 Review Article
Review Article
Efficacy, Safety and Perspectives of Convalescent Plasma Therapy for Coronavirus Disease 2019
Joshua Z Yu1 and Timothy D Veenstra2*
1Phillips Exeter Academy, Exeter, NH, USA
2School of Pharmacy, Cedarville University, Cedarville, OH, USA
Timothy D Veenstra, School of Pharmacy, Cedarville University, Cedarville, OH 45314, USA.
Received Date: October 01, 2021; Published Date: October 22, 2021
Abstract
The coronavirus disease 2019 (COVID-19) pandemic, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in over 38 million confirmed cases and over one million deaths worldwide. With few available effective treatments, convalescent plasma (CP) from recovered patients is an inexpensive alternative for COVID-19 treatment. The emergency use of CP for COVID-19 treatment has been authorized by regulatory agencies even while clinical trials are ongoing. Most of the completed clinical trials have included only a small number of patients and have not been randomized. Due to limitations in study design, it has been difficult to draw definitive conclusions on the effectiveness of CP therapy (CPT) for COVID-19. There are various factors that affect the outcomes of CPT, including disease severity of the plasma recipient, pathophysiological status of the recipient, timing of CP administration, and units of CP transfused. While recent evidence demonstrated that early transfusion of CP with high antibody titers leads to improved clinical outcomes and reduced mortality of hospitalized COVID-19 patients, many studies did not measure the antibody levels in the administered CP. As results from existing studies suggest that CPT could be a safe and effective therapy for hospitalized patients with COVID-19, routine measurement of CP antibody titers, along with patient stratification, will be critical in future clinical trials.
Keywords: Convalescent plasma therapy; Severe acute respiratory syndrome; Coronavirus 2; Coronavirus disease 2019; Neutralizing antibodies
Abbreviations: CP: Convalescent plasma; CPT: Convalescent plasma therapy; COVID-19: Coronavirus disease 2019; SARS-CoV-2: Coronavirus 2; FDA: Food and Drug Administration; EUA: Emergency use authorization; MERS: Middle eastern respiratory syndrome; SARS: Severe acute respiratory syndrome; nAbs: Neutralizing antibodies; IND: Investigational new drug; RBD: Receptor binding domain; ADE: Antibody-dependent enhancement; SAE: Serious adverse event; TACO: Transfusion-associated circulatory overload; TRALI: Transfusion-related acute lung injury; ELISA: Enzyme-linked immunosorbent assay
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was first reported in December 2019 [1,2]. “The pandemic has resulted in over 65 million confirmed cases and over 1.5 million deaths worldwide as of December 6, 2021” and before [3]. While most patients have mild symptoms or are asymptomatic, approximately 20-30% of the patients have severe or critically ill diseases that require treatment [4,5]. While no vaccines have been approved for use, some are currently undergoing clinical trials with several of them already at phase III [6]. Without widespread vaccine availability throughout the general population, treatment of infected patients is vital to minimizing death during the current pandemic. Small molecule drugs, antibodies, and convalescent plasma (CP) are among the potential therapeutic options for COVID-19. Off-label drug use is a fast approach for treating COVID-19 since the drugs have previously been approved for use under the labeled clinical conditions. Hydroxychloroquine, azithromycin, and dexamethasone are examples of drugs approved for use in other conditions that are being used for emergent COVID-19 treatment. However, the efficacy and safety of such off-label uses need to be evaluated in clinical studies [7,8]. So far, only remdesivir has been approved as a new drug for the treatment of COVID-19 [9]. Monoclonal antibodies and antibody cocktails for COVID-19 are currently under development [10,11], and a few of them are under phase II and III trials [12]. The U.S. Food and Drug Administration (FDA) granted emergency use authorization (EUA) for the monoclonal antibodies bamlanivimab on November 9, 2021 [13], and casirivimab and imdevimab (in combination) on November 21, 2021 [14] for the treatment of mild to moderate COVID-19 in adults and pediatric patients. As an alternative treatment, COVID-19 CP is obtained from the donors who have recently recovered from SARS-CoV-2 infection. An obvious benefit of CP is it is readily available and inexpensive compared to the cost of developing new drugs for COVID-19.
