 Short Communication
Short Communication
Ecological Risk and Bioremediation of Per- and Polyfluoroalkyl Substances
Stephen G Zemba1,2, Sarah Pope2 and Appala Raju Badireddy3*
1Department of Environmental, Earth & Atmospheric Sciences, University of Massachusetts, Lowell, Massachusetts, USA
2Sanborn Head & Associates, Inc., Concord, New Hampshire, USA
3Department of Civil and Environmental Engineering, The University of Vermont, Burlington, Vermont, USA
Appala Raju Badireddy, Department of Civil and Environmental Engineering, the University of Vermont, Burlington, Vermont, USA.
Received Date: September 07, 2020; Published Date: October 20, 2020
Abstract
Per- and Polyfluoroalkyl substances (PFAS) have become widely recognized as environmental hazards due to concerns over human health effects, but the potential importance of ecological interactions of PFAS are increasingly being recognized. Numerous uncertainties persist, but indications are that PFAS are not likely to be directly toxic to aquatic organisms except in cases of concentrated releases to surface water near heavily contaminated sites. Several so-called “long-chain” PFAS, notably including perfluorooctane sulfonic acid (PFOS), have been found to bioaccumulate in aquatic systems and potentially pose indirect risks to predatory species through food chain exposure. Similarly, terrestrial species with limited feeding ranges may be at risk near concentrated areas of PFAS releases such as sites where PFAS-containing aqueous film-forming foam (AFFF) has been used. The ability of the environment to self-cleanse by breaking down PFAS is questionable, as many stable compounds resist biodegradation. Active efforts are underway to identify specific microbes capable of breaking the strong carbon-fluorine bonds in PFAS in the hope of developing bioremediation technologies for in situ applications. Not many PFAS have been studied, however, and significant data gaps exist regarding the toxicity, bioconcentration, and biodegradation of compounds such as PFOS that have been investigated. Substantial research is needed to understand the risks that PFAS present to the environment and to promote degradation of these persistent compounds.
Keywords: PFAS; PFOA; PFOS; Perfluorinated substances; Ecological risk, Bioremediation
Introduction
Per- and polyfluoroalkyl substances (PFAS) have become widely recognized as environmental hazards due to concerns over human health effects, with many governmental agencies establishing low ng/L (nanogram per liter or part-per-trillion) standards for drinking water. In commerce for seven decades and widely applied in industry and consumer products, PFAS have distributed and reached even remote locations. PFAS effects on the wider environment are increasingly being recognized as PFAS have been detected in even remote locations. In this paper, two factors relevant to the biological sciences are examined with an emphasis toward research needs. First, potential risks to biota are evaluated. Second, the feasibility of biodegrading these recalcitrant compounds is considered.
Part of the difficulty in addressing PFAS lies in the definition of scope, as there are literally thousands of different compounds that differ in structure and complexity. Generally, though, PFAS comprise two parts – a chain of carbon-fluorine (C-F) bonds that repels both water and fats, and a functionalized group that provides solubility and surfactant behavior. The broad variety of PFAS nevertheless displays wide-ranging properties with respect to environmental systems. Research initially focused on two compounds – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – that received extensive use in commerce until the production ceased in the United States in the early 2000s. PFAS that replaced PFOA and PFOS generally have shorter C-F chains and/or are not fluorine saturated, allowing for environmental degradation (though in some cases to other stable PFAS). Considering that there is an increase in ecological risk worldwide caused by the spread of PFAS via environmental pathways, and considering the main characteristics of these persistent compounds, it is reasonable to call these compounds as contaminants of emerging concern.
Discussion
Ecological Risks of PFAS
Though safe drinking water is the regulatory focus at present, the need to assess hazards to organisms other than humans is recognized. The ECOTOX database provides 12,645 and 6,812 entries for aquatic and terrestrial species, respectively, when the search is specified to include all PFAS [1]. When the search is narrowed to include only PFOS and PFOA (acid, ionic, and salt forms), ECOTOX provides 6,931 and 2,914 entries for aquatic and terrestrial species, respectively, or roughly half of the total PFAS entries. The number of toxicity tests in daphnia, fathead minnows, and other aquatic species for PFOS and PFOA is adequate to develop ambient water quality criteria, and Table 1 summarizes some values issued or proposed by regulatory agencies.
