 Research Article
Research Article
The First Sign of the Complication of Angiosperm-Related Ecosystem
Gang Han1,2, Haichun Zhang3, Lijun Zhang2, Lingji Li4 and Xin Wang3*
1Hainan Vocational University of Science and Technology 571126, China
2Hainan Tropical Ocean University, Sanya 572022, China
3State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China
4Ziguang Shi Yan School, Weihai 264411, China
Xin Wang, State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China
Received Date: April 18, 2024; Published Date: May 02, 2024
Abstract
Angiosperms and insects are two most diverse groups of macroscopic organisms, and their relationship plays an important role in the current ecosystem. An angiosperm is usually attacked by multiple insects in the current ecosystem, which is a proxy of the complication of the ecosystem. However, such a complicate relationship appears lacking in the ecosystem for early angiosperms, which are usually attacked by only one type of insects. Therefore, when the complicate angiosperm-related ecosystem emerged is an important unanswered question. Here we document a new platanaceous species, Arthollia dayangshuensis gen. et sp. nov from the Nenjiang Formation (late Santonian-early Campanian, Late Cretaceous) with three different kinds of damages, suggesting that the ecological relationship between angiosperms and insects was already complicate in the Santonian. This surprising discovery implies that angiosperm-related ecosystem has already existed in the Late Cretaceous. To this date, this is the first sign of such a complicating process.
Keywords: Insects; angiosperms; ecosystem; late cretaceous; China
Introduction
As the top two most diverse groups of macroscopic organisms in the current world, angiosperms and insects as well as their mutual interplays define the complexity the Earth ecosystem. Although the interactions between insects and plants have been dated back to the Palaeozoic [1], and the interaction between angiosperms and insects has been documented at least back to the beginning of the Late Cretaceous [2], the interplay between insects and angiosperms remains simple and monotonous [2], unlike in the complicate current ecosystem. When and how the ecosystem became complex are important questions that demand answers. Here we report a new angiosperm fossil that signifies the first complication in angiosperms-related ecosystem by its multiple types of damage on a single leaf. We hope this fossil and ensuing studies will open a window on the complicating process of the current angiospermscentered ecosystem.
Materials and Methods
The Nenjiang Formation is widely distributed in northeastern China, and previous works as well as recent isotopic dating results suggest that the age of the formation is late Santonian-early Campanian [3], a conclusion favored by the latest palynological analysis [4]. The associated estherian species, Halysestheria yui (Chang) Li, places the plant-fossil-bearing strata within the first member of the Nenjiang Formation (83.4-85.7 Ma) [5,6]. Angiosperm pollen in the palynoflora include Retitricolpites sp., Liquidambarpollenites sp., Borealipollis yaojianica, B. songliaoensis, B. sp., Callistopollenites tumidoporus, Lythraites giganteus, Consoliduspollenites songliaoensis, Pentapollenites asymmetricus, and Euphorbiacites majorporus [4].
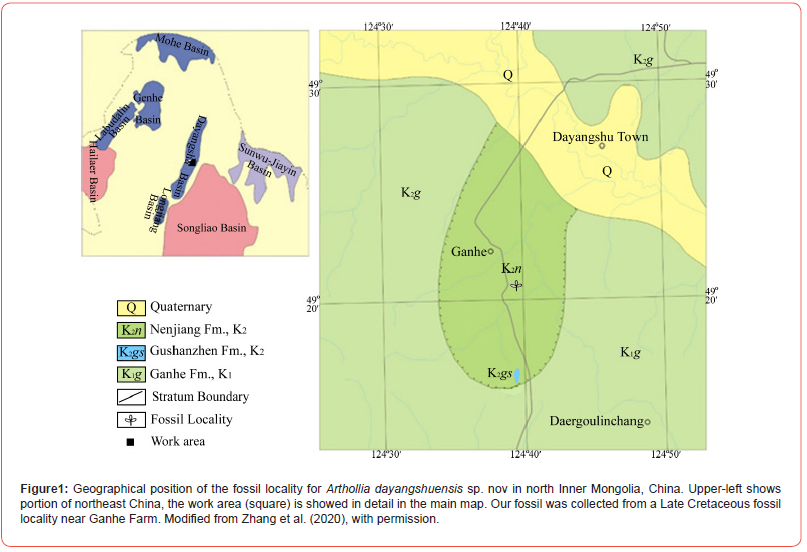
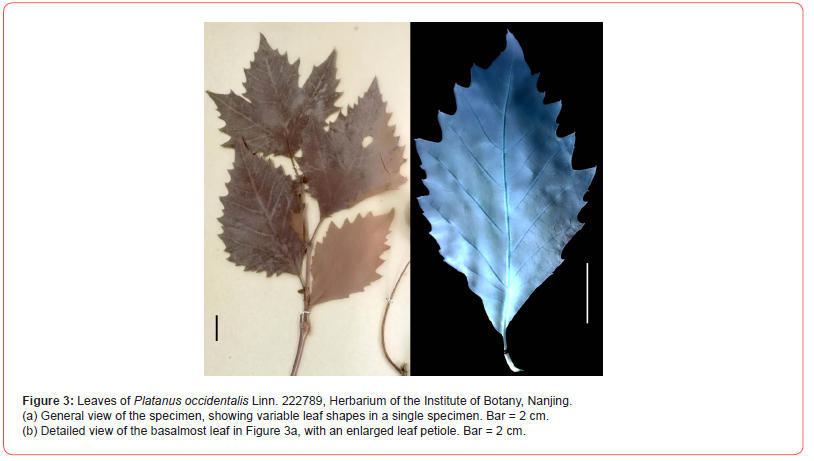