COVID-19 CP contains antibodies against SARS-CoV-2 antigens, which provides a certain degree of passive immunity when transfused into COVID-19 patients. CP has been used for over a century as a postexposure prophylaxis and for treating infectious diseases. Historically, CP was used to treat influenza and measles. More recently, CP therapy (CPT) was used to treat H1N1, Ebola virus, as well as other coronaviruses such as severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS) [15]. Due to the current pandemic, the US FDA authorized use of CP as an emergency investigational new drug for treating COVID-19 on March 24, 2021 [16]. So far, 170 clinical trials of COVID-19 CP have been registered [17]. In this article, the efficacy and safety of CPT for COVID-19 are discussed with an emphasis on relatively large recent clinical studies.
Mechanism of Action
The potential therapeutic effects of CP for treating or preventing COVID-19 infection may be mediated through multiple mechanisms. The distinct feature of therapeutic CP compared to normal plasma is that it contains polyclonal antibodies against a specific pathogen. In the case of COVID-19, antibodies in the plasma directly bind the virus through antibody-protein interaction to neutralize the virus’s ability to infect cells [18]. In addition to viral neutralization, non-neutralizing antibodies (non-nAbs) may also enhance the therapeutic effect of convalescent plasma [19]. A recent study revealed that SARS-CoV-2-specific antibodies within CP were capable of mediating Fc-dependent functions such as complement activation, phagocytosis, and antibody dependent cellular cytotoxicity [20]. Other components in the plasma that resulted from the donor’s viral immune response may modulate the immune system of the recipients, such as amelioration of cytokine storm via anti-inflammatory cytokines [18,21], thereby providing additional protectionary benefits to SARS-CoV-2 infected recipients.
Criteria for Convalescent Plasma Collection and Treatment
Certain criteria for donor selection, plasma characterization, and eligibility of recipients are required for COVID-19 CPT. A recent article summarized the workflow of CP collection and discussed the practical considerations on the selection of donors and plasma characterization [22]. The policies for use of CPT for COVID-19 and eligibility criteria for donors evolved rapidly as the 2020 pandemic grew. The US FDA issued an EUA on August 23, 2021 [23] and a new guidance on November 16, 2021 [24] to provide recommendations on use of COVID-19 CP for treating hospitalized patients through three pathways: EUA, clinical trials under the traditional investigational new drug (IND) pathway, and expanded access IND (including an intermediate-size population IND or single patient IND) for patients with serious or immediately life-threatening COVID-19 disease who are not eligible or who are unable to participate in randomized clinical trials. Therefore, the recipients of CP must be hospitalized COVID-19 patients. The eligibility criteria for COVID-19 CP donor selection, CP antibody testing, and CP recipients are outlined in Figure 1.
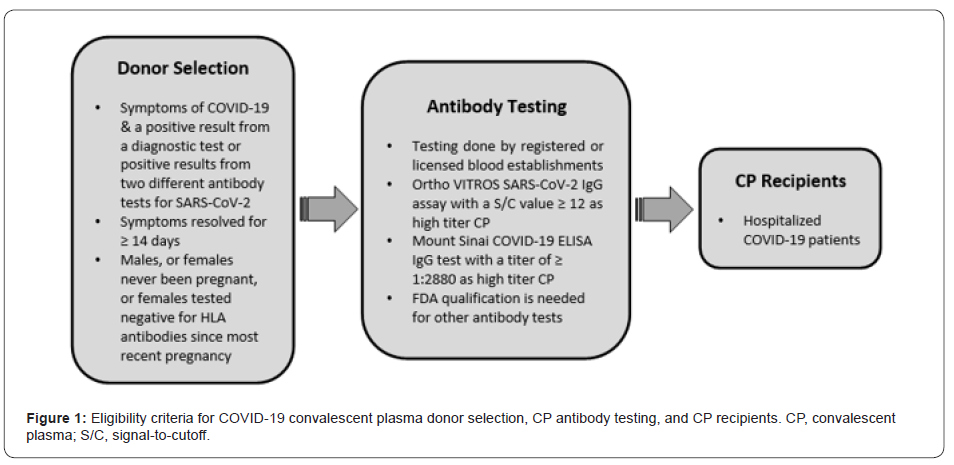
Before an individual is deemed eligible to donate plasma for COVID-19 treatment, standard blood donation requirements must be met. COVID-19 CP must be collected by registered or licensed blood establishments in accordance with applicable regulations, policies, and procedures. Testing for relevant transfusiontransmitted infections must be performed. The US FDA has set the specific qualification for COVID- 19 CP donors. Firstly, individuals who have had COVID-19 symptoms and a positive test result from an FDA approved, cleared, or authorized diagnostic test may donate CP. Individuals who did not have a prior positive diagnostic test and/or never had symptoms of COVID-19 may be qualified to donate if they have had reactive (positive) results in two different FDA-approved, cleared, or authorized tests that detect SARS-CoV-2 antibodies. Secondly, complete resolution of symptoms at least 14 days prior to donation is required. However, a negative COVID-19 diagnostic test result is not necessary to qualify the donor. Thirdly, male donors, female donors who have never been pregnant, or female donors who have been tested negative for HLA antibodies since their most recent pregnancy may also donate CP.
