 Research Article
Research Article
Invitro Evaluation of Antimicrobial Activity of Aqueous Extracts of Reishi Mushroom (Ganoderma Lucidum) Against a Select Gram Positive and Negative Bacteria
Akamu J Ewunkem*, Ilunga Tshimanga, Biruktawit Samson, Brittany Justice and Dinesh K Singh
Department of Biological Sciences, Winston Salem State University, North Carolina, USA
Akamu J Ewunkem, Department of Biological Sciences, Winston Salem State University, 601 S. Martin Luther King Jr. Drive Winston-Salem, NC 27110, North Carolina, USA
Received Date: February 20, 2024; Published Date: March 05, 2024
Abstract
Reishi mushroom (Ganoderma lucidum) are important medicinal mushrooms distributed worldwide. In this context, the present study evaluates antimicrobial effects of aqueous extract of reishi mushroom against several Gram positive and Gram-negative bacteria using micro dilution method. The antimicrobial activity of water extract of reishi extract was first observed as early as 3h of incubation. The antibacterial activity of the extract increased linearly with the increasing concentration of extracts. The results revealed that Gram-positive bacteria were more sensitive to reishi mushrooms than Gram-negative bacteria. The HPLC analysis of the aqueous extract of reishi revealed the presence of beta [1-3] glucans, ganoderic acid and triterpenoids that may have contributed to the antimicrobial activity. Overall results suggest that aqueous extract of reishi mushroom has considerable antimicrobial potential against gram positive bacteria.
Keywords: Mushroom; antimicrobial; reishi; bioactive compounds
Introduction
Microbial infections are the second leading cause of death in humans as well as farm animals worldwide. In human population, infectious diseases account for one in eight global deaths [1,2]. In the war against microbes, specifically bacteria, antibiotics have been a choice therapy against infections for a very long time [3-6]. However, the alarming increase in antibiotic resistance has forced us to look for some alternative antimicrobial agents. Numerous alternatives are being tried/developed to overcome antimicrobial resistance. Reishi mushrooms have been highly rated among medici nal mushrooms in traditional Chines/Japanese medicine for a long time. The bioactive constituents/compounds found in mushrooms are being tried and tested treating cancers, alleviating side effects of chemotherapy and radio therapy [7-11]. Ganoderma lucidum (Curtis) P. Karst commonly known as “Reishi” mushroom, is one of the most famous medicinal mushrooms that has a long history of use for promoting health and longevity in Asian countries. Reishi is an excellent source of diverse phytochemicals which have shown effectiveness in the prevention and treatment of Alzheimer disease, cancer, diabetes, and many other conditions [12-14]. Only a few reports have evaluated antimicrobial activities of G. lucidum using solvents like alcohol, water, hexane, polysaccharide, and chloroform [15-18]. These reports have provided important information about the functional concentrations of extracts. These reports however recorded antimicrobial activity of Reishi extracts at 18–24-hours of incubation. We were interested to evaluate concentrations of G. lucidum extract that will exhibit the fastest antimicrobial activity following treatment alongside its effectiveness against select microbial population. We were also interested in studying bioactive agents of G. lucidum responsible for antibacterial activities. This is with a view to evaluate efficacy of medicinal herbs in comparison to the antibiotics which may accelerate the emergence and spread of antibiotic-resistant bacteria.
Materials and Methods
Collection of Mushroom and Identification
The fresh fruiting bodies of Ganoderma lucidum mushrooms named reishi were collected from natural growth in Winston Salem, North Carolina, USA. The identification of G. lucidum was performed by the authors according to the methods of classical herbarium taxonomy; the micro- and macro morphology of collected specimens were compared to standard descriptions in the taxonomic monographs.
Preparation of water extracts
Dried fruiting bodies of reishi mushrooms were grounded in a grinder into a fine powder prior to extraction. Five grams of the powdered samples were extracted with water (50 mL) at 30 °C at constant shaking of 150 rpm for 24 h. The extracts were centrifuged at 3000 rpm, 4 °C for 15 min to give a clear supernatant that was filtered through Whatman filter paper number 4. The clear filtrate was concentrated by evaporation under vacuum, then the crude extract was stored at 4 °C for further use.
Tested microorganisms and growth conditions
Gram-Positive bacteria (such as Staphylococcus aureus, Bacillus megaterium and Micrococcus luteus) and Gram-negative bacteria (such as Escherichia coli, Klebsiella pneumoniae and Salmonella typhi) were used in this study. The bacteria were obtained from the Department of Biological Sciences, Winston Salem State University. Each bacterial strain was grown from stock culture by streaking them on nutrient agar (NA) and incubated at 37 °C for 16 to 18 hours (overnight). Then, a single colony of each bacterium was cultured overnight in Nutrient broth (NB) at 37 °C. The grown cultures were used for the antimicrobial assay.