The specimen of Arthollia dayangshuensis sp. nov is a leaf impression, 93 mm long and 48 mm wide, preserved on a yellowish gray siltstone slab. The slab was recovered from an outcrop of the Nenjiang Formation near the 4th Team, Ganhe Farm, Hulunbeier, Inner Mongolia, China (N49°20′36.39″,E124°39′32.69″, Figure 1), with default permit from Ganhe Farm. The age of the formation is 83.4-85.7 Ma [3]. The specimen was photographed using a Nikon D300S digital camera, a Nikon SMZ1500 stereomicroscope with a Nikon DS-Fi1 digital camera. All figures were organized using a Photoshop 7.0 software. The specimen (ZZB-P001) is deposited in the Natural History Museum, Weihai Ziguang Shi Yan School. For comparison, leaves of Platanus occidentalis Linn. (Voucher ID 222789) deposited in the Herbarium of the Institute of Botany, Nanjing were photographed. The study complies with relevant institutional, national, and international guidelines and legislation.
Results
Arthollia dayangshuensis sp. nov. (Figure 2)
Specific diagnosis
Leaf simple. Petiole basally enlarged. Lamina elliptic in shape, coarsely serrate, widest in middle. Leaf base obtuse-decurrent, and leaf apex missing. Venation pinnate, with 4 orders of veins. Midrib rigid, percurrent. Five pairs of secondary veins opposite, craspedodromous, parallel, forming an angle of 30-35° with midrib, tapering distally, rarely furcating near lamina margin, running to teeth on lamina margins. Each basalmost secondary vein giving rise to up to 4 exmedial veins. Tertiary veins percurrent, alternate, almost perpendicular to secondary veins. Quaternary veins perpendicular to tertiary veins, forming polygonal meshes. Simple veinlet in some areoles.
Description
The leaf is simple, approximately 93 mm long and 48 mm wide, including a petiole and an almost completely preserved lamina (Figure 2a). The petiole is 20 mm long, basally 3.3 mm wide, tapering slightly into only 1.3 mm at the base of the lamina, slightly enlarged at the base (Figures 2a, 2b, 2e). The lamina is 73 mm long and 48 mm wide, elliptic in shape, coarsely serrate, widest in the middle (Figures 2a, 2g, 2h). The leaf base is obtuse-decurrent, while the leaf apex is missing (Figures 2a, 2g). The venation is pinnate, with 4 orders of veins (Figures 2a, 2c, 2f-2i). The midrib runs almost percurrent, rigid, 1.2 mm wide near the base, slightly tapering and curving (Figure 2a). Five pairs of secondary veins are opposite, craspedodromous, parallel, forming an angle of 30-35° with the midrib, 0.3-0.6 mm wide, tapering distally, rarely furcating near the lamina margin, running to teeth on the lamina margins (Figures 2a, 2c, 2h). Each of the basalmost secondary veins gives rise to up to 4 exmedial veins, each of which joins branches of superadjacent exmedial vein to form loops before reaching to the leaf margin (Figure 2a). The tertiary veins are alternately arranged on and almost perpendicular to the secondary veins, forming a scalariform pattern, 75-380μm wide, connecting the neighboring secondary and thus forming meshes 0.3-0.9 x 0.4-1.3 mm (Figures 2a, 2c), while some tertiary veins may connect the secondary with the tertiary and forming acute angles (Fig. 2f). The quaternary veins are orthogonally reticulate, almost perpendicular to the tertiary veins, forming polygonal meshes (Figures 2a, 2c, 2i). Simple veinlets are observed in some areoles (Figures 2c, 2i).