Antibody testing is required to assess the effectiveness of CPT for COVID-19. Titers of neutralizing antibodies (nAbs) may better correlate with treatment outcomes of CPT, however, assays for nAbs (e.g., plaque reduction neutralization test) are often low-throughput, cell-based tests that are not routinely available. Many high throughput quantitative antibody binding assays such as enzyme-linked immunosorbent assays (ELISAs) are available; however, there are questions that remain which specific antibody types within CP correlate with higher treatment efficacy. The reliability of the antibody test is also critical as different assays for the same antibody type (e.g., IgG of anti-receptor binding domain (RBD) of SARS-CoV-2 spike protein) may provide discordant results. A total of 61 SARS-CoV-2 antibody tests have been granted emergency use authorization (EUA) by the FDA as of December 6, 2020 [25]. Most of these measure IgG antibodies, while some test IgM or total antibody. The cPass test (GenScript USA Inc.) is the only one granted EUA for measuring total nAbs.
The FDA has provided EUA guidelines for testing anti-SARSCoV2 antibodies in CP. Firstly, all plasma donations must be tested by registered or licensed blood establishments for anti-SARSCoV- 2 antibodies as a manufacturing step to determine suitability before release. Secondly, blood establishments must test antibodies using either the Ortho VITROS SARS-CoV-2 IgG or the Mount Sinai COVID-19 ELISA IgG antibody test. Using the Ortho VITROS SARS-CoV-2 IgG test, serum samples found to have a signal-tocutoff (S/C) value of 12 or greater qualify as high titer COVID-19 CP, while samples with a S/C value below 12 qualify as low titer. Using the Mount Sinai COVID-19 ELISA IgG antibody test, serum samples found to have a titer of 1:2880 or greater qualify as high titer COVID-19 CP while samples with a titer below 1:2880 qualify as low titer. Thirdly, blood establishments considering the use of a test other than the aforementioned two tests to qualify COVID- 19 CP should contact the FDA to determine the acceptability of the test, which if accepted, would require an amendment to the EUA. In summary, while the FDA recommends two specific antibody assays, it will also consider applications for antibody testing using other tests if the test can provide acceptable, validated results. It should be noted that FDA recommends testing for anti-SARS-CoV-2 antibodies to determine suitability of CP under the IND pathways, but it does not specify the exact antibody tests as used for COVID-19 CP under EUA. Thus, the antibody units that do not qualify as COVID-19 CP under the EUA may qualify for investigational use under an applicable IND.
Efficacy of COVID-19 Treatment
Clinical studies have been evaluating the effectiveness of CP for COVID-19 treatment. Most studies have focused on treatment of hospitalized adult patients with severe or life-threatening disease. However, there have been studies evaluating CP for treatment of pediatric patients with SARS-CoV-2-associated acute respiratory distress syndrome (ARDS). The results from four pediatric patients suggest that CPT is possibly efficacious [26]. Clinical studies of CPT have also been conducted for groups such as cancer patients who were infected with SARS-CoV-2. The study of 24 cancer patients with severe or life-threatening COVID-19 concluded that CPT may be a promising therapy, however, the study did not include a control group [27]. Immunocompromised or B-cell depleted COVID-19 patients many benefits from CPT [28,29] as these patients do not mount an appropriate immune response to viral infections.
Although CPT for COVID-19 appears promising, some studies observed no obvious therapeutic effect. A recent study showed that CPT of 64 hospitalized patients with severe COVID-19 at a median of 7 days after symptom did not improve in-hospital mortality or the overall rate of hospital discharge compared to a matched control group of 177 patients [30]. However, CPT did significantly increase rate of hospital discharge for patients ≥65 years of age. Another study in which CP was administered to 40 severe COVID-19 patients at a median of 10 days after symptom onset and 2.5 days after admission to the intensive care unit, also found that CPT was not associated with clinical benefits in terms of improvement in respiratory support status within 28 days, 28-day all-cause mortality, and viral clearance [31].