In vitro antimicrobial activity assay of mushroom’ extracts
Antimicrobial activities of water extract of reishi against Gram-positive pathogenic bacteria strains (S. aureus, B. megaterium and M. luteus) and Gram-negative pathogenic bacteria strains (E. coli, K. pneumoniae and S. typhi) were determined by establishing the minimum inhibitory concentration (MIC) of the extracts using micro dilution method in triplicate using 96-well plates. Test samples were loaded in rows with respective concentrations 0%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80% ,90% and 100% of aqueous extract of reishi mushroom. The 96-well plates were incubated at 37 °C, 1160 rpm for 24h. Bacterial growth was assessed by measuring turbidity at 600 nm for hours 0, 3, 12, and 24, using a GloMax®-Multi Microplate (Promega, USA).
High Performance Liquid Chromatography (HPLC) System
HPLC was carried out at Chemistry Laboratory in Greensboro North Carolina using Agilent 1100 series with a C18 column (4.6 mm × 250 mm i.d., 5 μm) at 35 °C to identify and quantify the bioactive compound present in reishi mushroom as it displayed the highest antimicrobial activity. The mobile phase consisted of HPLC water (A) and acetonitrile (B) at a flow rate of 1 ml/min. The multi-wavelength detector was monitored at 280 nm.
Statistical Analysis
All analyses were carried out in triplicates and the data are expressed as mean ± standard deviation (SD). GraphPad Prism software was used for statistical analyses. Statistical analysis was performed using the 2-tailed, independent t-test. Statistical significance was set at P < 0.05.
