 Minireview Article
Minireview Article
Electroanalytical Method for The Detection of Phenol: A Brief
Kalidas Mainali*, Marwan Husni Gagaa and Sohrab Haghighi Mood
Biological System Engineering, Washington State University, USA
Kalidas Mainali, Biological System Engineering, Washington State University, USA.
Received Date:October 06, 2022; Published Date:November 02, 2022
Abstract
Phenolic compounds are the most common species found in plants, agro-food matrices, and biological samples. Many conventional and advanced techniques, such as cyclic voltammetry and enzyme-based biosensor, are available to detect phenolics from complex matrices. However, detecting the low concentration of phenolics in complex matrices is challenging. Among them, the electroanalytical method is rapid and allows us to detect phenolics from hazardous organic compounds and biological sources. This method offers high selectivity and low detection limits in determining phenolics contaminants. In this brief, the mechanism of electrochemistry, along with voltammetry, is discussed.
Keywords:Phenolic compounds, Cyclic voltammetry, Electroanalytical method
Introduction
COVID-19 (corona virus disease 2019) is a contagious disease caElectrochemical techniques are essential tools in all chemical and biochemical research laboratories. These techniques are used not only for the fundamental studies of the oxidation and reduction process but also in studying the kinetics and thermodynamics of the electron and ion transfer process. Among the different electrochemical techniques applied in the analytical field, the principal ones are polarographic and voltammetric techniques. These techniques are popular and widely used because of their excellent sensitivity, rapid analysis time, and simultaneous determination of several analytes [1]. Nowadays, polarographic techniques have been substituted by sophisticated voltammetric techniques. It is a branch of electrochemistry, firstly developed by the discovery of polarography in 1922 by Jaroslav Heyrovsky. However, a significant improvement was made in the early 1960s. The electrochemistry mechanism provides a powerful and versatile tool in complex mix ture analysis that detects low concentrations. Additionally, one of the main advantages of the electrochemical technique is the direct analysis of the sample without tedious and time-consuming steps and subsequent separation. However, pretreatment is required when using complex sample mixtures. Compared to chromatographic techniques, this process is still faster, cheaper, and more accessible. Also, the effect of interference among molecules is low in electrochemical techniques. If required, voltammetric methods can be easily incorporated with other procedures like their use as a detector in chromatographic separations [2].
Voltammetric Techniques
Voltammetric experiments are usually carried out in simple electrochemical cells. Generally, electrochemical analysis can be done by two main classes of measurement. One is potentiometry (in which the potential that develops between two electrodes is measured), and another is amperometry (in which the current that flows between two electrodes is measured). Voltammetry is an experiment in which the potential applied to the working electrode is swept at a constant sweep rate, and the resulting current is calculated as a potential function [3]. A typical electrochemical cell consists of a working electrode, a reference electrode, and an auxiliary (counter electrode). The working electrode is an electron conductor at which the reaction or transfer of interest occurs. Similarly, in all voltammetric experiments, it is necessary to keep one of the electrodes at a constant potential. The electrode that involves in it is called a reference electrode. The auxiliary electrode is one at which a counter-reaction occurs at the working electrode. This electrode’s primary purpose is to balance the system’s total charge.
The primary working mechanism in the cell depends on the presence of electroactive species. The applied potential will change the concentration of the monitored electroactive species at the electrode surface by electrochemically reducing or oxidizing them. This process (changing the concentration of any electroactive participant at the working electrode surface) will cause mass transport toward the electrode. And the current flow through the electrode is directly proportional to the analyte concentration. Therefore, this dependence on measured current and analyte concentration makes voltammetric techniques widely used in the analytical field. The schematic representation of a typical electrochemical cell is shown below [1]:
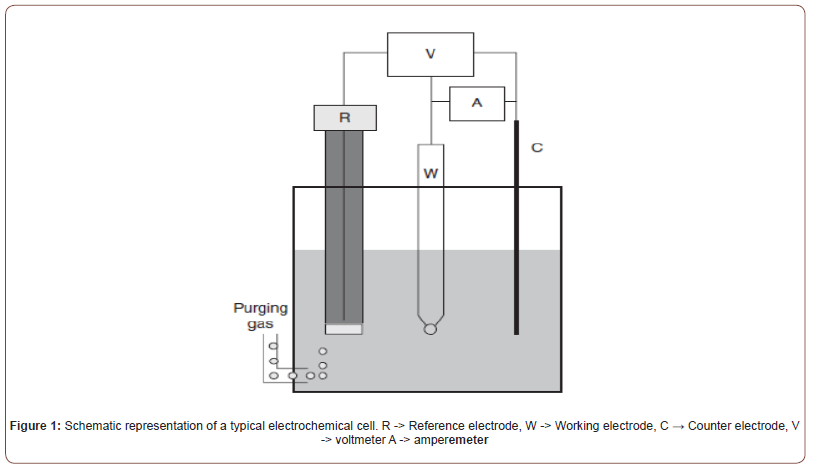
Figure 1: Schematic representation of a typical electrochemical cell. R ⟶ Reference electrode, W ⟶ Working electrode, C → Counter electrode, V ⟶ voltmeter A ⟶ amperemeter
Cyclic voltammetry is one of the most widely used techniques in electrochemical studies. Since this technique gives insight into the half-reactions at the working electrode, providing information about the chemical and physical phenomenon in the electrochemical reaction is also called electrochemical spectroscopy.