These studies revealed that the efficacy of CPT seems to be inconclusive. Many factors potentially confound CPT efficacy for COVID-19, including antibody levels, disease severity of the patient, pathophysiological status of the patient, timing of CP administration, units of CP transfused, and various primary and secondary endpoints of investigation. Some of the studies did not include a proper control group, only focusing on case observations. Furthermore, the CP was not characterized in many of the studies, and the antibody titer was not measured. In addition, the small size of these studies resulted in an underpowered statistical analysis.
A study including 1568 severely or critically ill COVID-19 patients, of which 1430 patients received standard treatment only and 138 received additional CP transfusion showed that CPT reduced the mortality rate by ~50% compared to the standard treatment [32]. Furthermore, the admission rate of non intensive care unit (ICU) patients to ICU was only 2.4% for those receiving CPT compared to 5.1% for the standard treatment group. They also found CP could effectively improve the respiratory symptoms of severe patients but not for critically ill patients. This finding is consistent with other studies that showed that intubated or patients with life-threatening COVID-19 were not likely to benefit from CPT [33,34]. A more recent study analyzed 3,529 patients with COVID-19 pneumonia, of whom 868 received CP containing an IgG antibody titer ≥1:400 [35]. They found that CPT significantly decreased the 28-day mortality (25.5%) compared to patients not receiving CPT (38.0%). However, there was a statistically significant difference (p<0.001) in the ages of patients in the CPT group (i.e., 56 years old) than those not receiving CPT (i.e., 64 years old).
In contrast, there have been studies with relatively large cohorts that concluded CPT had no significant benefits for COVID-19 patients. In a randomized clinical trial of 103 patients with severe or life-threatening COVID-19, no significant difference in time to clinical improvement within 28 days, 28-day mortality, and time to discharge between the 52 patients receiving CPT and the 51 patients in the control group was observed [33]. The study used plasma units with an S-RBD–specific IgG titer of ≥1:640. Among the patients with severe disease, however, 91.3% (21/23) of the CPT group showed time to clinical improvement within 28 days while only 68.2% (15/22) of the control group showed the same progress. In the PLACID trial of patients with moderate COVID- 19, 229 and 235 patients were randomly enrolled in control and CPT arms, respectively [36]. CPT did not reduce 28-day mortality or progression to severe disease overall, even for the 67 patients that received CPT with nAb titers ≥1:80. It should be noted that patients already had median nAb titer of 1:90 at enrollment, which was higher than the median nAb titers of CP from donors (1:40). Additionally, comorbidities, especially diabetes, were higher in the CP transfused study arm. Similarly, the recent ConCOVID trial showed that 79% of patients tested for nAbs already had median titers comparable to the 115 CP donors (1:160 vs 1:160, p=0.40), although they experienced symptoms for only 10 days [37]. This study was prematurely terminated after enrolling 86 patients and found that CPT did not confer benefits in 60-day mortality, hospital stay, or 15-day disease severity. The above studies suggest CP antibody titers, particularly nAb titers, could be a critical factor for effectively treating COVID-19 patients, and suggest higher antibody titers in CP than that in the recipients may be a prerequisite for CPT efficacy.
Clinical studies demonstrated that CP with high antibody titers has a potential positive impact on clinical outcomes including recovery, survival, viral clearance, and disease progression if given to patients early in the course of COVID-19 disease. In a randomized controlled trial for 81 patients using CP units with a nAb titer of >1:80 and a median titer of 1:292, found that none of the patients (n = 38) transfused with CP progressed to a requirement for mechanical ventilation or death, while 14% of the 43 patients in the control group did [38]. Mortality rates at both days 15 and 29 were 0% for the CPT group and 9.3% for the control. A recent clinical study enrolled 35,322 patients with 52.3% in the ICU and 27.5% receiving mechanical ventilation at the time of CP transfusion [39]. The study revealed that both 7-day and 30-day mortality rates in patients transfused within 3 days of COVID-19 diagnosis were significantly lower (8.7%, 21.6%) than in patients transfused ≥4 days after diagnosis (11.9%, 26.7%). It was also observed that transfusion of CP with high IgG antibody levels (>18.45 S/C) was associated with reduced mortality. Although no treatment control group (without CP transfusion) was included in this study, the results demonstrated a relationship between CP admission time and antibody levels with improved clinical outcomes.