Results
Antibacterial Activity
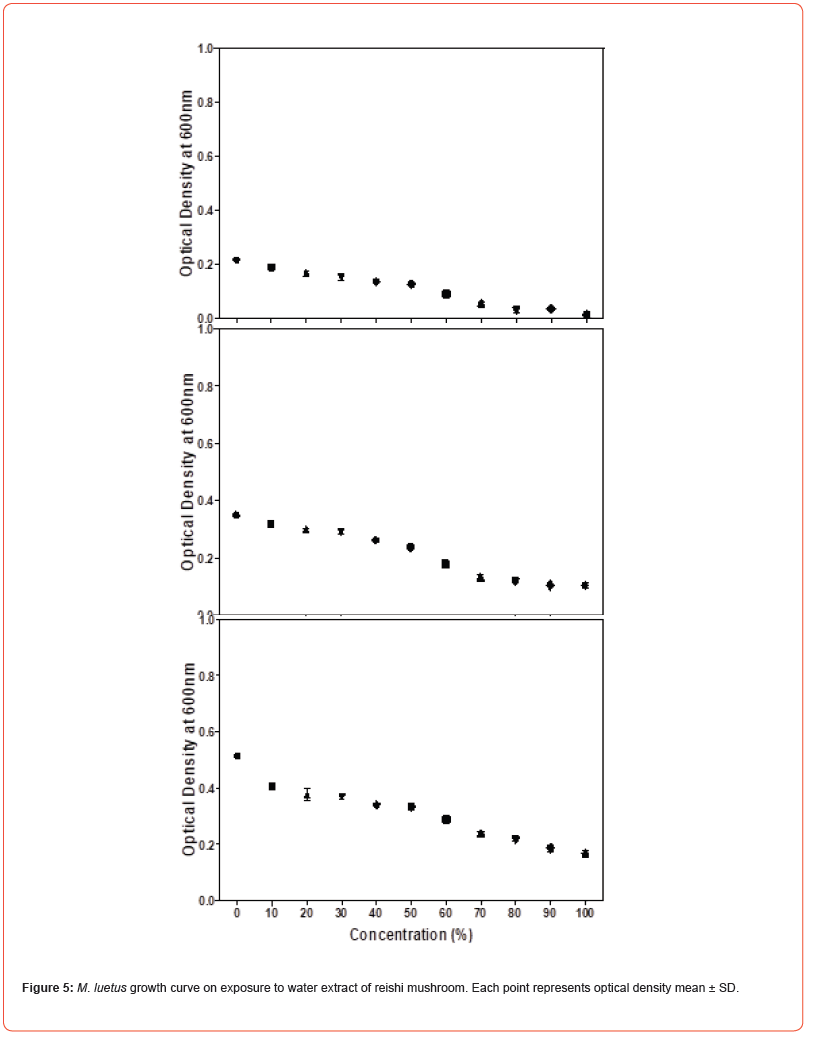
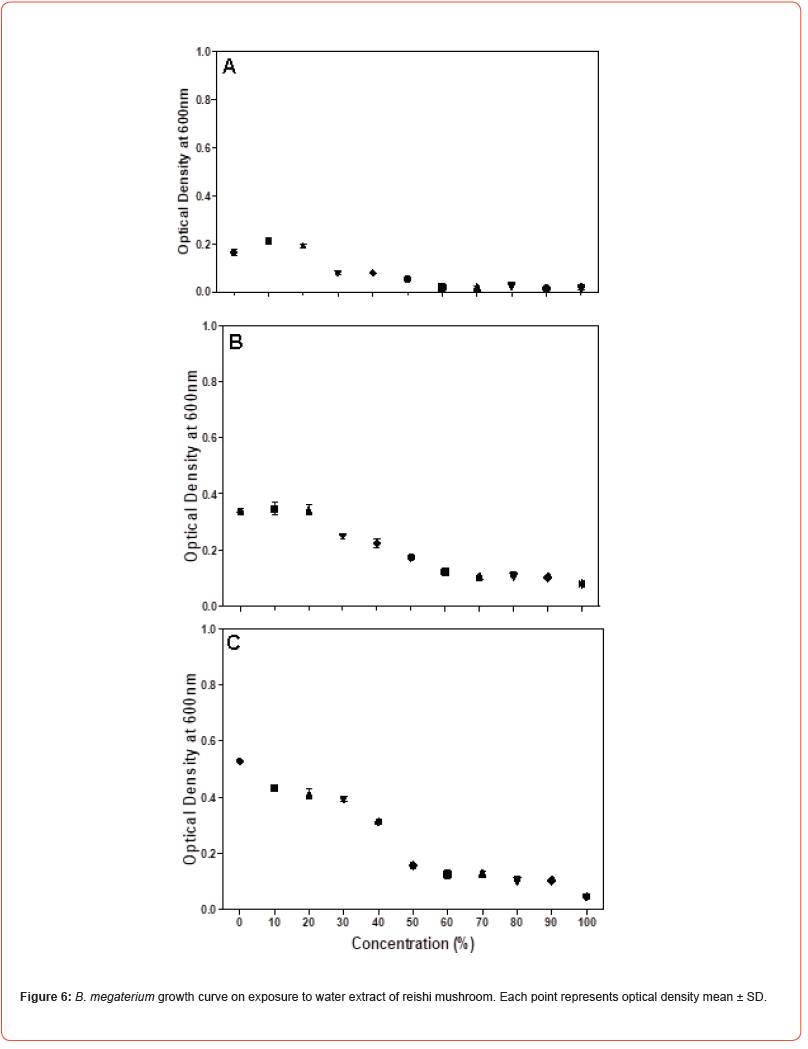
Microdilution was performed to determine the MIC of the water extract of reishi mushroom against Gram-negative bacteria namely Klebsiella pneumoniae, Escherichia coli and Salmonella typhi and Gram-positive bacteria namely Staphylococcus aureus, Micrococcus luteus and Bacillus megaterium. Microdilution was performed from 0 to 100% of the extract. Overall, the data showed statistically significant (P<0.05) antimicrobial efficacy of water extract of reishi mushroom against both bacterial strains compared to the control. Antimicrobial effect of water extract of reishi was first established within 3 hours of exposure and continued to 10 and 24 hours (Figures 1-3). An initial increase of bacterial growth at 0% and treatment with 10-100% of the extract resulted in statistically significant (p<0.05) reduction when compared to the control (Figures 1-3). Among the tested human pathogens, Gram positive bacteria were more susceptible (MIC values 20-60%) (Figures 4-6) than Gram negative bacteria (with MIC values of (40 -90%) (Figures 1-3). Though all the tested organisms were inhibited by water extract of reishi mushrooms, S. aureus (Figure 4) and E. coli (Figure 1) were the most inhibited Gram-positive and Gram-negative bacteria respectively.