Table 1: Summary of Ambient Water Quality Criteria for PFOA and PFOS..
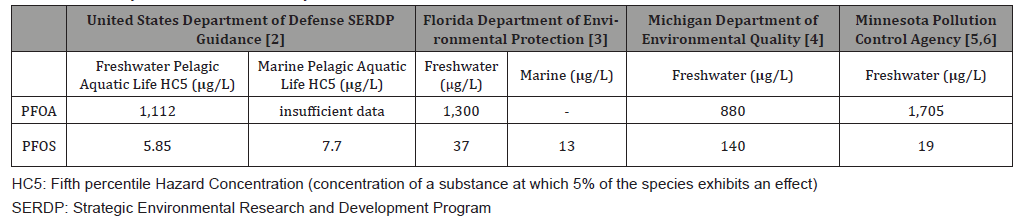
In general, the aquatic criteria are 2-3 orders of magnitude greater than drinking water standards for PFOS and 5-6 orders of magnitude greater for PFOA. Per our findings, only locations near substantial PFAS releases such as military bases that used aqueous fire-fighting foams (AFFF) have PFAS concentrations in surface water comparable to or greater than these criteria. Hence, for the general environment that receives PFAS from diffuse sources such as atmospheric deposition, PFAS are not likely to be directly toxic to aquatic organisms.
PFAS such as PFOA and PFOS adhere to and accumulate in soils. A framework for ecological risk assessment of PFAS [2] has been developed to assess potential risks in terrestrial environments, and receptors such as small birds and mammals with limited feeding ranges are likely at greatest risk in areas where PFAS concentrations are elevated in soil (such as former firefighting training areas). These soils also act as potential continuing release sources to groundwater and surface water.
Aquatic food web exposure may be important for PFOS, as a recent review recommends a water-to-fish bioconcentration factor of 1,100 liter per kilogram (L/kg). Several other so-called “longchain” compounds – PFDA (450 L/kg), PFUnDA (2,700 L/kg), PFDoDA (18,000 L/kg), PFTrDA (21,627 L/kg), PFTeDA (23,000 L/ kg), and PFDS (2,630 L/kg) – also have demonstrated tendencies to bioaccumulate in fish [2]. Top-of-the-food chain predatory species, such as fish-eating mammals and raptors, may be at greatest risk
Further research is necessary, however, to develop robust estimates of toxicity reference values (TRVs) for wildlife and biotransfer factors to relate PFAS flows from environmental media through the food chain. TRVs for PFOS and PFOA for terrestrial organisms are limited in scope, and the relevance of toxicity testing in laboratory animals such as rodents to other animals is not clear given wide-ranging half-lives and metabolism among species. Avian TRVs are lacking for even PFOS and PFOA, and information on TRVs for other PFAS is either scant or non-existent. Potential transformation of PFAS precursors into stable compounds is also a possible factor. As anecdotal food for thought, the highest recorded PFAS concentrations in loon eggs were found near a former military installation with PFAS contamination, but the understanding of PFAS uptake by loons and transfer to their eggs is lacking [7].
Bioremediation of PFAS
A second area of research interest is the potential biodegradation of stable PFAS compounds. Initial observations suggested the strength of the C-F bonds rendered biodegradation of PFAS by bacteria impossible, but recent research suggests otherwise. Stepwise microbial defluorination has been observed for fluorotelomeric structures with CH2 groups on the C-F backbone rather than perfluorinated substances [8]. Fluorotelomeric alcohols including carboxylic and sulfonic acids are all found to be partially biotransformed into perfluoroalkyl acids under both aerobic and anaerobic conditions. However, the responsible microorganisms and enzymes and underlying mechanisms are still unclear.