A more recent report detailed a 60-day follow up study of 351 hospitalized patients of which 91% of these patients were transfused with CP possessing an antibody titer ≥1:1350 [40]. The median S/C ratio of transfused CP units as measured by Ortho VITROS IgG assay was 24.0; far exceeding the FDA-required cutoff of 12.0 as high titer CP. A very strong positive correlation was observed between the ELISA anti-RBD IgG and the Ortho VITROS IgG tests (R=0.88; P<0.001). Analysis of 60-day mortality of the 341 transfused patients and 594 matched controls confirmed the previous finding that transfusion of COVID-19 patients 72 hours after hospitalization with high titer anti-spike protein RBD IgG CP significantly reduces mortality. The study also identified 44 hours post-hospitalization as an optimal time window for administering high titer CPT. The study was not a randomized controlled trial, thus potentially relevant covariates such as background standard of care over time may not have been completely addressed.
Safety of COVID-19 Treatment
One of the safety concerns with any CP treatment is transfusiontransmitted infections. Transfusion related transmissible infections are very low in the US since transfusion-transmitted pathogens (e.g., HIV, hepatitis B, and hepatitis C viruses) are screened during donor selection process [22]. Although virus screening for SARSCoV- 2 is not mandatory for donor selection, complete resolution of symptoms at least 14 days before the donation is required. In addition, reports showed that only 1% of symptomatic patients had detectable SARS-CoV-2 RNA in their blood [41]. Therefore, transfusion-transmitted infection of the SARS-CoV-2 virus is very low. Considering the recipient is already infected with SARS-CoV-2, the risk of further health risk from transfusion-transmitted SARSCoV- 2 infection is neglectable.
Noninfectious safety risks that are common to any standard plasma transfusions include allergic reactions, transfusionassociated circulatory overload (TACO) and transfusion-related acute lung injury (TRALI). Although the risk of TRALI and TACO are generally low, they are a major cause of transfusion-related mortality since no specific therapies are available. TRALI is manifested by acute hypoxemia and pulmonary edema within six hours of transfusion [42]. The mechanism is thought to be related to neutrophils releasing reactive oxygen species and inflammatory mediators that damage the pulmonary endothelium in response to the CP. HLA antibody screening, as required for female donors with a history pregnancy, potentially reduce the risk of TRALI. Since there are some common risk factors between TACO and COVID-19, careful attention should be paid to management of fluid volume [22].
A potential safety concern specific to COVID-19 CPT is antibody-dependent enhancement (ADE) infection and blunted immune response of the recipient to the infection, which potentially increase the risk of reinfection. ADE has been reported for various viral infections including dengue, Zika, influenza, etc. The main cause of ADE is due to prior infection with a virus of a different serotype [15]. There are two distinct mechanisms of ADE and both are believed to be related to non-nAbs or sub-nAbs [43]. In one mechanism, the Fc portion of the antibody binds to the Fc gamma receptor IIa (FcγRIIa) of the host cell, which enhances viral entry. In the second mechanism, ADE is caused by enhanced immune activation through excessive Fc-mediated effector functions and immune complex formation in an antibody-dependent manner. A recent in vitro study demonstrated CP-enhanced entry of SARSCoV- 2 into immune cells. Viral entry correlated with titer levels of SARS-CoV-2 spike protein-specific antibodies in CP acquired from severely-affected elderly patients [44]. Although in vitro models demonstrated ADE for SARS and COVID-19, there have been no clinical ADE reports using CPT for SARS, MERS, or COVID-19. However, currently there are technical and clinical challenges to differentiate severe viral infection from ADE infection [45].
A recent report provided update data on safety outcomes of CPT for 20,000 severe or life-threatening hospitalized COVID-19 patients [46], including 5,000 hospitalized patients from a previous report [47]. The study reported 141 (<1%) serious adverse events (SAEs) within 4 hours of completion of the COVID-19 CPT. Of these SAEs, 78 were non-mortality events including 36 TACOs, 21 TRALIs, and 21 severe allergic transfusion reactions. The remaining 63 SAEs were mortalities (~0.3% of all transfusions) with 10 of these mortalities related to the COVID-19 CPT. Within 7 days of completion of the COVID-19 CPT, 1247 other SAEs were reported. Of these, 113 were thromboembolic or thrombotic events with 38 of them related to the CPT; 457 were sustained hypotensive events requiring intravenous pressor support with 54 events related to the CPT; and 677 patients suffered a cardiac event with 80 of these related to the CPT. Thus, the vast majority of the thromboembolic or thrombotic complications (n=75) and cardiac events (n=597) were unrelated to the CPT.