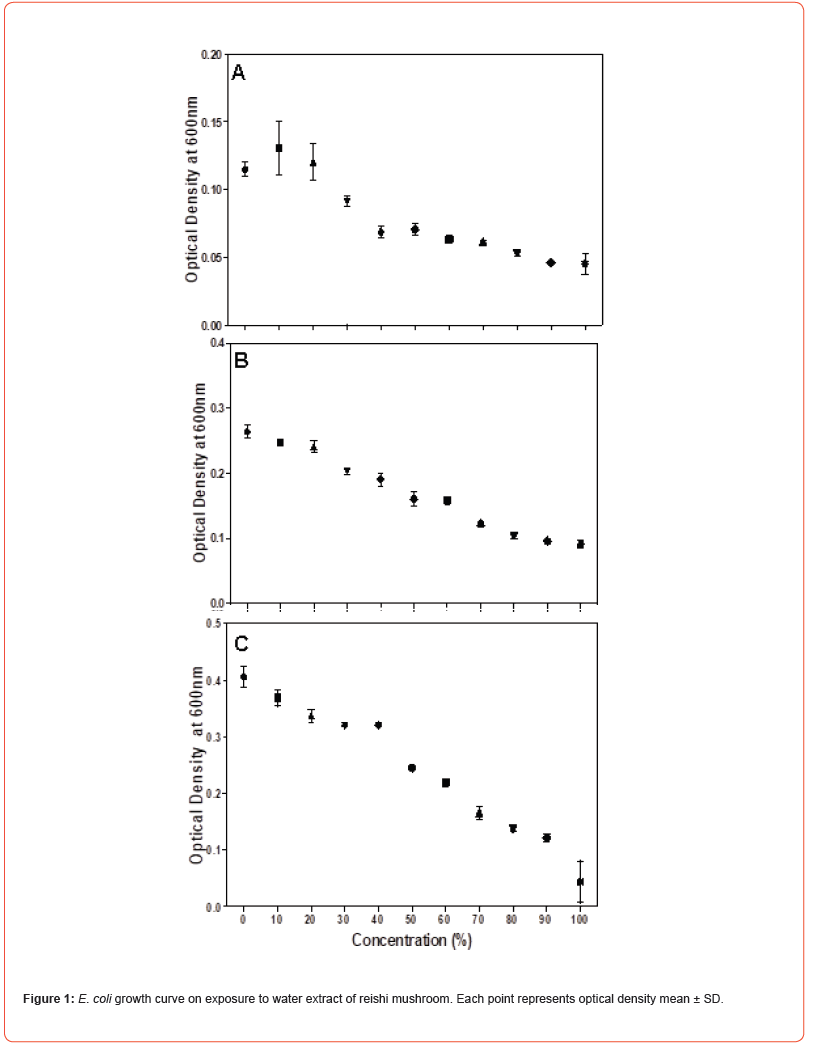
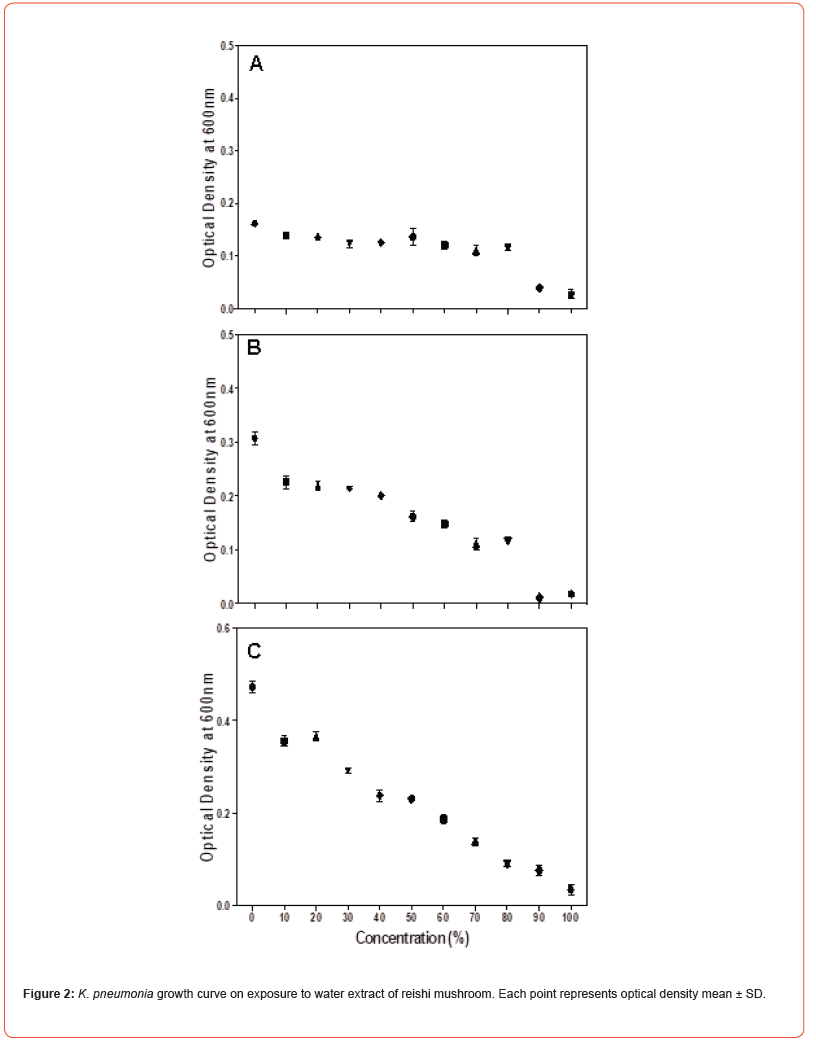
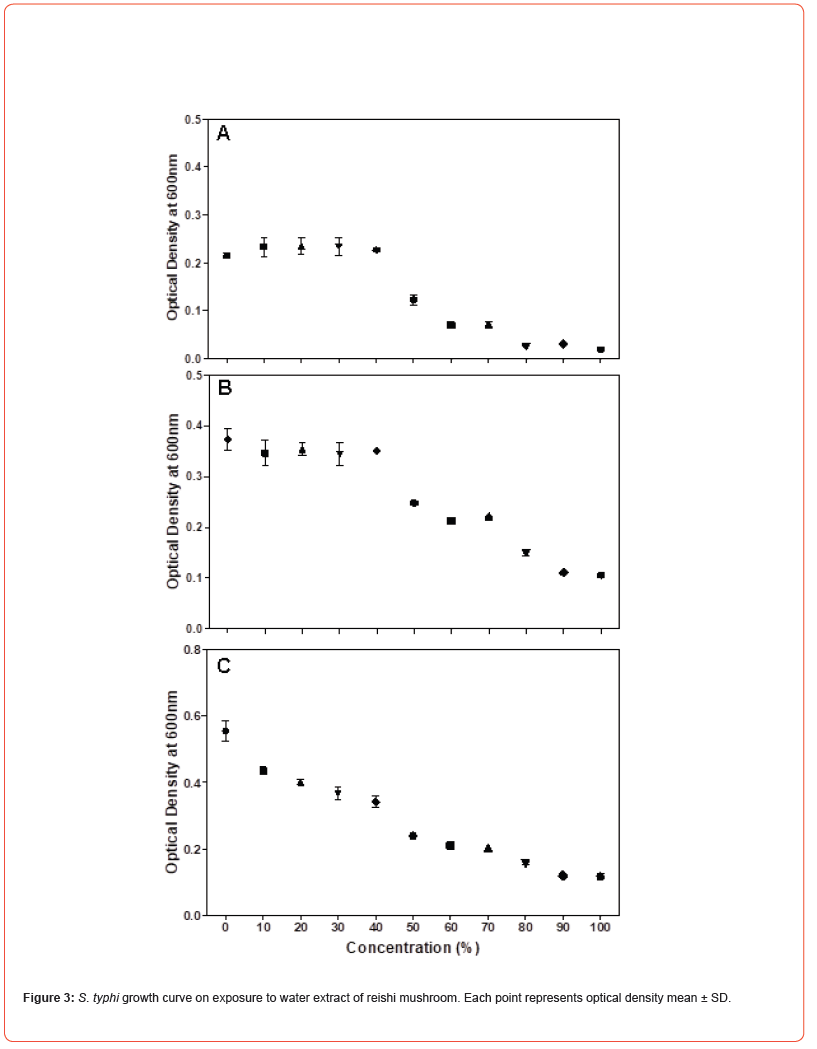
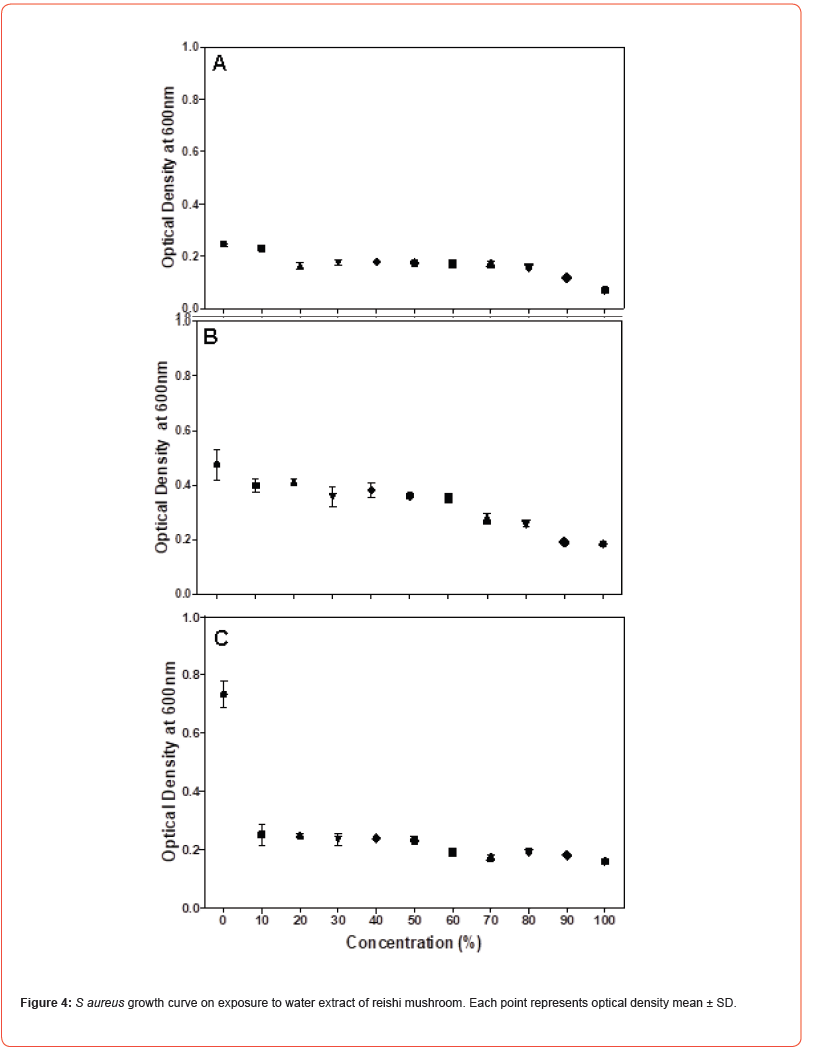
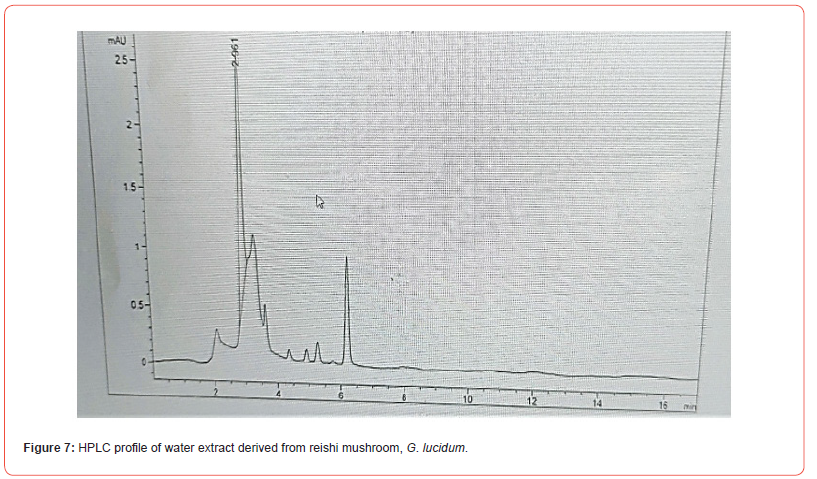
HPLC analysis
The content of the polyphenolic compounds in water extract of reishi and retention times determined by HPLC analysis are shown in figure 7. There are two main spikes; the one on the left is related to beta [1-3] glucans and the right is the Ganoderic acid and all the triterpenoids to the right of the two larger spikes.
Discussion
Bacterial infections still represent a major health problem, amplified by the phenomenon of antibiotic resistance. Mushrooms are now emerging as a promising source of antimicrobial compounds. While there is an abundance of mushrooms with medicinal properties, one of the most well-known is Ganoderma lucidum commonly called reishi which has been used for hundreds of years in Asian countries. The extracts of most mushrooms’ species including reishi showed relatively strong antimicrobial activity over a specified period of 18 to 24 hours [19-22]. This research identified that there is currently a shortage of data obtained from scientific studies on the antimicrobial action of the mushrooms as early as 3 hours to 24 hours after treatment. Herein water extract of reishi mushroom was evaluated against both Gram-positive and Gram-negative bacteria at 3-24hr. The water extract of reishi mushroom displayed antimicrobial activity