small plastic bucket containing 100ml of normal saline (8.5g of sodium chloride salt in 1000ml of distilled water). Each batch was agitated and washed well to ensure that as much material on the surface of the leaves sample as possible were discharge into the normal saline solution. We placed them to stand on the bench for few hours for proper sedimentation to occur in accordance with the procedure described by Alli et al. [2]. The suspension was strained through a sterile sieve to remove extraneous debris. About 10ml of the filtrate was dispensed into a clean centrifuge tube and centrifuged at 3000rpm for 5 minutes. The supernatant was discarded into a disinfectant jar and the deposit, resuspended [3]. A drop of the resuspended sediment was placed on a clean glass slide and covered with a cover slip. A drop of lugol’s iodine solution was placed on a slide and a drop of the resuspended deposit was added and covered with a cover slip. The prepared slides were examined microscopically for the presence of ova, cysts of parasites [4].
Results
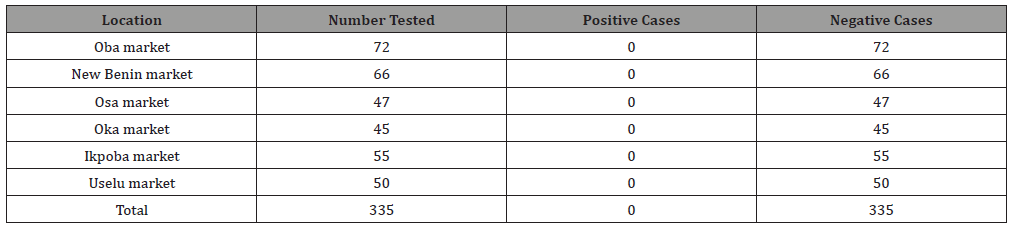
A total of 335 samples were examined for parasitic contamination, out of which none was positive for parasitic contamination. The location of the samples did not significantly affect the parasitic contamination of the study. Samples from all the six markets had no parasitic contamination (Table 1) (Figure 1).

Table 1: Relationship of location with parasitic contamination.
Discussion
The contamination of vegetables by parasites has long been established. Amongst the classes incriminated are Protozoa, Cestodes, Trematodes and Nematodes [5, 6]. These parasites could infect humans because of consumption of contaminated, uncooked, or improperly washed vegetables [7]. Preservation methods of M macrostachyum leaves cannot be overemphasized as the preservation methods employed for commercially sold M macrostachyum leaves has a direct correlation with the likelihood of parasitic contamination. Our study found that M macrostachyum leaves are sold fresh in the early hours of the day, with very few or no remainder for the next day. We found that this habit may prevents chances of humid environmental condition and temperature that could otherwise encourage the preservation and proliferation of geohelminths if stored for long. Our study found that M macrostachyum sold in popular markets in Benin City were free from parasitic contamination. An overall prevalence rate of 0% of parasitic flora of M. macrostachyum sold in popular markets in Benin. However, the overall result is not in exact representation of the findings of previous studies because the areas of study differ both in geographical location, climatic, environmental conditions, the general behavioral attitude to hygiene and the social-economic activities of sellers/traders. In contrast with our study, some other researchers had earlier on reported a 2.7% parasitic contamination of some commercially sold vegetables in Kerela state, India [8], 2.5% for cysts and 4.3% for parasite ova were reported in Southern Nigeria by OS Omowaya and PA Audu. The location of the samples did not significantly affect the parasitic contamination in our study as samples from all six selected markets were not contaminated and had the same percentage. Though, vendors may get their supplies from different sources, this study shows that parasitic contamination may not involve the diversity of source. Vendors serves as intermediaries between the farmers and the consumers, they play an important role in the chain of distribution of produce also may also play a role in the contamination and distribution of contaminants. The questionnaire distributed during this study provided more information on the sources and cleanliness of M macrostachyum leaves available in the marketplace. Our study has shown that an average of 10% of the end users purchased their produce from middlemen, 55% got theirs directly from farmers while 35% obtained theirs from the harvesters. It was also discovered that the vendors washed their produce irrespective of the source before display for sale.