The overall 7-day mortality rate was 13.0%, but higher among more critically ill patients. The study concluded that COVID-19 CPT is safe for hospitalized patients and support the hypothesis that earlier administration of plasma within the clinical course of COVID-19 is likely to reduce mortality.
Perspectives
As discussed earlier, the efficacy of CPT mainly relies on nAbs. Many studies did not measure the antibody levels of the transfused CP, which at least partially accounts for the inconsistent outcomes of CPT. Studies revealed that the patients’ immune response is largely heterogeneous to SARS-CoV-2 infection, which results in differential levels of antibodies in the CP [48-50]. Thus, determination of antibody titer using either a viral neutralization assay or a neutralizing antibody surrogate assay prior to transfusion is essential.
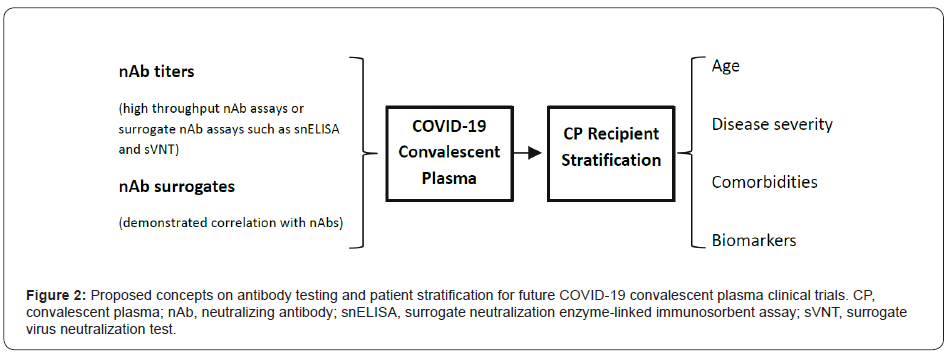
While the conventional viral neutralization assays are cell-based and relatively low throughput, measuring specific antibodies as nAb surrogates is more feasible to meet emergent time requirement for CPT. However, studies demonstrated that antibody titers obtained from binding assays such as ELISAs do not always correlate with nAb titers [51-54]. Novel antibody assays (e.g., UPT POCT assay) that show better correlation with nAb levels are being developed [55]. Novel high throughput nAb assays or surrogate neutralization assays, such as the cell-free neutralization PCR assay, surrogate neutralization ELISA, and surrogate virus neutralization test, are also being currently developed [56-58]. These assays circumvent the use of live virus and cells for nAb analysis, thus significantly increasing the assay’s throughput. It is proposed that the levels of nAbs or nAb surrogates should be evaluated, and correlation between antibody surrogates and nAbs levels should be validated in future clinical studies to enhance treatment efficacy of COVID-19 using CPT as shown in Figure 2.
Research has shown that COVID-19 patients do not uniformly respond to CPT. When CPT patients were classified as responders, partial responders, and non-responders, it was found that responders had significantly higher percentage lymphocyte and lower percentage neutrophil contents in their blood prior to treatment [32]. The levels of C-reactive protein and abnormal metabolic function markers were significantly higher in nonresponders prior to CPT. These results suggest that stratifying COVID-19 patients based on certain biomarkers could improve the overall effectiveness of CPT. Studies also show that CPT provides more benefits for severely, rather than critically ill, COVID-19 patients [32-34] as well as additional benefits for elderly patients [30]. Patient stratification based on age, disease severity, comorbidities, or biomarkers should be considered in future clinical trials that test CPT efficacy (Figure 2).
Current data indicate that the risk of SAEs associated with COVID-19 CPT (e.g., TACO, TRALI, severe allergic reactions, thromboembolic or thrombotic events, and cardiac events) is low. Further research is required to understand the mechanisms of these SAEs and how the recipients respond to CPT. The information would be valuable for discovering solutions to reduce the risk associated with COVID-19 CPT.
It is proposed that future randomized case-control clinical trials that evaluate CPT efficacy for COVID- 19 treatment measure antibody levels and properly stratify patients. In addition, the timing of CPT post-COVID- 19 infection and the number of units of CP should also be recorded.