against Escherichia coli and Salmonella typhi, Staphylococcus aureus, Micrococcus luteus and Bacillus megaterium after 3–24 h of exposure at different concentrations of water extract of reishi mushroom ranging from 10-100%. Moreover, with increasing time all the tested bacteria strains showed a significant difference with each other in terms of antimicrobial efficiency.
Our results indicate that exposure time influences antibacterial activities of water extract of reishi mushrooms. The inhibitory effect of the extract against the tested bacteria increases over time with greater inhibitory activity at 24 hours of incubation. Suggesting that water extract of reishi gradually prevents the growth of bacteria by interacting with key physiological processes. Based on the reports of [23] antimicrobial agents showed continuous bacteria inhibition against bacteria from 24 to 74 hours. Our studies confirmed that Gram-positive bacteria compared to Gram-negative bacteria were highly more susceptible to the water extract of reishi mushroom which corroborate with the previous reports [24,25]. This may be because of the inherent nature of the peptidoglycan layer which allows antimicrobial agents to enter inside the cell and results in protein denaturation and disruption in the cell membrane [26]. Furthermore, the lipopolysaccharides layer and periplasmic space of gram-negative bacteria induce relative resistance of gram-negative bacteria [27,28].
The antibacterial activity of water extract of reishi mushroom against bacteria may be an indication of the presence of broad-spectrum antimicrobial compounds. Our HPLC profile revealed peaks which were identified to represent ganoderic acid, less beta [1-3] glucans and triterpenoids. These results were in line with several previous investigations in mushrooms, which recorded the presence of these bioactive compounds [29-31]. The antimicrobial activities of ganoderic acid, less beta [1-3] glucans and triterpenoids derivatives are mediated by inhibition of crucial cellular processes which are essential to microbial survival such as oxygen uptake and oxidative phosphorylation and DNA synthesis [32-35]. The antimi crobial activity of ganoderic acid is more potent against Gram-positive bacteria than negative bacteria [36]. This is supported by a previous study where the extract of mushrooms has good antimicrobial activities against Gram-positive bacteria [37]. It should be highlighted that the identified compounds may exhibit synergistic action as observed in biochemical compounds [38,39]. The antimicrobial potency of water extract of reishi mushroom is believed to be due to bioactive compounds. It is worth noting that this extract was potentially effective in suppressing growth of resistant pathogens where modern antibiotic therapy has failed suggesting that reishi mushroom extract could be an alternative as antimicrobials against pathogenic micro-organisms resistant to conventional treatments.