There are three types of insect damages (Figures 2a, 2c, 2d, 2g, 2i). The first type is hole feeding (DT 57) [7] with the trapped trace at the divergence of the secondary vein from the midvein (Figures 2a, d). The second type is margin feeding (DT12, DT14) [7] (Figure 2g). DT12 is characterized by the circular, shallow to deep excisions from the leaf margin and with no more than 180 degrees of arc (Figure 2g), and DT14 by the excision of leaf to the midvein (Figures 2a, d) [7]. The third type is skeletonization (DT16) (5), in which all the tissues except vascular bundles are consumed and reaction rim is poorly developed (Figures 2a, c, i). The skeletonization is situated in the central region of left half-lamina and is in direct contact with the midvein and major secondary veins (Figures. 2a, c, i).
Remarks
The new species is very similar to Arthollia insignis (Plate 3, Figures 1-5 [8]) in terms of the venation and leaf shape, but differs from the latter in strictly opposite arrangement of the secondary veins. This difference, in addition to its geographical distribution, justifies a new species, A. dayangshuensis sp. nov.
Etymology
dayangshu-, for Dayangshu, the name of the basin from which the fossil was collected.
Holotype: ZZB-P001 (Figure 2a).
Type locality:
Ganhe Farm, Hulunbeier, Inner Mongolia, China (N49°20′36.39″ ,E124°39′32.69″).
Stratigraphic horizon:
The Nenjiang Formation, Santonian-early Campanian, Upper Cretaceous (83.4-85.7 Ma).
Depository
Natural History Museum, Weihai Ziguang Shi Yan School.
Discussions
The basal position of the Platanaceae in Eudicots makes the family very important in the systematics of Eudicots [9-12]. The extant Platanaceae includes only one genus, Platanus, with 7-9 species scattered in the temperate to tropical regions of North America, Europe as well as southeastern Asia [12,13]. The diversity of the family used to be much greater than today [14-17]. Their fossil record starts from the Early Cretaceous, and is widely distributed in Greenland, Europe, North America, and Asia [10,11,13,15-35]. The oldest record goes back to the Aptian [36], but the records become abundant since the Albian [9,17,22,23,29-33,35,37]. The ubiquitous presence of the Platanaceae in the Early-Middle Cretaceous floras is in line with its basal position in Eudicots. Sometimes platanaceous leaves may constitute the dominant species in some megafloras of the Dakota Formation [35].
The new fossil leaf reported here is comparable to Arthollia, a platanaceous fossil leaf genus recognized in northeast Russia [8] in general leaf shape and venation. The leaf margins of extant Platanus species vary from entire to serrate [15]. Arthollia dayangshuensis is similar to the basal leaf of extant Platanus (especially P. occidentalis., Figure 3) in its unlobed lamina, general leaf shape, straight secondary veins almost reaching lamina margin, regular tertiary veins forming meshes, and enlarged petiole base, but differs in coarsely serrate lamina margin and poorly-developed teeth, and thus different from well-toothed Platanus of North America, Greenland, and Asia [15]. The enlarged petiole base is a feature shared among Arthollia dayangshuensis, P. fraxinifolia and P. neptuni [15], suggestive of the presence of intrapetiolar axillary bud in these taxa. This feature is a proxy of deciduous habit and seasonal climate [35]. Fossil leaves of nymphaealean affinity (in progress) associated with Arthollia dayangshuensis suggest that Arthollia dayangshuensis may be a tree living in a niche not far away from water body, which may be common in this region at that time, a landscape quite different from the current prairie.
