 Short Communication
Short Communication
Calmodulin and its Binding Proteins
Gunjan Saini*
Department of Medicinal Chemistry & Molecular Pharmacology, Purdue University, West Lafayette, Indiana, 47906, USA
Gunjan Saini, Department of Medicinal Chemistry & Molecular Pharmacology, Purdue University, West Lafayette, Indiana, 47906, USA.
Received Date:May 22, 2023; Published Date:June 09, 2023
Introduction
Most widely studied calcium binding protein is Calmodulin (CaM), that binds and activates more than 100 different proteins [1-4]. It is expressed in all eukaryotic cells and involved in many physiological and cellular processes like apoptosis, cell proliferation, neuronal signaling and muscle contractions [5-6]. It is a low molecular weight (16kDa), and extraordinarily conserved proteins, across the evolutions [7].
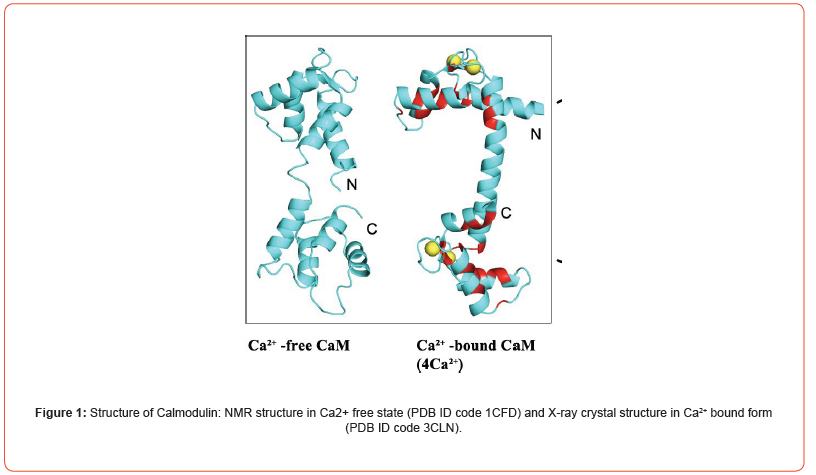
CaM consists of two globular domains: N- and C-terminal, separated by linker helix (Figure 1). Each domain consists of a pair of EF-hands, that can bind two Ca2+ ions [8-10]. Both N- and C- domains show different binding kinetics to Ca2+ ions. Both domains have 10-fold difference in Ca2+ affinity and N-terminal domain binds faster than C-terminal lobe. Ca2+ binds to both pairs of EF- hand in a cooperative manner. Upon binding to Ca2+ ions, it undergoes a conformational change which opens several hydrophobic patches that facilitates its binding to several different downstream binding proteins (more than 100) [11-12]. This Ca2+ ions binding is readily reversed by chelation of Ca2+ ions with a chelating agent, EDTA. The flexible linker helix helps to wrap the CaM around the target binding protein. Many other binding mechanisms are also known [2]. Furthermore, downstream binding protein alters the binding affinity of CaM towards Ca2+ ions.
CaM binds up to vast number of downstream proteins, including enzymes that play important role in regulation of cellular processes like smooth muscle contraction, lipids metabolism and cell proliferation [3,5,6]. In this research article, we will discuss the role of this extraordinary, conserved CaM protein in contractility of muscles and in long term potentiation (LTP). CaM dependent protein kinases have specific sequences that bind to the calmodulin. These specific sequences are known as calmodulin binding regions. The first calmodulin binding domain was identified and sequenced in 1985 in Kreb’s laboratory with a collaboration effort of researchers at University of Washington in the structure of rabbit skeletal muscle MLCK [13].
Discussion
One of the most important steps in the contraction of smooth muscles is phosphorylation of the light chain of the myosin. This phosphorylation is done by Ca2+ -calmodulin dependent myosin light chain kinase (MLCK) [14]. This MLCK becomes stimulated when a calcium bound calmodulin is attached to it. Contractility of smooth muscle is dependent on the presence of Ca2+ and its binding to the calmodulin and activation of Myosin light chain kinase (MLCK) [14].
