 Research Article
Research Article
p53 Alteration, a Potential Prognostic Indicator for Prostate Cancer from Thai Population
Wilairat Leeanansaksiri1, Hatcha Sriplung2, Yuwadee Klomwises3, Sittisak Honsawek4 and Chavaboon Dechsukhum5*
1School of Preclinics, Institute of Science, Suranaree University of Technology, Nakhon Ratchasima, Thailand
2Department of Epidemiology, Prince of Songkla University, Songkhla, Thailand
3Statistics department, School of science, King Mongkut’s Institute of Technology Ladkrabang, Thailand
4Department of Biochemistry, Chulalongkon University, Thailand
5School of Pathology, Institute of Medicine, Suranaree University of Technology, Nakhon Ratchasima, Thailand
Chavaboon Dechsukhum MD, Ph.D. School of Pathology, Institute of Medicine 111 University Avenue, Suranaree University of Technology, Muang Nakhon Ratchasima 30000, Thailand
Received Date: April 01, 2024; Published Date: May 01, 2024
Abstract
Prostate cancer is the second most common cancer in men worldwide. The management of prostate cancer in complicated due the highly heterogenous nature of molecular mechanism in oncogenesis and tumor progression. Understanding the molecular pathways underlying these processes is critical for the effective management of this cancer. Growth factor receptor signaling pathways especially initiated by EGFR and IGF- 1R play crucial role in growth, differentiation and survival of prostate epithelial cells, therefore dissection of the alterations of the molecules in this pathway received major attention in prostate cancer research. The aim of this study is to compare the protein expression pattern of EGFR and IGF-1R between malignant and benign prostate epithelial cells as well as the impact on the survical of the patients. We performed protein expression analysis on EGFR and IGF-1R by immunohistochemical method on tissue microarray specimens. As p53 mutation is common molecular alteration in prostate cancer, we included p53 mutational analysis in this study. The tissue array blocks were constructed from the archival paraffin embedded tissue obtained from the 128 prostate cancer patients as well as 28 patients diagnosed as benign prostatic hyperplasia (BPH). Univariate and multivariate analyses were used to evaluate the association between the expression status of these genes and the overall survival of the patients. EGFR and IGF-IR were detected in 36.5% and 18.3% of the cancer specimens, respectively. No association between EGFR and IGF-IR expression and cancer mortality was detected. In contrast, p53 nuclear immunoreactivity was detected in 24.2 % of malignant tumor cases and was shown to correlate with the cancer death in both univariate and multivariate analyses (p = 0.0076, 0.027, respectively). This data suggests that p53 mutational status detected by immunohistochemical method in prostate cancer is associated with the poorly differentiated phenotype and can serve as a prognostic indicator for the overall survival of the patients.
Keywords: Prostate cancer; p53; EGFR; IGF_1R; Prognostic indicator
Introduction
Prostate cancer has become a major health problem, as it is the second commonly diagnosed malignancy in men worldwide and the fifth leading cause of cancer death among men worldwide [1]. The incidence of prostate cancer greatly varies up 25 folds between races and geographic populations [2]. However, the mortality rate varies about 10-fold across the countries [3]. Moreover, much larger relative increases in incidence and mortality rates were seen in Asian countries [3]. In Thailand, the nationwide incidence rates of prostate cancer have increased at an average annual percent change of 2.7% over the past two decades [2]. The mean annual ASR increased from 4.9 prostate cancer cases per 100,000 person-years in 1995 to 1999 to 7.1 prostate cancer cases per 100,000 person- years in 2010 to 2012 [4]. However, reports from the Thai National Cancer Institute show regional differences in the incidence of prostate cancer, with a higher incidence rate in southern Thailand compared with the northeast region (ASR, 10.4 v 4.1 prostate cancer cases per 100,000 person-years, respectively) [4]. Therefore, there is a trend of increasing incidence of prostate cancer in the Thai population in some parts of the country, and Thai patients presented at more advanced stages [5].
The incidence of prostate cancer has increased due to sensitive screening procedures. Current estimates suggest that as many as 40% of American men over the age of 50 years have histologically detectable prostate cancer. However, only 8% of these prostate cancer patients will develop clinically significant tumors. Unfortunately, about 10% of newly diagnosed prostate cancer patients already have metastatic lesions. Once metastases develop, the median survival of these patients is limited to 6-12 months [6]. For this reason, establishment of prognostic indicators which can identify a subset of patients with aggressive cancer is warrant. Although prostate cancer is initially hormonally responsive, the subsequent development of hormone refractory phenotype in the majority of prostate cancer patients will limit the efficacy of this therapeutic measure. As prostate cancer becomes the major health problem worldwide, research work that can provide a better insight into the prostate carcinogenesis will be urgently required for the development of better tumor marker and prognostic indicators as well as the more effective treatment of patients.
Analysis of molecular characteristic of prostate cancer indicates that multiple genetic alterations are required for prostate cancer development and progression. This processes likely involves activation of oncogenes and inactivation of tumor suppressor genes. The common alterations include p53 mutation [7-10] Bcl2 overexpression [11-13], androgen receptor (AR) alteration [14- 16], c-Myc amplification [17-19], MMCAI/PTEN down regulation [20,21] and loss of heterozygosity at specific loci. Some of these molecular alterations are associated with the tumor aggressiveness, however further large-scale studies are required to verify their clinical relevance. As the growth-signaling pathway has been shown to be critical in malignant transformation, characterization of the deregulation of this pathway has received major attention. Among the major growth factors and growth factor receptors involved in prostate cancer development are IGF-R1 (insulin-like growth factor receptor 1), EGFR (epidermal growth factor), IGFII (insulin-like growth factor 2), EGF (epidermal growth factor), TGF (transforming growth factor-beta) and FGF (fibroblast growth factor) [22,23]. Autocrine loops involving these growthsignaling pathways have been implicated in the development of prostate cancer and the androgen-independent phenotype.