The latest palynological study indicated that angiosperms played a minor role in the Nenjiang Flora, accounting for less than 1% of diversity in the palynoflora [4]. This is in stark contrast to the frequent occurrence of angiosperm leaves in the megaflora, on which further investigation is on-going. The occurrence of Arthollia dayangshuensis in north China, plus previous reports of platanaceous fossil records in USA, China, Russia, and Greenland, favors the circumboreal dispersal of the family, just like the Hamamelidae, proposed by Kvacek et al. [15]. The congeneric occurrence of Arthollia in north China and northeast Russia implies the geographical and ecological similarities shared between these regions. Three types of insect damages are recognized in this single specimen. The insect damages are hole feeding, margin feeding, and skeletonization (Figures 2a, 2c, 2d, 2g, 2i). These feeding damages are all due to external foliage feedings caused by mandibulate insects [7]. The hole feeding and margin feeding generally remove tissues and/or veins (Figure 2g), unlike skeletonization which is reluctant to chew the vein with nutritional barriers, such as lowness in nutrition (Figure 2c) [38-40], structure in sclerosis [39,41]. The most diverse leaf-chewing insects are mainly Coleoptera (beetles), but other major groups include Orthoptera, Phasmida, and Lepidoptera [42]. Skeletonization tends to be formed by holometabolous insects such as Coleoptera, Hymenoptera, Diptera, and Lepidoptera [39,43-45]. Due to the non-distinctive and nonrecognizable damage pattern of external feedings, and considering no insect records in the Nenjiang Formation of Inner Mongolia, China, it is difficult to figure out the specific feeders now.
The varied damage types (Figures 2c, 2d, 2g) suggest that there were at least two chewing mouthpart types and at least three different insects were feeding on the leaf of Arthollia dayangshuensis. Checking out the previous record of insect damage on angiosperm leaves [2], it is clear that there is only one type of insect damage on each leaf, suggesting either that there is already host specificity at the beginning of the Late Cretaceous, or the ecological relationship between angiosperms and insects is primary and not diversified yet. The contrast between the present fossil material and previous record indicates that, at least by the Late Santonian, Arthollia (Platanaceae, Eudicots, Angiosperms) already has become the food resources for three different insects. This indicates that the Santonian ecosystem is already more complicated than that of the early angiosperms in term of food web and energy flow.
Conclusion
Arthollia dayangshuensis is a new fossil leaf recovered from the Nenjiang Formation (late Santonian-early Campanian) of Inner Mongolia. This fossil, together with previous data, adds evidence on the palaeogeography, history as well as ecology of the Platanaceae during the Late Cretaceous. The relationship between fossil angiosperms and insects has become complicated by the end of the Santonian, suggesting that, far before the eve of the Cenozoic, the angiosperms-related palaeoecosystem had started its complication and reached certain complexity.
Acknowledgement
We appreciate Dr. Gang Li for identifying the associated estherian specimens, Dr. Xiaoqin Sun and Dr. Xiaoyu Dong for access to the specimens of Platanus occidentalis Linn. in Herbarium of the Institute of Botany, Nanjing., Ms Hui Jiang and Dr. Torsten Wappler for discussing the insect damages on the leaf, and Dr. Hongshan Wang for help with this manuscript. This research is supported by the Strategic Priority Research Program (B) of Chinese Academy of Sciences (XDPB26000000) and the National Natural Science Foundation of China (42288201) awarded to XW, and the Key Discipline Construction Program of Hainan Provincial Department of Education in 2017-Marine geology as well as the Research to Start Project 2018- Hainan Tropical Ocean University RHDXB201802 awarded to GH.
References
- Labandeira C (1998) Early history of arthropod and vascular plant association. Annual Review of Earth and Planetary Sciences 26(1): 329-377.
- Labandeira CC, Dilcher DL, Davis DR, Wagner DL (1994) Ninety-seven million years of angiosperm-insect association: Paleobiological insights into the meaning of coevolution. Proceedings of the National Academy of Sciences, United States 91(25): 12278-12282.