There is a great scientific and practical interest to know whether microbes can breakdown perfluorinated compounds in diverse environmental media. Although reductive defluorination of perfluorinated compounds is biologically feasible according to the thermodynamic calculations [8], to date there is no convincing evidence reported for microbial cleavage of the C-F bond in the PFAS compounds, in terms of release of fluoride ions and formation of transformation products under environmental conditions. However, thermodynamic calculations indicate that energy released from reductive defluorination of fluorinated compounds can support growth of microorganisms catalyzing the reaction. The energy is estimated to be in the range of 80 and 160 kJ/mol fluoride, assuming equimolar concentrations of polyfluorinated and perfluorinated compounds and environmentally realistic conditions (pH 7; F- = 0.1 mM; and H2 = 10 Pa) [9]. Therefore, it is reasonable to predict that these PFAS compounds should be biodegradable under anaerobic conditions. However, there are critical questions about the microorganisms catalyzing defluorination that still remain unanswered. For instance, how fast microorganisms will evolve that are able to benefit from the energy released during defluorination reaction, and from where and how they should recruit the enzymatic machinery necessary to catalyze this reaction and harness the energy produced. It is expected that microorganisms that thrive on anaerobic processes should be able to degrade perfluorinated compounds under anaerobic conditions [9].
A recent culture enrichment study has shown that the Pseudomonas parafulva have the capability to utilize PFOA as the sole carbon source via defluorination reactions and their defluorination efficiency increased from 32% to 48% in the presence of glucose [10]. The activated sludge microorganisms in a sewage treatment plant have been shown to degrade PFOA under anaerobic conditions, but the degradation efficiency was low [11,12]. Furthermore, compositional analysis of microbial communities isolated from 40 PFAS-contaminated groundwater samples revealed that PFAS had a negligible impact (p > 0.05) on the taxonomic homogeneity of the overall community including Burkholderiales, Methylophiales, Xanthomonadales and Pseudomonadales [13]. This study also showed that the positive and negative correlations between individual PFAS and bacterial communities could provide important insights related to development of biodegradation technologies for PFAS impacted sites. For instance, the Oxalobacteraceae family has been shown to be relatively more abundant in areas with higher PFAS concentrations. This family is generally more tolerant to environmental stresses. While the Desulfococcaceae family have shown significant positive associations with C4-C7 PFAS, no association with PFOS or PFOA was observed. These findings suggest that C4-C7 could be a potential carbon source for this family via defluorination reactions. More recently it has been shown that the Acidimicrobium (Acidimicrobiales family), in the presence of ferric ions, ammonium, and anthraquinone-2,6-disulfonate or hydrogen under well-controlled anaerobic conditions, is also capable of degrading both PFOS and PFOA [14]. Furthermore, the Acidimicrobium and another bacterium in the EB1017 family in PFAS-contaminated groundwater showed a positive correlation with PFOA but not the other PFAS, which is unsurprising because the conditions are not well-controlled in the field settings [13]. These results suggest that at low PFOA concentrations the biostimulation is possible under environmental conditions.
Fungal degradation of PFAS is also drawing increased attention due to the wide spectrum of substrate utilization catalyzed by extracellular ligninolytic enzymes [15]. White-rot fungus Phanerochaete chrysosporium under aerobic conditions has been able to biotransform 45% of 6:2 fluorotelomer alcohols into shorter-chain metabolites such as perfluorobutyric acids and perfluoropentanoic acids. Enzyme-catalyzed oxidative coupling reactions have been shown to degrade PFOS and PFOA at higher levels in aqueous systems relative to soils suggesting that PFAS adsorption to soil particles may affect the removal efficiencies [17].
There is an urgent need for establishing fundamental understanding of the microorganisms, genetics, and biochemistry involved in the cleavage of C-F and C-S bonds under aerobic and anaerobic conditions. Further research must be conducted to fully understand the biodegradation and transformation of PFAS in the environmental media. This type of research could provide important clues in the development of cost-effective, biologically mediated remediation technologies as well as the impact of PFAS to the environment.