Conclusion
In this research work, water extract of reishi mushroom was investigated for early antimicrobial activities for the first time. The results proved that antimicrobial activity of water extraction of reishi mushroom against Gram-positive and Gram-negative bacteria was first observed within 3 hours of treatment and continued to 24 hours due to the presence of various bioactive agents. Hence, reishi muchroom can be used to discover natural products that can be used by pharmaceutical industries to combat pathogens. Further studies which aimed at investigating antioxidant and anticancer activities of water extract of reishi mushroom have been initiated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors of this paper are thankful to the department of Biological Sciences and the department of Chemistry at Winston Salem State University for their invaluable support and for providing all the research facilities.
References
- Kainz K, Bauer MA, Madeo F, Carmona Gutierrez D (2020) Fungal infections in humans: the silent crisis. Microbial Cell 7(6): 143-145.
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, et al. (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399(10325): 629-655.
- Lorian V (1975) Some effects of subinhibitory concentrations of antibiotics on bacteria. Bulletin of the New York Academy of Medicine 51(9): 1046-1055.
- Prosser BL, Taylor D, Dix BA, Cleeland R (1987) Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrobial Agents and Chemotherapy 31(10): 1502-1506.
- Campoy S, Adrio JL (2017) Antifungals. Biochemical pharmacology 133(1): 86-96.
- Kolář M (2022) Bacterial infections, antimicrobial resistance and antibiotic therapy. Life 12(4): 468-480.
- Adetunji CO, Olaniyan OT, Anani OA, Bodunrinde RE, Osemwegie OO, et al. (2022) Integrated processes for production of pharmaceutical products from agro-wastes. Biomass, Biofuels, Biochemicals pp. 439-461.
- Kour H, Kour D, Kour S, Singh S, Hashmi SAJ, et al. (2022) Bioactive compounds from mushrooms: an emerging bioresources of food and nutraceuticals. Food Bioscience 50(PART B): 102124-102130.
- Xu J, Shen R, Jiao Z, Chen W, Peng D, et al. (2022) Current Advancements in Antitumor Properties and Mechanisms of Medicinal Components in Edible Mushrooms. Nutrients 14(13): 2622-2630.
- Ahmed AF, Mahmoud GAE, Hefzy M, Liu Z, Ma C (2023) Overview on the edible mushrooms in Egypt. Journal of Future Foods 3(1): 8-15.
- Yu Y, Liu Z, Song K, Li L, Chen M (2023) Medicinal value of edible mushroom polysaccharides: a review. Journal of Future Foods 3(1): 16-23.
- Ahmad R, Riaz M, Khan A, Aljamea A, Algheryafi M, et al. (2021) Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytotherapy Research 35(11): 6030-6062.
- Vu TM, Hervé V, Ulfat AK, Lamontagne Kam D, Brouillette J (2023) Impact of non-neuronal cells in Alzheimer’s disease from a single-nucleus profiling perspective. Frontiers in Cellular Neuroscience 17(2): 1208122-1208128.
- Johra FT, Hossain S, Jain P, Bristy AT, Emran T, et al. (2023) Amelioration of CCl4-induced oxidative stress and hepatotoxicity by Ganoderma lucidum in long evans rats. Scientific Reports 13(1): 9909-9915.
- Ofodile LN, Uma NU, Kokubun T, Grayer RJ, Ogundipe OT (2005) Antimicrobial activity of some Ganoderma species from Nigeria. Phytotherapy Research 19(4): 310-313.
- Vazirian M, Faramarzi MA, Ebrahimi SES, Esfahani HRM, Samadi N, et al. (2014) Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds. International Journal of Medicinal Mushrooms 16(1): 77-84.
- El Dein MMN, El Fallal AA, El Sayed AK, El Esseily SR (2023) Antimicrobial Activities of Ganoderma mbrekobenum Strain EGDA (Agaricomycetes) from Egypt. International Journal of Medicinal Mushrooms 25(9): 31-41.
- Rašeta M, Mišković J, Čapelja E, Zapora E, Petrović Fabijan A, et al. (2023) Do Ganoderma Species Represent Novel Sources of Phenolic Based Antimicrobial Agents?. Molecules 28(3264): 1-19.
- Nowacka N, Nowak R, Drozd M, Olech M, Los R, et al. (2015) Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PloS one 10(10): e0140355-e0140360.
- Al Azad S, Ping VCA (2022) Antioxidant Properties and Antimicrobial Activity in the Extracts of Two Edible Mushroom, Pleurotus sajor caju and Schizophyllum commune. Advances in Bioscience and Biotechnology 13(09): 352-361.
- Saludares GG, Amper CD, Lituanas IM (2023) Antimicrobial performances of Ganoderma lucidum extract against fruits and leaves pathogens. In IOP Conference Series: Earth and Environmental Science 1145 (1): 012019-012025.