- Zhang LJ, Han G, Chu H, Wang X (2020) On the age of angiosperm bearing strata in Ganhe region in middle of the Dayangshu Basin. Journal of Stratigraphy 44(1): 326-336.
- Sun L, Wang C, Bian X (2020) A new palynological assemblage from the Nenjiang Formation of Dayangshu Basin, and its Geological Implication. Acta Geologica Sinica (English edition) 94(1): 198-199.
- Li G (2005) Halysestheria yui from the Nenjiangian Taipinglinchang Formation of Heilongjiang Province, China. Acta Geologica Sinica (English edition) 44(1): 322-324.
- Xi D, Wan X, Li G, Li G (2019) Cretaceous integrative stratigraphy and timescale of China. Science China Earth Sciences 62(1): 256-286.
- Labandeira CC, Wilf P, Johnson KR, Marsh F (2007) Guide to insect (and other) damage types on compressed plant fossils. (Smithsonian Institution, Washington, DC) Version 3.0. 25.
- Herman AB, Golovneva, LB (1988) A new genus of the Late Cretaceous cycamore-like plants from the north-east of the USSR. Bot Zh 73(2): 1456-1467.
- Crane P, Donoghue MJ, Doyle JA, Friis EM (1989) Angiosperm origins. Nature 342: 131.
- Magallόn Puebla S, Herendeen, PS, Crane PR (1997) Quadriplatanus georgianus gen. et sp. nov.: staminate and pistillate platanaceous flowers from the late Cretaceous (Coniacian-Santonian) of Georgia, U.S.A. International Journal of Plant Sciences 158(3): 373-394.
- Magallón S, Crane PR, Herendeen PS (1999) Phylogenetic pattern, diversity, and diversification of Eudicots. Annals Missouri Botanical Garden 86(2): 297-372.
- Judd WS, Campbell SC, Kellogg EA, Stevens PF (1999) Plant systematics: a phylogenetic approach. Sinauer Associate Inc., Sunderland, MA.
- Manchester SR (1986) Vegetative and reproductive structure of an extinct plane tree (Platanaceae) from the Eocene of western North America. Botanical Gazette 147(2): 200-226.
- Crane PR (1988) Origin and evolution of gymnosperms (ed CB Beck) Columbia University Press 5(1): 218-272.
- Kvacek Z, Manchester SR, Guo SX (2001) Trifoliolate leaves of Platanus bella (Heer) comb. N. from the Paleocene of North America, Greenland, and Asia and their relationships among extinct and extant Platanaceae. International Journal of Plant Sciences 162(2): 441-458.
- Kvacek Z, Manchester SR (2004) Vegetative and reproductive structure of the extinct Platanus neptuni from the Tertiary of Europe and relationships within the Platanaceae. Plant Systematics AND Evolution 244(1): 1-29.
- Wang X (2008) Mesofossils with platanaceous affinity from the Dakota Formation (Cretaceous) in Kansas, USA. Palaeoworld 17(3-4): 246-252.
- Lesquereux L (1892) The flora of the Dakota Group. Washington DC, USA.
- Kvacek Z (1970) A new Platanus from the Bohemian Tertiary. Palaeontol. Abhandl. B 3(1): 435-439.
- Dilcher DL, Eriksen L (1983) Sycamores are ancient trees. The Museum of Western Colorado Quarterly Spring pp. 8-11.
- Knobloch E, Mai DH (1986) Monographie der Früchte und Samen in der Kreide von Mitteleuropa. Rozpravy Ustredniho Ustavu Geologickeho.
- Friis EM, Crane PR, Pedersen KR (1988) Reproductive structures of Cretaceous Platanaceae. Der Kongelige Danske Videnskabernes Selskab, Biologiske Skrifter 31(1): 1-32.
- Friis EM, Eklund H, Pedersen KR, Crane PR (1994) Virginanthus calycanthoides gen. et sp. nov ---- a calycanthaceous flower from the Potomac Group (early Cretaceous) of eastern North America. International Journal of Plant Sciences 155(2): 772-785.
- Crane PR (1989) Paleobotanical evidence on the early radiation of nonmagnoliid dicotyledons. Plant Systematics AND Evolution 162(1/4): 165-191.