Conclusion
Convalescent plasma is a promising safe and effective therapy for COVID-19. Without knowing antibody levels, particularly nAbs, data from the past clinical studies have prevented researchers from drawing definitive conclusions on the effectiveness of CPT. While further investigation on the timing of CP transfusion and CP units is important, stratification of CP recipients based on age, disease severity, comorbidities, or biomarkers will be critical for future clinical trials and successful application of CPT of COVID-19.
Acknowledgement
The authors would like to thank Phillips Exeter Academy and Cedarville University for support during the writing of this manuscript.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 382(8): 727-733.
- Phelan AL, Katz R, Gostin LO (2020) The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA 323(8): 709-710.
- WHO Coronavirus (COVID-19).
- Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13): 1239-1242.
- Livingston E, Bucher K (2020) Coronavirus disease 2019 (COVID-19) in Italy. JAMA 323(14): 1335.
- gov (COVID-19).
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. (2021) Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 384(8): 693-704.
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, et al. (2020) Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 383(21): 2041-2052.
- (2020) FDA approves first treatment for COVID-19.
- Baum A, Ajithdoss D, Copin R, Zhou A, Lanza K, et al. (2020) REGN-COV2 antibodies prevent and treat SARS- CoV-2 infection in rhesus macaques and hamsters. Science 370(6520): 1110-1115.
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, et al. (2021) SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med 384: 229-237.
- (2020) Clinicaltrials.gov.
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19.
- Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19.
- Mucha SR, Quraishy N (2020) Convalescent plasma for COVID-19: Promising, not proven. Cleve Clin J Med 87(11): 664-670.
- Coronavirus (COVID-19) Update: Daily Roundup.
- (2000) Clinicaltrials.gov.
- Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, et al. (2020) Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev 19: 102554-102563.
- Gunn BM, Yu WH, Karim MM, Brannan JM, Herbert AS, et al. (2018) A Role for Fc Function in Therapeutic Monoclonal Antibody-Mediated Protection against Ebola Virus. Cell Host Microbe 24(2): 221-233.
- Natarajan H, Crowley AR, Butler SE, Xu S, Weiner JA, et al. (2020) SARS-CoV-2 antibody signatures robustly predict diverse antiviral functions relevant for convalescent plasma therapy. medRxiv.
- Bandopadhyay P, D'Rozario R, Lahiri A, Sarif J, Ray Y, et al. (2021) Nature and dimensions of the cytokine storm and its attenuation by convalescent plasma in severe COVID-19. J Infect Dis 224(4): 565-574.
- Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, et al. (2020) Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 130(6):2757-2765.
- (2020) Hinton DM, FDA, DHHS. Emergency use authorization for COVID-19 convalescent plasma.
- (2020) Investigational COVID-19 convalescent plasma-guidance for industry.
- (2020) In Vitro Diagnostics EUAs.
- Diorio C, Anderson EM, McNerney KO, Goodwin EC, Chase JC, et al. (2020) Convalescent plasma for pediatric patients with SARS-CoV-2-associated acute respiratory distress syndrome. Pediatr Blood Cancer 67(11): e28693.
- Tremblay D, Seah C, Schneider T, Bhalla S, Feld J, et al. (2020) Convalescent plasma for the treatment of severe COVID-19 infection in cancer patients. Cancer Med 9(22): 8571-8578.
- Fung M, Nambiar A, Pandey S, Aldrich JM, Teraoka J, et al. (2020) Treatment of Immunocompromised COVID- 19 patients with Convalescent Plasma. Transpl Infect Dis 23(2): e13477.
- Hueso T, Pouderoux C, Péré H, Beaumont AL, Raillon LA, et al. (2020) Convalescent plasma therapy for B- cell depleted patients with protracted COVID-19 disease. Blood 136(20): 2290-2295.
- Rogers R, Shehadeh F, Mylona EK, Rich J, Neill M, et al. (2020) Convalescent plasma for patients with severe COVID-19: a matched cohort study. Clin Infect Dis 73(1): e208-e214.
- Omrani AS, Zaqout A, Baiou A, Daghfal J, Elkum N, et al. (2021) Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J Med Virol 93(3): 1678-1686.
- Xia X, Li K, Wu L, Wang Z, Zhu M, et al. (2020) Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood 136(6): 755-759.
- Li L, Zhang W, Hu Y, Tong X, Zheng S, et al. (2020) Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA 324(5): 460-470.
- Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, et al. (2020) Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med 26(11): 1708-1713.
- Salazar MR, González SE, Regairaz L, Ferrando NS, Veronica V, et al. (2020) Effect of convalescent plasma on mortality in patients with COVID-19 pneumonia. medRxiv.
- Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, et al. (2020) Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 371: m3939.
- Gharbharan A, Jordans CCE, Geurtsvankessel C, den Hollander JG, Karim F, et al. (2020) Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv.
- Avendano-Sola C, Ramos-Martinez R, Munez-Rubio E, Ruiz-Antoran B, Malo de Molina R, et al. (2020) Convalescent plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv.
- Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. (2020) Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medRxiv.
- Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, et al. (2020) Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol 191(1): 90-107.
- Wang W, Xu Y, Gao R, Lu R, Han K, et al. (2020) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323(18): 1843-1844.
- Gupta A, Karki R, Dandu HR, Dhama K, Bhatt ML, et al. (2020) COVID-19: benefits and risks of passive immunotherapeutics. Hum Vaccin Immunother 16(12): 2963-2972.
- Lee WS, Wheatley AK, Kent SJ, DeKosky BJ (2020) Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 5(10): 1185-1191.
- Fan Wu RY, Mei Liu, Zezhong Liu, Yingdan Wang, Die Luan, et al. (2020) Antibody-dependent enhancement (ADE) of SARS-CoV-2 infection in recovered COVID-19 patients: studies based on cellular and structural biology analysis. medRxiv.
- Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, et al. (2020) A perspective on potential antibody- dependent enhancement of SARS-CoV-2. Nature 584(7821): 353-363.
- Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, et al. (2020) Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc 95(9): 1888-1897.
- Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, et al. (2020) Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 130(9): 4791-4797.
- Madariaga MLL, Guthmiller JJ, Schrantz S, Jansen MO, Christensen C, et al. (2021) Clinical predictors of donor antibody titer and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial. J Intern Med 289(4): 559-573.
- Balcerek J, Trejo E, Levine K, Couey P, Kornberg ZV, et al. (2020) Hospital-based donor recruitment and pre- donation serologic testing for COVID-19 convalescent plasma. Am J Clin Pathol 155(4): 515-521.
- Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, et al. (2020) Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584(7821): 437-442.
- Gniadek TJ, Thiede JM, Matchett WE, Gress AR, Pape KA, et al. (2021) SARS-CoV-2 neutralization and serology testing of COVID-19 convalescent plasma from donors with nonsevere disease. Transfusion 61(1): 17- 23.
- Jungbauer C, Weseslindtner L, Weidner L, Gänsdorfer S, Farcet MR, et al. (2021) Characterization of 100 sequential SARS-CoV-2 convalescent plasma donations. Transfusion 61(1): 12-16.
- Salazar E, Kuchipudi SV, Christensen PA, Eagar T, Yi X, et al. (2020) Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor binding domain IgG correlate with virus neutralization. J Clin Invest 130(12): 6728-6738.
- Wang Y, Zhang L, Sang L, Ye F, Ruan S, et al. (2020) Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 130(10): 5235-5244.
- Zhang P, Li B, Min W, Wang X, Li Z, et al. (2020) Rapid evaluation of neutralizing antibodies in COVID-19 patients. medRxiv.
- Zeng C, Evans JP, Pearson R, Qu P, Zheng YM, et al. (2020) Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers and convalescent plasma donors. JCI Insight 5(22): e143213.
- Abe KT, Li Z, Samson R, Samavarchi-Tehrani P, Valcourt EJ, et al. (2020) A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 5(19): e142362.
- Tan CW, Chia WN, Qin X, Liu P, Chen MI, et al. (2020) A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 38: 1073-1078.
-
Joshua Z Yu, Timothy D Veenstra. Efficacy, Safety and Perspectives of Convalescent Plasma Therapy for Coronavirus Disease 2019. Sci J Biol & Life Sci. 2(1): 2021. SJBLS.MS.ID.000529. DOI: 10.33552/SJBLS.2021.02.000529
-
Convalescent plasma therapy; Severe acute respiratory syndrome, Coronavirus 2, Coronavirus disease 2019, Neutralizing antibodies, COVID-19 treatment, Clinical trials, New drugs, SARS-CoV-2 antigens, COVID-19 patients, Antibody-protein interaction, Viral immune response
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