Conclusion
This study has shown that M macrostachyum leaves have minimal tendencies of serving as a source of parasitic infection when sold commercially. Although, washing of these leaves with just water alone is inadequate to remove all contaminating pathogens, proper hygiene and good environmental sanitation should be maintained by all vendors to avoid contamination and distribution of parasitic contaminants [9-19].
Acknowledgement
None.
Conflict of Interest
None.
References
- Amoah P, Drechsel P, Abaidoo RC, Klutse A (2007) Effectiveness of common and improved sanitary washing method of selected cities of West Africa for the reduction of coliforms bacteria and helminth eggs on vegetable. Tropical Medicine and International Health 12(2): 40-50.
- Ogbolu DO, Alli OA, Ogunleye VF, Olusoga-Ogbolu FF, Olaosun I (2009) The presence of intestinal parasites in select vegetables from open markets in South Western Nigeria. Africa Journal of Medical Science 38(4): 319-324.
- Gharavi M, Jahani M, Rokni M (2002) Parasitic Contamination of Vegetables from Farm and Markets in Tehran. Iranian Journal Public Health 31(3): 4.
- Cheesbrough M (2006) Diseases. In: District laboratory practice in Tropical countries. Cheesbrough M (2nd), Cambridge University Press, United Kingdom, pp. 183-200.
- Okoronkwo MO (2000) Detection and enumeration of parasitic eggs in irrigated vegetables and salad crops in Plateau State, Nigeria. Journal of Medical Laboratory Science 9: 30-36.
- Gupta S, Satpati S, Nayek S, Garai D (2010) Effect of wastewater irrigation on vegetables in relation to bioaccumulation of heavy metals and biochemical changes. Environmental Monitory Assess 165(1-4): 169-177.
- Daryani A, Ettehad GH, Sharif M, Ghorbani L, Ziaeni H (2008) Prevalence of Intestinal Parasites in Vegetables consumed in Ardabil, Iran. Food Control 19(8): 790-794.
- Sunil B, Thomas DR, Latha C, Shameem H (2014) Assessment of parasitic contamination of raw vegetables in Mannuthy, Kerala state, India. Veterinary World 7(4): 253-256.
- Arora DR, Brij BA (2010) Introduction to helminths. Med Parasitol 5(3): 119-220.
- Davami M, Mosayyebi M, Mahdavipour A (2000) Prevalence of parasitic infections in consumed vegetables in Ardabil city. J Rah Avarde Danesh 3: 18-22.
- Erdogrul OR, Sener H (2005) The contamination of various vegetables with Enterobius vermicularis, Ascris eggs, Entamoeba histolytica cysts and Giardia. Food control 16(6): 557-560.
- FAO: Food and Agriculture Organization, World Health Organization (2004) The state of food insecurity in the world: Monitory Progress towards the World Food Summit and MDGs. Rome.
- Neva FA, Brown HW (1994) Basic Clinical Parasitology. (6th), Connecticut: Appleton and lange, Norwalk, California, USA, pp. 356-358.
- Neva FA, Brown HW (2008) Basic Clinical Parasitology. (6th), Connecticut: Appleton and lange, Norwalk, California, USA, pp. 356-358.
- Omowaye OS, Falola OO (2012) Prevalence of helminth and protozoa cysts and ova on vegetables sold in middle-belt Nigeria. Cibtech J Bio-protocols 1(1): 37-43.
- Said D (2012) Detection of parasites in commonly consumed raw vegetables. Alexandria J Med 48(4): 345-352.
- World Health Organization (1999) Report of the WHO informal consultation on the use of chemotherapy for the control of morbidity due to soil-transmitted nematodes in humans.
- World Health Organization (2000) Intestinal parasites.
- World Health Organization (1987) Public Health Significance of intestinal parasitic infections. WHO Expert Committee. Bulletin of WHO 65(5): 575-588.
-
Nekpen Osarugue O, Beck A Akhiwu, Isaac Gadzekpo, Onas Ikele. Parasitic Flora of Megaphrynium Macrostachyum Leaves sold in Popular Markets in Benin City, Nigeria. Sci J Biol & Life Sci. 1(4): 2020. SJBLS.MS.ID.000520.
-
Megaphrynium Macrostachyum, Parasitic flora, Microscopically, Intestinal parasites, Proper hygiene, Food-borne parasites, Rhizomes, Seeds, Helminthes eggs
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