One of these is the secretion of TGF from the prostate epithelial cells, which can bind to the EGFR as an autocrine fashion [24]. Moreover, the shift from androgen dependent to androgen independent status has been proposed as the result of the use of growth factor signaling pathway as a compensatory mechanism. This hypothesis is supported by the fact that the crosstalk between both pathways actually exists [25-30]. However, further studies to elucidate the molecular mechanism underlying the castration-resistance phenotype and the potential clinical application is awaiting. As growth signalling pathway initiated by the aforementioned growth factor receptors play the crucial role for growth and differentiation of prostate epithelial cells and the alteration of growth signalling pathway is likely involved in the tumorigenesis and progression of the prostate cancer, we propose that expression levels of these growth factor receptor proteins, IGF- 1R and EGFR are altered during the development and progression of the prostate cancer. Therefore, this study aims to analyze levels of protein expression of EGFR and IGF-1R by immunohistochemical study on tissue microarray obtained from both malignant cells and the benign counterparts. Additionally, the potential role of this alteration as the prognostic indicator of prostate cancer was also evaluated.
Moreover, the p53 mutational status as detected by immunohistochemical study was also conducted as the mutational alteration of p53 is one the most frequent molecular change among human cancer including the prostate cancer. As the prostate cancer incidence varies greatly among different ethnic populations and between subpopulations among the same ethnic groups, the molecular information of prostate cancer in Thai population representing the low-risk group of prostate cancer would provide the insight of the molecular pathway underlying the diversity of prostate cancer risk and also the progression and clinical variation of prostate cancer. In this study, we conducted expression analysis for EGFR, IGF-1R and p53 in 128 cases of human primary prostate cancer samples from Thai population and 39 samples of benign counterparts. These results were analyzed in conjunction with patient survival data. Therefore, the prognostic value of the protein expression of these genes can be evaluated.
Materials and Methods
Tissue preparation
The specimens included in this study were the formalin-fixed paraffin embedded prostate tissue obtained from consecutive prostate cancer patients by transurethral resection (TUR-P). All cancer specimens were obtained from Thai patients who were diagnosed and treated at Songklanagarind hospital, Prince of Songkla University, Hatyai, Songkhla, Thailand during 1994-2002. The total number of cancer patients was 128 cases. The age of patients varied from 41-101 yrs (median=72.5). The median follow up time was 27 months (0-130 months). The combined Gleason grade of the prostate cancer varies from 6-10 (the number of cases which had combined Gleason score 6, 7, 8, 9, 10 = 25, 23, 42, 16, 22, respectively). The fraction of prostatic tissue involved by carcinoma varied from 10-100% (mean = 84%). The pretreatment evaluation included serum PSA measurement and bone scan for detection of metastatic disease. Pretreatment serum PSA varied from 2 to 5315 (mean = 263). 56 out of 128 patients (43.6%) had metastatic diseases at the initial diagnosis. Most of the patients came to the hospital due to obstructive uropathy symptom that was likely due to the lack of the screening program during the period of this study. All patients were categorized as an advanced disease according to high serum PSA level and high tumor volume as assessed by TUR-P specimens. The summary of clinicopathological data of cancer patients is shown in Table 1. Benign prostate samples obtained from patients with nodular hyperplasia (28 cases) and from benign counterparts of prostate cancer samples (11 cases) were also included in this study. Thus total number of benign cases was 39. All cancer patients were treated with androgen ablation therapy (bilateral orchidectomy). The research projected was approved by the ethical committee of Prince of Songkla University (institutional review board, IRB).
Table 1:Patients and clinicopathological parameters.
Tissue microarray block construction
The paraffin blocks from each case of prostate cancer patients were used to prepare the tissue array blocks using a tissue microarray precision instrument (Beecher instrument company, MD, USA). The desired areas of tumor or benign tissue were selected by mapping the paraffin block with the corresponding pathological slides, which were stained with hematoxylin and eosin. Recipient wells with a diameter of 1 mm were punched into blank paraffin blocks. Cylinders of the tissue having a diameter of 1 mm were punched from the prostate donor tissue blocks from an area of interest and inserted into each empty 1 mm cylinder of the receipt block. Three tissue cylinders from tumor tissue of the primary Gleason grade were sampled to construct the array. The benign tissue was also sampled in the same way. The tissue sections of 5 m thickness were then prepared from the microarray block which were then subjected to hematoxylin and eosin staining (H&E) and subsequent immunohistochemical analysis. The H&E slides were used to confirm that the areas of interest were actually obtained.
Immunohistochemical staining
The tissue microarray slides were subjected to immunohistochemical study using avidin-biotin horseradish peroxidase complex (ABC) technique with 3,3-diaminobenzidine tetrahydrochloride as the chromogen (Vecta staining kit, Vector Laboratory, Burlingame, California, USA). The procedure was performed according to the manufacturer’s protocol. Briefly, sections (5μm thick) from formalin-embedded tissue microarray blocks were mounted on the silanized slides, and baked overnight at 60˚C. They were then deparaffinized in xylene, rehydrated in a decreasing ethanol series and rinsed in phosphate buffered saline (PBS), pH 7.4. Endogeneous peroxidase was blocked using 0.3% H2O2 in PBS for 5 minutes. The tissue slides were then subjected to antigen retrieval by heating with microwave oven at high and medium-low power for 3 and 10 min respectively in the presence of TE buffer (pH 9.0). The slides were then washed with distilled water, followed by blocking for non-specific binding with 10% normal horse serum. The tissue slides were then incubated overnight at 4˚C with monoclonal mouse antibody against EGFR, clone EGFR1 (Novocasta, Newsastle, UK) or IGF-1R, clone αIR3 (Oncogene Research product, EMD biosciences, Inc., San Diego, CA, USA) or p53, clone PAb 240 (Dako cytomation, Glostrup, Denmark). The dilution of anti-EGFR and anti-IGF-1R antibodies was 1:100 and for anti-p53 antibody was 1:500. The slides were then incubated with a biotinylated rabbit anti-mouse IgG secondary antibody (Vecta staining kit) and an avidin-biotin-peroxidase complex (Vecta staining kit). Visualization of the immunoreaction was achieved using diaminobenzidine tetrahydrochloride. Sections were then counterstained with hematoxylin, dehydrated through graded alcohol, cleared in xylene, and mounted. The membrane staining in the basal layer of benign squamous epitheliums of the skin and benign mammary ductal epitheliums was used as a positive control for the EGFR and IGF-1R analyses, respectively. The positive nuclear staining in esophageal cancer tissue obtained from cancer specimens with previously confirmed to possess p53 mutation by DNA sequencing was used as a positive control in p53 immunohistochemical study.