- Yakobi SH, Mkhize S, Pooe OJ (2023) Screening of Antimicrobial Properties and Bioactive Compounds of Pleurotus Ostreatus Extracts against Staphylococcus Aureus, Escherichia coli, and Neisseria Gonorrhoeae. Biochemistry Research International 2023(1): 1777039-1777045.
- Wang Q, Larese Casanova P, Webster TJ (2015) Inhibition of various gram- positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. International journal of nanomedicine 10(1): 2885-2894.
- Zalazar AL, González MM, Gliemmo MF, Campos CA (2018) A colorimetric assay using tetrazolium salts with an electron mediator to evaluate yeast growth in opaque dispersed systems. Journal of Food Science & Technology 3(1): 233-240.
- Waithaka PN, Gathuru EM, Githaiga BM, Onkoba KM (2022) Antimicrobial Activity of Mushroom (Agaricus bisporus) and Fungi (Trametes gibbosa) Extracts from Mushrooms and Fungi of Egerton Main Campus, Njoro Kenya. 5th KyU International Conference 6(3): 20-26.
- Koohsari H, Ghaemi EA, Sheshpoli MS, Jahedi M, Zahiri M (2015) The investigation of antibacterial activity of selected native plants from North of Iran. Journal of medicine and life 8(2): 38-42.
- Exner M, Bhattacharya S, Christiansen B, Gebel J, Goroncy Bermes P, et al. (2017) Antibiotic resistance: What is so special about multidrug- resistant Gram-negative bacteria?. GMS hygiene and infection control 12(1): Doc05-Doc10.
- Tabbouche SA, Gürgen A, Yildiz S, Kiliç AO, Sökmen M (2017) Antimicrobial and antiquorum sensing activity of some wild mushrooms collected from Turkey. MSU J Sci 5(2): 453-457.
- Sharif S, Shahid M, Mushtaq M, Akram S, Rashid A (2017) Wild mushrooms: A potential source of nutritional and antioxidant attributes with acceptable toxicity. Preventive nutrition and food science 22(2): 124-130.
- Kebaili FF, Tahar N, Toumi ME, Redouane R, Chawki B, et al. (2021) Antioxidant Activity and Phenolic Content of Extracts of Wild Algerian Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes). International Journal of Medicinal Mushrooms 23(6): 79-88.
- Erbiai EH, Amina B, Kaoutar A, Saidi R, Lamrani Z, et al. (2023) Chemical Characterization and Evaluation of Antimicrobial Properties of the Wild Medicinal Mushroom Ganoderma lucidum Growing in Northern Moroccan Forests. Life 13(5): 1217-1225.
- Chamidah A, Hardoko H, Prihanto AA (2017) Antibacterial activities of β- glucan (laminaran) against gram-negative and gram-positive bacteria. In AIP Conference Proceedings 1844(1): 1-8.
- Mahizan NA, Yang SK, Moo CL, Song AAL, Chong CM, et al. (2019) Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 24(14): 2631-2635.
- Serrano Márquez L, Trigos A, Couttolenc A, Padrón JM, Shnyreva AV, et al. (2021) Antiproliferative and antibacterial activity of extracts of Ganoderma strains grown in vitro. Food Science and Biotechnology 30(5): 711-721.
- Rezghi Jahromi MH, Mozafary M (2021) Ganoderma lucidum and antimicrobial activity. Journal of Ethno-Pharmaceutical Products 2(2): 35-41.
- Upadhyay M, Shrivastava B, Jain A, Kidwai M, Kumar S, et al. (2014) Production of ganoderic acid by Ganoderma lucidum RCKB-2010 and its therapeutic potential. Annals of microbiology 64(2): 839-846.
- Manjunathan J, Kaviyarasan V (2010) Solvent based effectiveness of antibacterial activity of edible mushroom Lentinus tuberregium (Fr.). International Journal of PharmTech Research 2(3): 1910-1912.
- Pezzani R, Salehi B, Vitalini S, Iriti M, Zuñiga FA, et al. (2019) Synergistic effects of plant derivatives and conventional chemotherapeutic agents: an update on the cancer perspective. Medicina 55(4): 110-115.
- Gupta S, Summuna B, Gupta M, Annepu SK (2018) Edible mushrooms: cultivation, bioactive molecules, and health benefits. Bioactive molecules in food 1(4): 1-33.
-
Akamu J Ewunkem*, Ilunga Tshimanga, Biruktawit Samson, Brittany Justice and Dinesh K Singh. Invitro Evaluation of Antimicrobial Activity of Aqueous Extracts of Reishi Mushroom (Ganoderma Lucidum) Against a Select Gram Positive and Negative Bacteria. Sci J Biol & Life Sci. 3(3): 2024. SJBLS.MS.ID.000565.
-
Mushroom; antimicrobial; reishi; bioactive compounds
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