- Herendeen PS (1991) Charcoalified angiosperm wood from the Cretaceous of eastern North America and Europe. Review of Palaeobotany and Palynology 70(3): 225-229.
- Pigg KB, Stockey, RA (1991) Platanaceous plants from the Paleocene of Alberta, Canada. Review of Palaeobotany and Palynology 70(1-2): 125-146.
- McIver EE, Basinger JF (1993) Flora of the Ravenscrag Formation (Paleocene), Southwestern Saskatchewan, Canada. Paläontographica Canadian 10(1): 1-167.
- Pedersen KJ, Friis EM, Crane PR, Drinnan AN (1994) Reproductive structures of an extinct platanoid from the early Cretaceous (latest Albian) of eastern North America. Review of Palaeobotany and Palynology 80(3-4): 291-303.
- Crane PR, Herendeen PS (1996) Cretaceous floras containing angiosperm flowers and fruits from eastern North America. Review of Palaeobotany and Palynology 90(3-4): 319-337.
- Crane PR, Friis, EM, Pedersen KR (1994) Palaeobotanical evidence on the early radiation of magnoliid angiosperms. Plant Systematics AND Evolution 8(1): 51-72.
- Crane PR, Friis EM, Pedersen KR (1995) The origin and early diversification of angiosperms. Nature 374(6517): 27-33.
- Crane PR, Manchester SR, Dilcher DL (1988) Morphology and phylogenetic significance of the angiosperm Platanites hebridicus from the Palaeocene of Scotland. Palaeontology 31(2): 503-517.
- Crane PR, Pedersen KR, Friis EM, Drinnan AN (1993) Early Cretaceous (early to middle Albian) platanoid inflorescences associated with Sapindopsis leaves from the Potomac Group of eastern North America. Systematic Botany 18(2): 328-344.
- Manchester SR (1999) Biogeographical relationships of North American Tertiary Floras. Annals Missouri Botanical Garden 86(2): 472-522.
- Wang H (2002) Diversity of angiosperm leaf megafossils from the Dakota Formation (Cenomanian, Cretaceous), North Western Interior, Ph. D thesis, University of Florida, USA.
- Hickey LJ, Doyle JA (1977) Early Cretaceous fossil evidence for angiosperm evolution. The Botanical Review 43(1): 3-104.
- Dilcher DL (1979) Early angiosperm reproduction: an introductory report. Review of Palaeobotany and Palynology 27(3-4): 291-328.
- Mattson Jr WJ (1980) Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics 11(1): 119-161.
- Scheirs J, Vandevyvere I, De Bruyn L (1997) Influence of monocotyl leaf anatomy on the feeding pattern of a grass-mining agromyzid (Diptera). Annals of the Entomological Society of America 90(1): 646-654.
- Strong D, Lawton J, Southwood T (1984) Insects on plants: community patterns and mechanisms. Harvard University Press, Cambridge, MA, United States.
- Esau K (1965) Vascular differentiation in plants. Holt, Rinehart and Winston, New York.
- Mónica R Carvalho, Peter Wilf, Héctor Barrios, Donald M Windsor, Ellen D, et al. (2014) Insect leaf-chewing damage tracks herbivore richness in modern and ancient forests. PloS one 9(5): e94950-e94959.
- Hohn F, Wagner D (2000) Larval substrates of herminiine noctuids (Lepidoptera): macrodecomposers of temperate leaf litter. Environmental Entomology 29(2): 207-212.
- Shade RE, Wilson MC (1967) Leaf-vein spacing as a factor affecting larval feeding behavior of the cereal leaf beetle, Oulema melanopus (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America 60(3): 493-496.
- Feng Z, Schneider JW, Labandeira CC, Kretzschmar R, Rößler R (2014) A specialized feeding habit of Early Permian oribatid mites. Palaeogeography Palaeoclimatology Palaeoecology 417(1): 121-125.
-
Gang Han, Haichun Zhang, Lijun Zhang, Lingji Li and Xin Wang*. The First Sign of the Complication of Angiosperm-Related Ecosystem. Sci J Biol & Life Sci. 3(5): 2024. SJBLS.MS.ID.000571.
-
Insects; angiosperms; ecosystem; late cretaceous; China
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