The immunohistochemical staining interpretation
EGFR and IGF-IR expression was semi quantitatively measured by estimating the fraction of positive cells and the intensity of immunoreactivity. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). Positive staining was further categorized into 2 scores according to the fraction of positive cells (1, <50% positive cells; 2, ≥ 50% positive cells) as recommended by the published article [31]. A total immunoreactivity score was calculated by multiplication of the two values (staining intensity × fraction of positive cells). Therefore, the total immunoreactivity score can be measured as the value of 0, 1, 2, 3, 4, 6. The p53 nuclear immunoreactivity was interpreted as positive if the number of positive nuclear staining was more than 10% of tumor cells as suggested by the published article [32]. The scores of immunoreactivities assigned for each case were the average values of all three microarray spots examined.
Statistical analysis
Pearson’s Chi-square was used to evaluate the possible association between gene expression and the Gleason grade of tumor. The association between p53 nuclear immunoreactivity and tumor grade was also analyzed by the same method. If the expected number in the 2×2 contingency table less than 5, Fisher’s exact test was used. Log-rank test was used to evaluate the possible association between the expression of the genes of interest (EGFR and IGF-IR) and the overall survival of the patients in univariate analysis. The Cox Hazard-regression model including relative risk, probability and 95% confidence interval was used for multivariate analysis of variables.
Results and Discussion
Prevalence of WT1, EGFR, IGF-1R expression and p53 nuclear accumulation in malignant and benign prostatic tissue
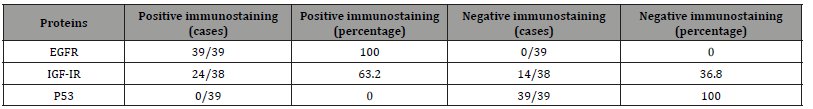
For the EGFR and IGF-IR expression analysis, a total of 126 cases were evaluated. EGFR expression was detected in 46 out of 126 cases (36.5%) and IGF-IR was detected in 23 out of 126 cases (18.3%). The pattern of EGFR and IGF-IR immunoreactivity was found to be predominantly membrane staining with diffuse cytoplasmic staining in a small subset of samples. Diffuse cytoplasmic staining was found in 4 out of 126 samples (2.9%) and 7 out of 126 samples (5.14%) for EGFR and IGF-1R, respectively. The expression of EGFR in benign glands was found exclusively in basal epitheliums with cell membrane staining pattern in all samples (39 cases) whereas expression of IGF-1R was detected only in basal epitheliums with cell membrane staining pattern in 24 out of 38 samples (63.16%). The p53 analysis showed the overall nuclear immunoreactivity of 24.2% (31 out of 128 cases). Moreover, the rate of p53 nuclear immunoreactivity increased in the tumor with higher Gleason grade, in that the tumor with Gleason grade of 3, 4, 5, showed the rate of p53 immunoreactivity of 3.2, 20, and 51%, respectively. The summary of gene expression profile and p53 immunoreactivity result was shown in Tables 2 and 3. The possible correlation between expression of EGFR and IGF-1R and the Gleason grade of the tumor was tested by Pearson’s Chi-square. We used primary Gleason grade (a predominant grade of the tumor) in the analysis as the tumor tissue analyzed was taken from the areas of primary Gleason grade of the tumor tissue. However, no statistically significant correlation between the expression of EGFR and IGF-1R and the histologic grade of the tumors was demonstrated (p = 0.098 and 0.068, respectively). Additionally, no difference in expression score of EGFR and IGF-1R among groups of different Gleason score was observed (Figure 1a, b). Besides this, the possible association between bone metastasis and combined Gleason score was tested, which showed that Gleason score of bone metastasis group was higher than non-metastasis group (Figure 1c).
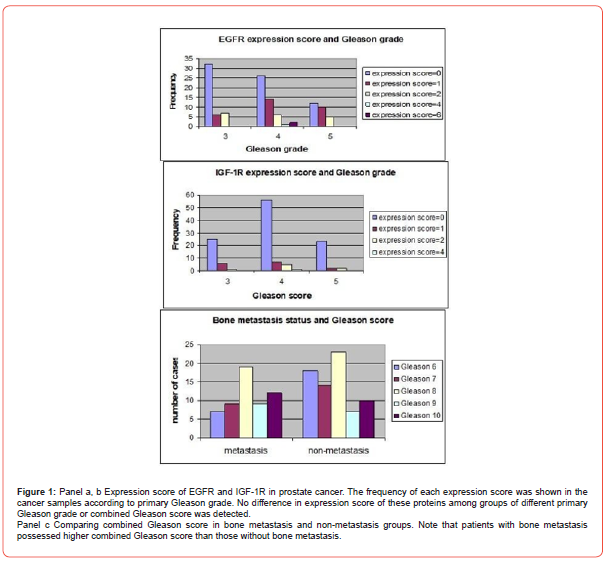
Table 2:Prevalence of EGFR and IGF-1R expression and p53 immunoreactivity in malignant prostatic tissue.