Electrochemical Determination of Phenol and Phenolic Compounds: A Mechanistic Approach
Electrochemical methods are reliable tools for the fast and lowcost assay of the mixture’s phenolic compounds (phenolics). These techniques are low cost, sensitive, and enable rapid analysis of the sample [4]. Generally, analyses can be performed in a stationary system using techniques such as cyclic voltammetry or biosensor applications based on enzyme catalysis, differential pulse voltammetry, etc. Since the beginning of humankind, people have used to do bioanalysis by using nerve cells to detect scents or enzymatic reactions in the tongue to taste food. Based on this, researchers are still interested in developing a simple, sensitive, specific, and portable system such as biosensors for determining phenolic compounds. In addition, these techniques are cost-efficient and fast compared to the gas chromatographic/mass spectrometric method. Biosensor is an analytical device that combines a biological sensing element with a transducer to produce a signal proportional to the analyte concentration. Biomolecules such as enzymes, antibodies, receptors, and microorganisms are generally used as biological sensing elements. Among them, enzymes are widely used biological sensing elements. In this process, a specific “bio” element (enzyme) recognizes the analyte, and the “sensor” element transduces the change in the biomolecule into an electrical signal. The bio element is particular to the analyte and does not recognize other analytes [5]. Due to its specific and peculiar properties, the use of enzymatic biosensors has increased over time.
In literature, methods for detecting phenolic compounds have been reported based on several types, such as tyrosinase, laccase, etc. Among many analytical methods for measuring phenolic compounds in the electrochemical biosensor, tyrosinase is very popular. Tyrosinase is an enzyme that catalyzes phenol 0-hydroxylation yielding o-diphenol, which is subsequently oxidized to a quinone. Thus obtained Quinone further reduces as follows:
Phenol + tyrosinase (O2) ⟶ Catechol Catechol+ tyrosinase (O2) ⟶ O-quinone+H2O o-quinone+2H+ +2e ⟶ Catechol
So, phenol detection depends on monitoring the quinone products or the consumption of the oxygen co-factor.
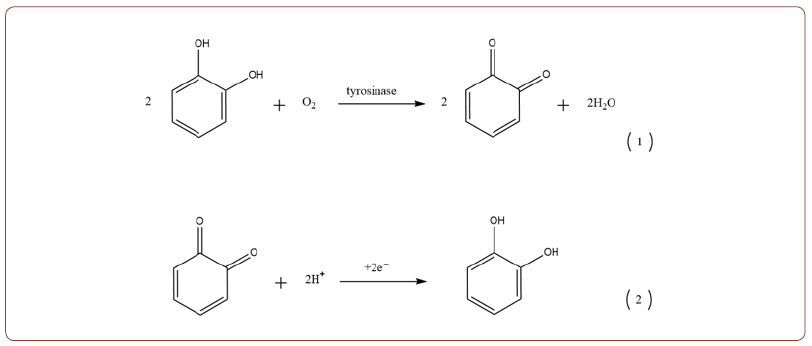
Debg Q, et al. [6], successfully developed Cyro-hydrogel, a tyrosinase- based biosensor. As shown above, tyrosinase catalyzes the oxidation of phenol and o-diphenol to quinones. The electrode response was based on the amperometric detection of the biocatalytically generated quinone products. At the enzyme electrode, according to above reactions 1 and 2, reactions start quickly, and the response can be detected via voltammetry [6]. In addition to tyrosinase, peroxidase, laccase, and polyphenol oxidase have also been reported as modified biosensors to determine phenolic compounds. Electrodes modified with these enzymes have the advantage that phenolic compounds can be detected between -0.2 and 0.05 V versus SCE. The use of laccase is of significant importance because of its sensitivity to chlorinated organic compounds. So, laccase can react with phenolic compounds that are not reactive with tyrosinase [7]. In literature, the use of laccase biosensors shows excellent potential for detecting and quantifying phenolic compounds. The main transduction methods for laccase biosensors are electrochemical (amperometric, voltammetric, potentiometric, and conductometric), optical and thermal. However, amperometric is the most used and studied method for laccase biosensors [8].