Conclusion
PFAS are ubiquitous in the environment, and recent concerns over effects on human health have led questions over potential risks to biota. Some PFAS bioaccumulate, making food chain exposures to predatory species a potential threat. Ecological risks of PFAS are exacerbated by their environmental persistence. Natural degradation processes of stable PFAS are not significant, but engineered biodegradation processes show some promise. Substantial research is needed to understand the risks that PFAS present to the environment and to promote degradation of these persistent compounds.
Acknowledgements
This work was supported by the University of Vermont Office of Vice President (OVPR) REACH grant and in-kind services from Sanborn, Head & Associates, Inc.
Conflict of Interest
No competing financial interests exist.
References
- United States Environmental Protection Agency (2020). ECO Toxicology Knowledgebase (ECOTOX).
- Conder J, Arblaster J, Larson E, Brown J, Higgins C (2019) Guidance for Assessing the Ecological Risks of PFASs to Threatened and Endangered Species at Aqueous Film Forming Foam-Impacted Sites. United States Department of Defense Strategic Environmental Research and Development Program (SERDP Project ER18-1614).
- Stuchal L, Roberts S (2019) Development of Surface Water Screening Levels for PFOA and PFOS Based on the Protection of Human Health. Center for Environmental & Human Toxicology, University of Florida. p. 9.
- Michigan Department of Environment, Great Lakes, and Energy (EGLE, formerly known as Michigan Department of Environmental Quality or MDEQ), Water Resources Division (2015). Rule 57 Water Quality Values (Aquatic Life).
- Stevens J, Coryell A (2007) Surface Water Quality Criterion for Perfluorooctanoic Acid. Minnesota Pollution Control Agency (STS Project No. 200604796).
- Stevens J, Coryell A (2007) Surface Water Quality Criterion for Perfluorooctane Sulfuric Acid. Minnesota Pollution Control Agency (STS Project No. 200604796).
- Edwardson K, Ali J, Diers T (2019) Plan to Generate PFAS Surface Water Quality Standards. New Hampshire Department of Environmental Services.
- Liu J, Mejia Avendaño S (2013) Microbial degradation of polyfluoroalkyl chemicals in the environment: A review. Environ Int 61: 98-114.
- Parsons JR, Sáez M, Dolfing J, de Voogt P in Reviews of Environmental Contamination and Toxicology Vol 196 (ed David M. Whitacre) 53-71 (Springer US, 2008).
- Bossert I, Haggblom MM, Young LY in Environmental Dehalogenation (eds M.M. Häggblom & I.D. Bossert) 33-52 (Kluwer, Dordrecht, 2003).
- Yi LB, Chai LY, Xie Y, Peng QJ, Peng QZ (2016) Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet Mol Res 15(2).
- O'Carroll DM, Nick Battye, David J Patch, Michael J Manefield, Kela P Weber, et al. (2020) Developing a roadmap to determine per- and polyfluoroalkyl substances-microbial population interactions. Sci Total Environ 712: 135994.
- Huang S, Jaffé PR (2019) Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ Sci Technol 53(19): 11410-11419.
- Colosi LM, Pinto RA, Huang Q, Weber WJ (2009) Peroxidase-mediated degradation of perfluorooctanoic acid. Environ Toxicol Chem 28(2): 264-271.
-
Stephen G Zemba, Sarah Pope, Appala Raju Badireddy. Ecological Risk and Bioremediation of Per- and Polyfluorinated Substances. Sci J Biol & Life Sci. 1(3): 2020. SJBLS.MS.ID.000514.
-
Hippodamia, Consequences, Predator, Aphid, Polyploidy, Plant Biomass, Soil nutrients, polyploidization, Herbivore, Negatively, Aphid-predators, Nigrotuberculatum, Alignment, Hexaploid, Herbivores
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