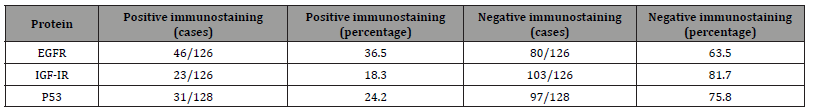
Table 3:Prevalence of EGFR and IGF-1R expression and p53 immunoreactivity in benign prostatic tissue Number of cases and percentage of EGFR, IGF-1R, nuclear p53 immunoreactivity in malignant and benign prostatic tissue was shown.
Prognostic value of EGFR, IGF-1R expression and p53 immunoreactivity
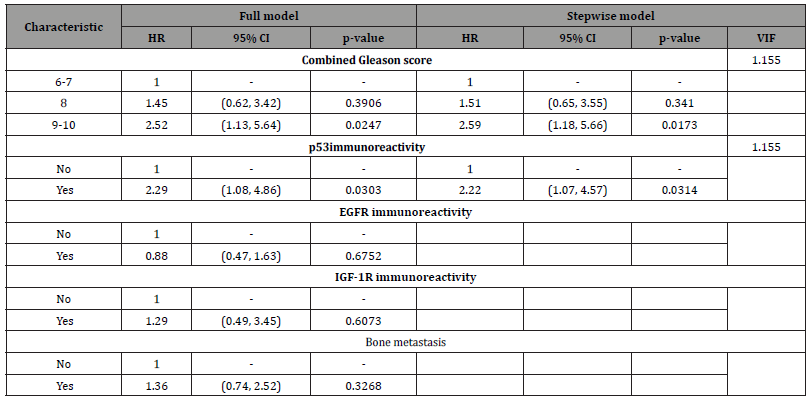
In univariate analysis using log-rank test, EGFR expression was not found to be a significant predictor of cancer mortality of the prostate cancer patients (p= 0.6000), (Figure 2). Next, the relationship between the expression of IGF-1R and overall survival of the patients was evaluated. The result demonstrated that there was no statistically significant association between IGF-IR expression and the outcome of the disease (p= 0.3000), (Figure 3). As p53 nuclear immunoreactivity was demonstrated to be more frequently detected in prostate cancer samples compared with benign tissue and was also associated with worse clinical outcome, the prognostic significance of p53 nuclear immunoreactivity was also determined in Thai prostate cancer patients. Intriguingly, in p53 nuclear immunoreactivity was shown to be a significant prognostic indicator with regard to the overall survival using logrank test (p = 0.0000), (Figure 4). For Gleason score, it was strong evidence that there was no statistically significant association between Gleason score and cancer mortality of the prostate cancer patients (p = 0.0010), (Figure 5). We further evaluated the possible independent prognostic significance of the expression of these proteins and p53 nuclear immunoreactivity in multivariate analysis using Cox Hazard-regression model. The other variables included in this model were combined Gleason score and bone metastasis status. First, full model with no interaction term was developed. Then, the important variables were selected by stepwise procedure. In addition, the inflation in the variances of the parameter estimates were measure by variance inflation factors (VIF). We can conclude that multicollinearity is not exit among the predictors where VIF value is close to 1, (Table 4). Furthermore, proportional hazards assumption of a Hazard-regression model was evaluated, where combined Gleason score (p = 0.29), p53 nuclear immunoreactivity (p = 0.27), and global variables (p = 0.29) indicated that the assumption was not violated. The results showed that EGFR, IGF-1R expression status and bone metastasis stage in prostate cancer cells did not have an impact on cancer-specific mortality (Table 4). However, p53 nuclear immunoreactivity was shown to be associated with increased risk for cancer death (p = 0.0314), (Table 4). Altogether, p53 nuclear immunoreactivity in prostate cancer cells strongly associated with a poor prognostic of prostate cancer with regard to overall survival and may serve as a prognostic indicator or marker to evaluate the progression of the disease. On the other hand, IGF-IR and EGFR expression did not have an impact on the cancer mortality in this patient population.