It has also been reported the use of the spectrophotometric method for phenol determination. However, the absorptivities of different phenolic compounds differ from each other. Using an amperometric biosensor based on tyrosinase helped tackle associated problems. Kochana J, et al. [9], studied the four different phenol compounds concentration like phenol, catechol, 3-Cresol and 4- chlorophenol by using biosensor. They concluded that different phenols influence the biosensor response independently of each other [9].
Similarly, Chen K, et al. [10], modified the electrode surface with graphene and tyrosinase. They developed an electrochemical biosensor that could be used to detect the concentration of phenols in water samples. It is because of excellent conductivity, sizeable effective surface area, and strong graphene adsorption. The electrode performed good electroanalytic activity and showed an electrochemical response to phenols. Since graphene is a single layer of carbon atoms organized in a closely packed honeycomb two-dimensional lattice, it has a unique nanostructure and extraordinary properties. Since every bit in a graphene sheet is on the surface, molecular interaction via π-π stacking occurs, and electron transfer through graphene is highly sensitive to adsorbed molecules. In this process, cyclic voltammetry was used to characterize the modification process of the electrode. All the optimal experimental parameters were also crucial to determine the concentration of phenol. The effect of scan rate, the impact of graphene dose, the result of tyrosinase dosage, and the development of pH were the most studied parameters to detect phenol concentration successfully [10]. It was also observed that the higher the dosage of tyrosinase, the better results were obtained.
Additionally, in the literature, phenol oxidation of aqueous solutions was also studied using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) on a glassy carbon electrode [11].
This study observed a linear relationship between the peak current and the concentration ranging from micro to milli-molar levels. It was observed that an increase in pH makes oxidation easy by shifting the oxidation peak potential to more and more cathodic values. At low concentrations, the oxidation was diffusion controlled; at high concentrations, adsorption of phenol became predominant.
The phenol quantification was carried out in literature using a boron-doped nanocrystalline diamond (BDND) electrode in the electroanalytical square wave voltammetry (SWV) technique. This study validated SWV and BDND electrodes with the Ion Chromatography assay [12]. The obtained results confirmed the importance of controlling the BDND electrode surfaces, which strongly affect the electrochemical behavior, proving the BDND electrode as a nanosensor. Recently, a novel indirect electrochemical protocol for the electroanalytical detection of phenol is also presented. This optical analytical protocol involves the reaction of phenolic compounds with 4-amino antipyrine, a pyrazoline substitution product, in the presence of potassium ferricyanide at the pH of forms a stable reddish- brown colored antipyrine dye. Thus formed dye is measured electrometrically at 510 nm. The color intensity produced is a function of the concentration of the phenolic material. The process is summarized in two steps [13].
Step 1: Electro-oxidation of the phenolic compounds and reaction with the 4-aminoantipyrine.
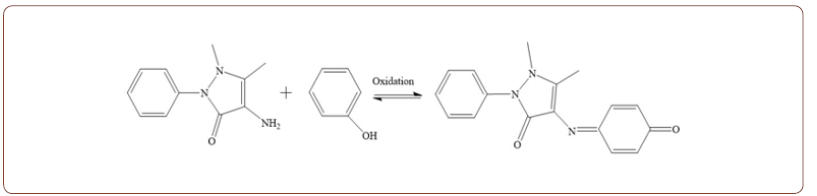
Step 2: Redox reaction of the aminoantipyrine phenolic complex (analytical signal)
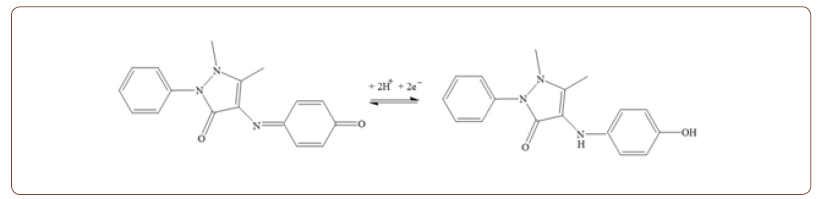
In this process, 4-amino antipyrine reacts with the target phenol to produce the quinone imine dye. Then, the dye is measured spectrophotometrically by using oxidizing agent potassium ferricyanide; this chemically oxides the target phenol to phenoxy radical, reacting with the 4-amino antipyrine. In this new electrochemically adapted optical protocol, the phenol is oxidized (step 1), and the dye is electrochemically active (step 2). This provides an indirect electroanalytical protocol for the electroanalytical sensing of phenols. On the other hand, direct voltammetric responses of the BDDE using the same experimental analysis without adding 4-amino antipyrine were also conducted on different phenolic compounds. Finally, it is concluded that the indirect approach is advantageous over direct electroanalytical sensing of phenols.