In the central nervous system (CNS), changes in the volume settings of the synapses that cause memory formation are initiated by pulses of calcium ions rising through NMDA glutamate receptors in post synaptic spines. Potentiation or depression of synapse is determined by the difference in the size and duration of the pulse of the calcium. Ca2+ -calmodulin -dependent protein kinase II (CaM kinase II) is involved in the neuronal signaling that underlies the learning and memory information [15]. CaM kinase II is a dodecameric oligomer that phosphorylates a wide number of substrates to regulate calcium mediated changes in the cellular function. CaM kinase is activated by the calcium bound calmodulin. When CaM binds to a subunit of CaM kinase, it activates its kinase activity that initiates the phosphorylation of the other proteins. CaM kinase II is also important for calcium homeostasis.
Acknowledgement
None.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alvaro Villarroel, Maurizio Taglialatela, Ganeko Bernardo-Seisdedos, Alessandro Alaimo, Jon Agirre, et al. (2014) The ever-changing moods of calmodulin: how structural plasticity entails transductional adaptability. J Mol Biol 426(15): 2717-2735.
- Henning Tidow, Poul Nissen (2013) Structural diversity of calmodulin binding to its target sites. FEBS J 280(21): 5551-5565.
- Zhengui Xia, Daniel R Storm (2005) The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci 6(4): 267-276.
- D Chin, A R Means (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10(8): 322-328.
- Means AR, Rasmussen CD (1988) Calcium, calmodulin and cell proliferation. Cell Calcium 9(5-6): 313-319.
- Ui-Tei K, Nagano M, Sato S, Miyata Y (2000) Calmodulin-dependent and -independent apoptosis in cell of a Drosophila neuronal cell line. Apoptosis 5(2): 133-140.
- Friedberg F, Rhoads AR (2001) Evolutionary aspects of calmodulin. IUBMB Life 51(4): 215-221.
- Y Sudhakar Babu, Charles E Bugg, William J Cook (1988) Structure of calmodulin refined at 2.2 A resolution J Mol Biol 204(1): 191-204.
- Finn BE, Drakenberg T, Forsen S (1993) The structure of apo-calmodulin. A 1H NMR examination of the carboxy-terminal domain. FEBS Lett 336(2): 368-374.
- Meador WE, Means AR, Quiocho FA (1992) Target enzyme recognition by calmodulin: 2.4 A° structure of a calmodulin-peptide complex. Science 257(5074): 1251-1255.
- Linse S, Helmersson A, Forsen S (1991) Calcium binding to calmodulin and its globular domains. J Biol Chem 266(13): 8050-8054.
- Persechini A, White HD, Gansz KJ (1996) Different mechanisms for Ca2+ dissociation from complexes of calmodulin with nitric oxide synthase or myosin light chain kinase. J Biol Chem 271(1): 62-67.
- Blumenthal DK (1993) Development and characterization of fluorescently labeled myosin light chain kinase calmodulin-binding domain peptides. Mol Cell Biochem 127: 45-50.
- Tansey MG, Luby Phelps K, Kamm KE, Stull JT (1994) Ca(2+)- dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. The Journal of Biological Chemistry 269(13): 9912-9920.
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, et al. (1995) Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proceedings of the National Academy of Sciences of the United States of America 92(24): 11175-11179.
-
Joshua Z Yu, Timothy D Veenstra. Efficacy, Safety and Perspectives of Convalescent Plasma Therapy for Coronavirus Disease 2019. Sci J Biol & Life Sci.3(1): 2023. SJBLS.MS.ID.000553. DOI: 10.33552/SJBLS.2023.03.000553
-
Convalescent plasma therapy; Severe acute respiratory syndrome, Coronavirus 2, Coronavirus disease 2019, Neutralizing antibodies, COVID-19 treatment, Clinical trials, New drugs, SARS-CoV-2 antigens, COVID-19 patients, Antibody-protein interaction, Viral immune response
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