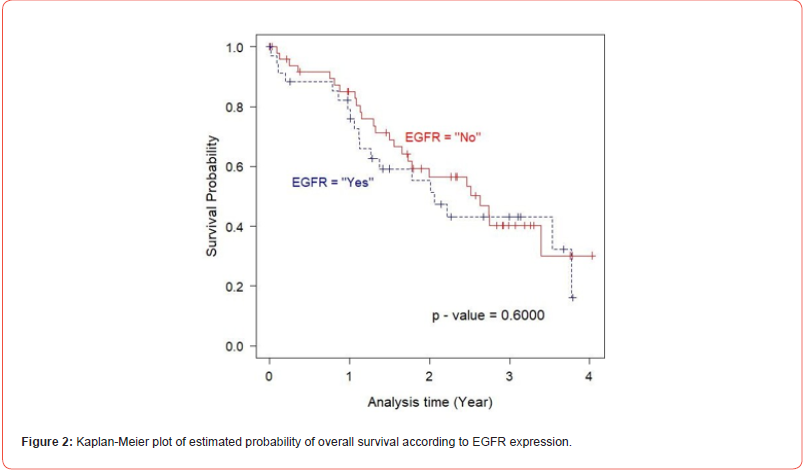
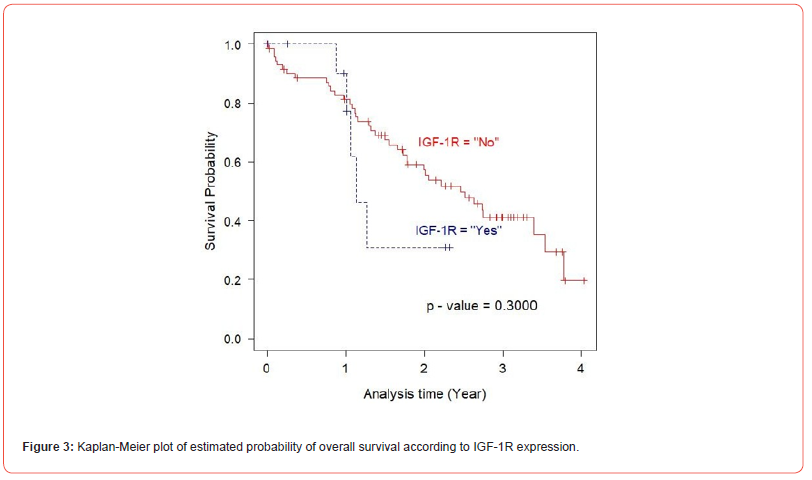
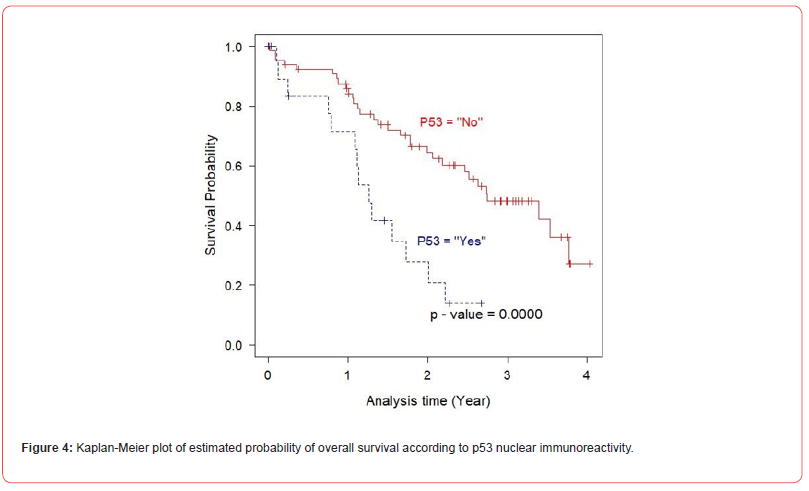
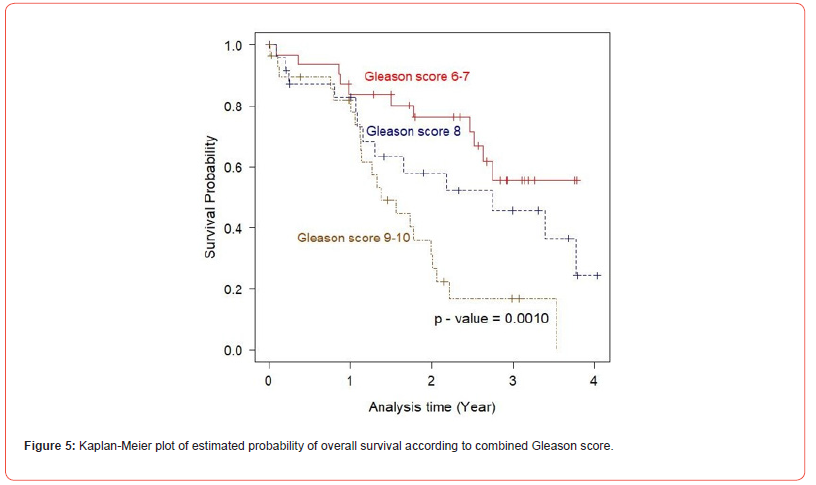
Table 4: Multivariate analysis of potential prognostic factors for overall survival of 128 prostate cancer patients.
P53 immunoreactivity but not EGFR and IGF-1R expression was correlated with the high combined Gleason score
As Gleason score is the well established grading system which indicates the aggressiveness of the disease, the possible association between EGFR, IGF-1R expression and p53 mutation and this histologic parameter was evaluated. We found that neither EGFR nor IGF-1R expression correlated with Gleason grade of the prostate cancer (p= 0.098 and 0.068, respectively). However, the rate of p53 nuclear immunoreactivity was found to be associated with high Gleason grade of the tumor, in that tumors with higher Gleason score possessed more frequent p53 nuclear immunoreactivity (p= 0.001). Tumor samples of Gleason grade 3, 4, 5 showed the prevalence of p53 nuclear immunoreactivity 3.2, 20 and 51%, respectively. This finding concurs with the data from most of previously published articles and confirms a pivotal role of p53 in prostate cancer progression. Additionally, the possible association between p53 nuclear immunoreactivity and bone metastasis status was tested. However, there was no significant difference in the rate of p53 nuclear immunoreactivity between bone metastasis and non-bone metastatic group (23.2 and 25%, respectively).
Gleason score was confirmed to be a good prognostic index for prostate cancer
As Gleason score is one of the widely accepted indicators for aggressiveness of the disease, the prognostic value of this grading system was evaluated in this Thai prostate cancer population. Logrank test for survival analysis was used to evaluate the prognostic significance of this parameter. In this analysis, the patients can be stratified into three groups, Gleason score 6-7, 8 and 9-10, in which high Gleason score group (combined Gleason score > 7) had shorter overall survival than low Gleason score group as shown in Figure 5 (p=0.0002). Additionally, impact of Gleason score and metastasis stage on the patient outcome was also analyzed in the multivariate analysis. Only two variables, high Gleason score (9 or 10) and p53 nuclear immunoreactivity were found to have independent prognostic value with regard to the overall survival of the patients (p = 0.015 and 0.027, respectively), (Table 4). However, no independent prognostic significance of bone metastasis status was observed (p = 0.106).