Although many attempts were made to develop phenol electrochemical biosensors, problems like serious adsorption, formation of electro-inactive tary polymers, and surface fouling have often been associated. Therefore, it is challenging to develop a phenol electrochemical sensor that overcomes these problems. However, using enzymes such as tyrosinase helps tackle this issue in some instant. However, many associated problems, such as instability at room temperature, complicated purification methods, and high cost, are the main drawbacks of this kind of biosensor for routine analysis. In literature, Vishnu N & Kumar AS [14], studied the lowcost sensor to detect phenol in complex matrices. In those studies, they proposed ultra-low-cost pencil graphite as an alternative electrochemical sensor for the mono-phenols. New ultra-low cost 6B grade pencil graphite was used as a disposable sensor for each measurement. This 6B-pencil graphite preanodized at 2V vs. Ag/ AgCl for 180 s showed efficient differential pulse voltammetric sensing of phenolic compounds. This sensor is suitable for the electrochemical detection of phenols in different areas [14].
In the literature, researchers studied a series of 25 phenol derivative compounds and their potential antioxidant capacity by using cyclic voltammetry and square-wave voltammetry. From these studies, it was concluded that the electrochemical behavior of the substituted phenol compounds occurs electrochemical (redox) mechanism at the graphite electrode. In this process, estimating the first oxidation peak potential (Epa) values of the studied compounds by electrochemical investigation methods confirms a qualitative classification of the compounds depending on the structure and the increase of their antioxidant capacity [15]. Researchers also studied the paper-based biosensor for detecting phenols from complex matrices like wine, paper, and plastic industries. Three filter papers were assessed in that study, but Whatman No. 2 showed the best response for biosensor development. Since bio-conjugation is an important field of research resulting in formulating bioconjugate for varied applications, a conjugate of enzymes and nanoparticles is increasingly essential in many applications. The paper-based biosensor was used for the colorimetric detection of phenolic compounds present in tap water and river water samples. The sensor was based on a layer-by-layer assembly approach on filter paper by physically trapping the mushroom tyrosinase in these layers. The sensor response is generally quantified as a color change from the specific binding of the enzymatically generated Quinone on the paper. Based on this, a paper biosensor was developed to detect phenol constituents using bioconjugate of tyrosinase and gold nanoparticles produced by a novel strain of Streptomyces. Thus formed Whatman No. 2 filter paper biosensor could detect in 3 min the phenol content from the complex matrices. Gold nanoparticles enhance tyrosinase activity for their greater efficacy and stability in detecting phenol contents [16].
Liu J, et al. [17], studied two large organic contaminants (mainly produced by industries) like, phenol and o-cresol, which could harm people and the environment. These studies proposed a new electrochemical approach that could simultaneously determine phenol and o-cresol. This method contained a commercial electrode surface modification process in which deposition of ZnO nanosheets on the screen-printed electrode (SPE) [17]. The response of phenol and o-cresol oxidation in cyclic voltammograms at ZnO nanosheets modified SPE almost 23 times and 34 times higher than the bare SPE. Thus, formed fabricated sensor exhibited an outstanding electro- catalytic performance toward electro-oxidation of phenol and o-cresol. The detection limit of the phenol and o-cresol was 4.1 nM and 5.5 nM, respectively. Also, the proposed sensor was successfully applied to detecting phenol and 0-cresol in actual wastewater [17].
Conclusion
Electrochemical techniques (cyclic voltammetry and square wave voltammetry) are versatile methods for detecting phenolic compounds in complex matrices. The process is a reliable and low-cost tool for detecting low concentration phenolics. The electrochemical behavior of different phenolic groups depends on the electrochemical parameters of the redox process occurring in the electrode. Furthermore, the enzyme-modified biosensor can be used to determine phenols in complex matrices such as biological sources, food waste, etc. Finally, it is worth mentioning that the biological activity of phenols, which can donate electrons to a broad set of receiver species that undergo reduction upon oxidation, is the primary source of selectivity during the electroanalytical process.