Discussion
The key findings in this study are discussed in the following points. First, the expression of EGFR and IGF-IR can be detected in a minor subset of malignant samples whereas expression of these proteins detected in the majority of benign samples albeit restricted to the basal epitheliums. As the luminal glandular epithelium was believed to be the one that turns to cancer, the finding in this study would support that EGFR functions as a survival factor in a subset of prostate cancer. This observation also supported the previous study that showed that the level of EGFR expression was associated with tumor extension and loss of differentiation [33,34]. On the other hand, the EGFR expression analysis by immunohistochemical study showed that EGFR protein was decreased in prostate cancer tissue as compared with benign tissue [35,36]. Prognostic significance of EGFR expression was also evaluated by various groups. However, no association between EGFR expression and overall survival of the patients was demonstrated in both univariate and multivariate analyses [37,38]. However, EGFR expression was shown to be an independent indicator for the relapse of prostate cancer by other study [33]. This discrepancy could be explained by the different gene expression analysis techniques used and the heterogeneous nature of samples among these studies. However, this issue has to be addressed by more extensive systematic studies. The observed cytoplasmic staining pattern of EGFR in our study was also demonstrated in other tumor samples. In pancreatic ductal adenocarcinoma, EGFR cytoplasmic staining in tumor cells was shown to be associated with poorly differentiated tumor as well as shorter overall survival of the patients [39]. The study in thyroid carcinoma, increased cytoplasmic localization was shown to be the result of enhanced receptor turnover and accumulation of cytoplasmic degradation product due to the presence of TGFalpha- EGFR autocrine loop [40]. This potential mechanism and clinical relevance of EGFR cytoplasmic localization has to be further investigated in prostate cancer. The IGF-IR expression was detected in a minor subset of prostate cancers in this study (23 out of 126 cases, 18.3%). The staining pattern was predominantly membranous, however diffuse cytoplasmic staining was found in a small subset of cases. In benign tissue, IGF-1R protein was detected in 63.2% of cases (24 out of 38 cases) albeit exclusively in basal epitheliums with membrane staining pattern. In univariate analysis (log-rank test), no association between IGF-1R expression and the disease outcome was detected.
Moreover, there was also no correlation between the expression status of this protein and Gleason grade of the tumor. Additionally, after testing in a multivariate setting, IGF-1R expression was not demonstrated to have a significant impact on the overall survival. This suggests that IGF-1R does not play a critical role in the malignant transformation in the majority of cancer cases and is unlikely be involved in the cancer progression among this studied population. The role of IGF-1R as a survival factor was supported by some previous studies which showed that overexpression of IGF- 1R and its ligand IGF-II was more frequently detected in malignant than in benign prostate tissue [41-43]. In contrast, downregulation of IGF-1R protein and/or mRNA was observed during malignant transformation and metastasis progression [44,45]. This similar trend was also observed in the TRAM model of prostate cancer progression in that IGF-1R mRNA level was lower in metastatic cancer as compared with the primary tumor [46]. This finding was also recapitulated in another model of prostate cancer progression [47]. To further support the biological relevance of this finding, by using the functional study, IGF-1R has been shown to be able to promote cell differentiation [48]. According to this scenario, in the absence of IRS-1, IGF-1R can induce the terminal differentiation via the Shc/mitogen-activated protein kinase kinase pathway. Therefore, downregulation of IGF-1R expression would be required to abrogate this growth inhibitory effect of IGF-1R or the IGF-1R expressing cells will not have selective advantage during tumor progression. According to this scenario, the presence of IGF-1R in some tumor samples may indicate a less aggressive phenotype.
However, the trend for the better survival in the IGF-1R negative group was also observed in this study albeit did not reach statistic significance. More comprehensive gene expression analyses in a large set of samples will be required to test this. The molecular mechanism and the biological relevance of cytoplasmic localization of IGF-1R in some prostate cancer samples is not clear at this standpoint, however this pattern of expression was also observed in the majority of breast cancer samples [49]. The clinical relevance of this finding has to be addressed by further studies in larger sample size.
The analysis for p53 mutation by immunohistochemical study showed that the p53 nuclear immunoreactivity can be detected in 24.2% of cancer cases. Intriguingly, the p53 nuclear immunoreactivity was found to be associated with shorter overall survival of the patients in univariate analysis. Moreover, the prognostic significance of this parameter also continued after testing in the multivariate model. Additionally, p53 nuclear immunoreactivity was also found to be associated with poorly differentiated tumor as defined by Gleason grade. This finding is in line with most of previous research work that found strong correlation between p53 nuclear accumulation and tumor aggressiveness characterized by cancer recurrence, Gleason grade and pathological stage of prostate cancer. However, our study is the first attempt to characterize these molecular alterations and their possible prognostic value in Thai prostate cancer population who is known to possess low risk for prostate cancer. Previous studies showed that lower rate of p53 and k-Ras mutations in prostate cancer obtained from Japanese population [50]. However, the rate of p53 nuclear immunoreactivity in Thai population was found to fall in the range of those found in Caucasian population. The larger series of Thai patient population should be analyzed to confirm this trend.
This study also confirms the prognostic significance of Gleason score on the disease outcome as anticipated.
The combined Gleason score is one of the established prognostic indexes in prostate cancer. In survival analysis (Kaplan- Meier plot), patients with combined Gleason score > 7 had higher mortality rate compared with those with combined Gleason score ≤ 7. In multivariate analysis, combined Gleason score of 9 or 10 (high Gleason score) continued to be an independent prognostic indicator in this patient population. Additionally, bone metastasis status was found to be a significant prognostic indicator for cancer mortality in this patient population after testing in a univariate setting. However, this association disappeared in multivariate model. This finding may imply that patients in non-bone metastasis group have also in advanced stage as reflected by high tumor volume evaluated by TUR-P specimens and high serum PSA level, therefore other prognostic parameters such as Gleason grade or other key molecular alterations like p53 mutation may have greater impact on the overall survival of the patients. This hypothesis was supported by the fact that the association between Gleason score and bone metastasis was observed in this study.
Conclusion
In summary, we found the nuclear immunoreactivity of p53 in highly aggressive prostate cancer cases with high mortality rate. This finding can serve as a significant prognostic indicator with regard to the overall survival of the patients. In addition, this study is the first attempt to detect and evaluate the prognostic significance of these molecular changes in Thai prostate cancer population, a low risk ethnicity of this cancer albeit high mortality rate. In contrast, EGFR and IGF-1R expression was not shown to have a prognostic value in this population. This finding strongly supports the pivotal role of p53 in prostate cancer in both high and low risk populations. The implication of this work is providing a highly potential prognostic method to identify the aggressive cases with high risk for cancer death, who need aggressive therapeutic interventions e.g. radical surgery and to avoid from inappropriate treatment in indolent cancer. In addition, p53 might have a therapeutic value by being a target for immunotherapy and gene therapy for prostate cancer treatment. Therefore, the future experiments should examine whether restoration of wild type p53 expression in these cancer cells will have a favorable therapeutic value.
Acknowledgements
We are grateful to the Prince of Songkla University and Professor Joy L Ware, Virginia Commonwealth University for their support of this study. Funding provided by Collaborative Research Fund, Prince of Songkla University, Thailand.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Statement
This study was carried out in accordance with the recommendations of Ethical Principles and Guidelines for the Use of clinical samples and was approved by institutional ethic committee of Prince of Songkla University, Thailand.
References
- Zhou CK, Check DP, Lortet Tieulent J, Laversanne M, Jemal A, et al. (2016) Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int J Cancer 138(6): 1388-1400.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3): 209-249.
- Banerjee S, Kaviani A (2016) Worldwide prostate cancer epidemiology: Differences between regions, races, and awareness programs. Int J Clin Exp Med Sci 2(1): 1-6.
- Imsamran W, Chaiwerawattana A, Wiagnon S, et al. Cancer in Thailand, 2010-2012 (2015) Bangkok, New Thammada Press, Thailand.
- Alvarez CS, Virani S, Meza R, Rozek LS, Sriplung H, Mondul AM (2018) Current and Future Burden of Prostate Cancer in Songkhla, Thailand: Analysis of Incidence and Mortality Trends From 1990 to 2030. J Glob Oncol 4(1): 1-11.
- Siegel RL, Giaquinto AN, Jemal A. CA (2024) Cancer statistics, 2024. Cancer J Clin 74(1): 12-49.
- Quinn DI, Stricker PD, Kench JG, Grogan J, Haynes AM, et al. (2019) p53 nuclear accumulation as an early indicator of lethal prostate cancer. Br J Cancer 121(7): 578-583.
- Guedes LB, Fawaz Almutairi, Michael C Haffner, Gaurav Rajoria, Zach Liu, et al. (2017) Analytic, Preanalytic, and Clinical Validation of p53 IHC for Detection of TP53 Missense Mutation in Prostate Cancer. Clin Cancer Res 23(16): 4693-4703.
- Strickler HJ, Jay JK, Linder MD, Tamboli D, Amin MB (1996) Determinating prognosis of localized prostate cancer by immunohistochemical detection of mutant p53. Urology 47(3): 366-369.
- Bauer JJ, Sesterhenn IA, Mostofi KF, McLeod DG, Srivastava S, Moul JW (1995) p53 nuclear protein expression is an independent prognostic marker in clinically localized prostate cancer patients undergoing radical prostatectomy. Clin Cancer Res 1(11): 1295-1300.
- Soliman L, De Souza A, Srinivasan P, Danish M, Bertone P, et al. (2021) The Role of BCL- 2 Proteins in the Development of Castration-resistant Prostate Cancer and Emerging Therapeutic Strategies. Am J Clin Oncol 44(7): 374-382.
- Kim JH, Lee H, Shin EA, Kim DH, Choi JB, et al. (2017) Implications of Bcl-2 and its interplay with other molecules and signaling pathways in prostate cancer progression. Expert Opin Ther Targets 21(9): 911-920.
- Kroemer G (1997) The proto-oncogene bcl-2 and its role regulating apoptosis. Nat Med 3(6): 614-620.
- Aurilio G, Cimadamore A, Mazzucchelli R, Lopez Beltran A, Verri E, et al. (2020) Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 9(12): 2653-2658.
- Jamroze A, Chatta G, Tang DG (2021) Androgen receptor (AR) heterogeneity in prostate cancer and therapy resistance. Cancer Lett 518(1): 1-9.
- Giovannucci E, Stampfer MJ, Krithivas K, Brown M, duhlD, et al. (1997) The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci USA 94(7): 3320-3323.
- Buttyan R, Cawczuk IS, Bensod MC (1987) Enhanced expression of c-myc protooncogene in high grade human prostae cancers. Prostate 11(4): 327-337.
- Flaming WH, Hamel A, MacDonald R, Ramsey E, Pettrigrew NM, et al. (1986) Expression of the c-myc protooncogene in human prostatic carcinoma and benign prostatic hyperplasia. Cancer Res 46(3): 1535-1538.
- Matusik RJ, Fleming WH, Hamel A, Westenbrink TG, Hrabarchuk B, et al. (1987) Expression of the c-myc-protooncogene in prostatic tissue. Prog Clin Biol Res 239(1): 91-112.
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, et al. (1997) Frequent inactivation of PTEN/MMCA1 in primary prostate cancer. Cancer Res 57(22): 4997-5000.
- Teng DH, Hu R, Lin H, Davis T, Iliev D, et al. (1997) MMAC1/PTEN mutation in human primary specimens and tumor cell lines. Cancer Res 57(23): 5221-5225.
- Soulitzis N, Karyotis I, Delakas D, Spandidos DA (2006) Expression analysis of peptide growth factors VEGF, FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int J Oncol 29(2): 305-314.