Acknowledgment
Special thanks to Melba Denson for the graphic support.
Conflict of Interest
The authors declare no conflict of interest.
References
- Gulaboski R, Carlos M (2008) Electrochemical techniques and instrumentation in Food Analysis in Handbook of Food Analysis Instruments book, Semih Otles.
- Pohanka M, Skládal P (2008) Electrochemical biosensors-principles and applications. J Appl Biomed 6(2): 57-64.
- Dobes J, Zitka O, Sochor J, Ruttkay-Nedecky B, Babula P, et al. (2013) Electrochemical tools for determination of phenolic compounds in plants. A review. Int J Electrochem Sci 8: 4520-4542.
- Karim F, Fakhruddin ANM (2012) Recent advances in the development of biosensor for phenol: a review. Reviews in Environmental Science and Biotechnology 11(3): 261-274.
- Dzyadevych SV, Arkhypova VN, Soldatkin AP, El'skaya SV, Martelet C, et al. (2008) Amperometric enzyme biosensors: Past, present and future. Irbm 29(2-3): 171-180.
- Deng Q, Guo Y, Dong S (1996) Cyro-hydrogel for the construction of a tyrosinase-based biosensor. Analytica Chimica Acta 319(1-2): 71-77.
- Sołoducho J, Cabaj J (2013) Phenolic compounds hybrid detectors. Journal of Biomaterials and Nanobiotechnology 4(3): 17-27.
- Rodríguez-Delgado MM, Alemán-Nava GS, Rodríguez-Delgado JM, Dieck-Assad J, Martínez-Chapa SO, et al. (2015) Laccase-based biosensors for detection of phenolic compounds. TrAC Trends in Analytical Chemistry 74: 21-45.
- Kochana J, Adamski J, Parczewski A (2012) A Critical View on the Phenol Index as a Measure of Phenol Compounds Content in Waters. Application of a Biosensor. Ecological Chemistry and Engineering S 19(3): 383-391.
- Chen K, Zhang ZL, Liang YM, Liu W (2013) A graphene-based electrochemical sensor for rapid determination of phenols in water. Sensors(Basel) 13(5): 6204-6216.
- Mathiyarasu J, Joseph J, Phani K, Yegnaraman V (2004) Electrochemical detection of phenol in aqueous solutions. Indian journal of chemical technology 11(11): 797-803.
- Azevedo AF, Souza FA, Matsushima JT, Baldan MR, Ferreira NG (2011) Detection of phenol at boron-doped nanocrystalline diamond electrodes. Journal of electroanalytical chemistry 658(1-2): 38-45.
- Kolliopoulos AV, Kampouris DK, Banks CE (2015) Indirect electroanalytical detection of phenols. Analyst 140(9): 3244-3250.
- Vishnu N, Kumar AS (2015) A preanodized 6B-pencil graphite as an efficient electrochemical sensor for mono-phenolic preservatives (phenol and meta-cresol) in insulin formulations. Analytical Methods 7(5): 1943-1950.
- Turdean GL, Casoni D, Sârbu C (2016) Structure–electrochemical properties correlations of some phenol derivatives investigated by electrochemical techniques. Journal of the Iranian Chemical Society 5(13): 945-956.
- Mazhari, BBZ, Agsar D, Ambika Prasad MVN (2017) Development of Paper Biosensor for the Detection of Phenol from Industrial Effluents Using Bioconjugate of Tyr-AuNps Mediated by Novel Isolate Streptomyces tuirus DBZ39. Journal of Nanomaterials 2017: 1352134.
- Liu J, Huang H, Zhong S, She X, Yin D (2016) Electrochemical Simultaneously Determination of Phenol and o-Cresol in Water Based on ZnO Nanosheets. Int J Electrochem Sci 11(5): 3921-3930.
-
Kalidas Mainali*, Marwan Husni Gagaa and Sohrab Haghighi Mood. Electroanalytical Method for The Detection of Phenol: A Brief. Sci J Biol & Life Sci. 2(4): 2022. SJBLS.MS.ID.000543 DOI: 10.33552/SJBLS.2022.02.000543
-
Phenolic compounds, Cyclic voltammetry, Electroanalytical method; Complex matrices; Biological sources; Organic compounds; Phenolics contaminants; Electrochemistry; Polarographic techniques; Voltammetric techniques; Complex mixture analysis; Chromatographic techniques; Chromatographic separations
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