- Kambhampati S, Ray G, Sengupta K, Reddy VP, Banerjee SK, et al. (2005) Growth factors involved in prostate carcinogenesis. Front Biosci 10(1): 1355-1367.
- Cohen DW, Simak R, Fair WR, Melamed J, Scher HI, et al. (1994) Expression of transforming growth factor-alpha and the epidermal growth factor receptor in human prostate tissues. J Urol 152(6 Pt 1): 2120-2124.
- Jenster G (2000) Ligand-independent activation of the androgen receptor in prostate cancer by growth factors and cytokines. J Pathol 191(3): 227-228.
- Nazareth LV, Weigel NL (1996) Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem 271(33): 19900-19907.
- Ye D, Mendelsohn J, Fan Z (1999) Androgen and epidermal growth factor down-regulate cyclin-dependent kinase inhibitor p27Kip1 and costimulate proliferation of MDA PCa 2a and MDA PCa 2b prostate cancer cells. Clin Cancer Res 5(8): 2171-2177.
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, et al. (1999) From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A 96(10): 5458-5463.
- Abreu Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL (1999) Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol 19(7): 5143-5154.
- Mandel A, Larsson P, Sarwar M, Semenas J, Sajid Syed Khaja A, et al. (2018) The interplay between AR, EGF receptor and MMP-9 signaling pathways in invasive prostate cancer. Mol Med 24(1): 34-40.
- Liao Y, Abel U, Grobholz R, Hermani A, Trojan L, Angel P, Mayer D (2005) Up-regulation of insulin-like growth factor axis components in human primary prostate cancer correlates with tumor grade. Hum Pathol 36(11): 1186-1196.
- de la Taille A, Buttyan R, Benson MC, Katz AE (1998) The role of tumor biomarkers as predictors of serum PSA recurrence after radical prostatectomy. Semin Urol Oncol 16(3): 137-144.
- Morris GL, Dodd JG (1990) Epidermal growth factor receptor mRNA levels in human prostatic tumors and cell lines. J Urol 143(6): 1272-1274.
- Di Lorenzo G, Tortora G, D Armiento FP, De Rosa G, Staibano S, et al. (2002) Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 8(11): 3438-3444.
- Mellon K, Thompson S, Charlton RG, Marsh C, Robinson M, et al. (1992) p53, c-erbB-2 and the epidermal growth factor receptor in the benign and malignant prostate. J Urol 147(2): 496-499.
- Ibrahim GK, Kerns BJ, MacDonald JA, Ibrahim SN, Kinney RB, et al. (1993) Differential immunoreactivity of epidermal growth factor receptor in benign, dysplastic and malignant prostatic tissues. J Urol 149(1): 170-173.
- Moul JW, Maygarden SJ, Ware JL, Mohler JL, Maher PD, et al. (1996) Cathepsin D and epidermal growth factor receptor immunohistochemistry does not predict recurrence of prostate cancer in patients undergoing radical prostatectomy. J Urol 155(3): 982-985.
- Shuch B, Mikhail M, Satagopan J, Lee P, Yee H, et al. (2004) Racial disparity of epidermal growth factor receptor expression in prostate cancer. J Clin Oncol 22(23): 4725-4729.
- Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, et al. (2004) The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas 29(1): e1-8.
- Ness GO, Haugen DR, Varhaug JE, Akslen LA, Lillehaug JR (1996) Cytoplasmic localization of EGF receptor in papillary thyroid carcinomas: association with the 150-kDa receptor form. Int J Cancer 65(2): 161-167.
- Kurek R, Tunn UW, Aumueller G, Renneberg H (2000) Insulin-liked growth factor-1 (IGF-1) and insulin-like growth factor receptor (IGF-R1) in prostate cancer. J Urol 163 (Suppl)35: 2000-2010.
- Figueroa JA, De Raad S, Speights VO, Rinehart JJ (2001) Gene expression of insulin-like growth factors and receptors in neoplastic prostate tissues: correlation with clinico-pathological parameters. Cancer Invest 19(1): 28-34.
- Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, et al. (2002) Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res 62(10): 2942-2950.
- Tennant MK, Thrasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, et al. (1996) Protein and messenger ribonucleic acid (mRNA) for the type 1 insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelium. J Clin Endocrinol Metab 81(10): 3774-3782.
- Chott A, Sun Z, Morganstern D, Pan J, Li T, et al. (1999) Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol 155(4):1271-1279.
- Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM (1999) The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res 59(9): 2203-2209.
- Plymate SR, Bae VL, Maddison L, Quinn LS, Ware JL (1997) Reexpression of the type 1 insulin-like growth factor receptor inhibits the malignant phenotype of simian virus 40 T antigen immortalized human prostate epithelial cells. Endocrinology 138(4): 1728-1735.
- Baserga R (2000) The contradictions of the insulin-liked growth factor I receptor. Oncogene 19(49): 5574-5581.
- Happerfield LC, Miles DW, Barnes DM, Thomsen LL, Smith P, et al. (1997) The localization of the insulin- like growth factor receptor 1 (IGFR-1) in benign and malignant breast tissue. J Pathol 183(4): 412-417.
- Inoue T, Segawa T, Shiraishi T, Yoshida T, Toda Y, et al. (2005) Androgen receptor, Ki67, and p53 expression in radical prostatectomy specimens predict treatment failure in Japanese population. Urology 66(2): 332-337.
-
Wilairat Leeanansaksiri, Hatcha Sriplung, Yuwadee Klomwises, Sittisak Honsawek and Chavaboon Dechsukhum*. The p53 Alteration, a Potential Prognostic Indicator for Prostate Cancer from Thai Population. Sci J Biol & Life Sci. 3(4): 2024. SJBLS. MS.ID.000570.
-
Prostate cancer; p53; EGFR; IGF_1R; Prognostic indicator
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






