 Mini Review
Mini Review
Quercetin, A Potential Metallo-β-Lactamase Inhibitor for Use in Combination Therapy Against β-Lactam Antibiotic-Resistant Bacteria
Bogdan M. Benin1, Trae Hillyer1,2, and Woo Shik Shin1,2*
1Department of Pharmaceutical Sciences, Northeast Ohio Medical University, Rootstown
1Integrated Pharmaceutical Medicine Program, Northeast Ohio Medical University, Rootstown
Woo Shik Shin, Integrated Pharmaceutical Medicine Program, Northeast Ohio Medical University, Rootstown, USA.
Received Date: November 02, 2021; Published Date: November 15, 2021
Abstract
Among the various mechanisms that bacteria use to develop antibiotic resistance, the multiple expression of β-lactamases has become pandemic, threatening public health and increasing patient mortality rates. Although combination therapy—in which a β-lactamase inhibitor is administered together with a β-lactam antibiotic—has proven effective against serine β-lactamases, inhibitors are not yet available for metallo-β- lactamases. Herein, we describe the potential for quercetin, a plant-derived flavinol, to be utilized in combination therapy as a metallo-β-lactamase inhibitor. Previous studies have demonstrated the numerous biological effects that this natural compound may exhibit; however, its anti-bacterial and inhibitor activity is only now coming under investigation. We therefore seek to provide an overview of the literature describing the current progress in our understanding of quercetin as a metal-chelator and a β-lactamase inhibitor and thus bring greater attention to this non-toxic and abundant natural product.
Keywords: Quercetin; Flavinols; Anti-Microbials; Combination Therapy; Metallo-β-Lactamase Inhibitor
Abbreviation: AMA: Aspergillo Marasmine A; EDTA: Ethylene Diaminetetraacetic acid; ESKAPE: Enterococcus Faecium, Staphylococcus Aureus, Klebsiella Pneumoniae, Acinetobacter Baumannii, Pseudomonas Aeruginosa, and Enterobacter Species; FDA: Food and Drug Administration; IMP: Imipenemase; MBL: Metallo-β-Lactamase; NDM-1: New Delhi Metallo-Beta-Lactamase-1; VIM: Verona Imipenemase
Antimicrobial Resistance and Metallo- β -Lactamases
Since discovering β-lactam antibiotics in the 1930s, a massive decline in mortality rates has transpired. These antibiotics still serve as the critical first-line treatment against common bacterial infections; however, resistance to β-lactam antibiotics, especially among the ESKAPE pathogens, threatens global public health while every year costing upwards of $4 billion due to community and hospital-acquired infections and causing about 23,000 deaths [1].
One of the major drivers of this resistance is the expression of β-lactamase enzymes that cleave β-lactam rings, rendering β-lactam antibiotics inactive. While numerous variations of β-lactamases exist, they are effectively classified into four classes: serine (Class A, C, and D) and metallo (class B) β-lactamase (MBL). The primary difference between these classes is that serine β-lactamases utilize a catalytic serine residue, whereas metallo-β-lactamases utilize metal-activated water as a nucleophile for β-lactam hydrolysis (Figure 1) [2].
The only viable approach for overcoming β-lactam drug resistance while utilizing the existing antibiotic arsenal is through the use of combination therapies in which β-lactamase inhibitors are administered together with β-lactam antibiotics. Today, three classes of FDA-approved serine β-lactamase inhibitors exist: β-lactams (sulbactam, tazobactam and clavulanate), 1,6-diazabicyclo octanes (avibactam, zidebactam, relebactam, nacubactam), and boron-based (vaborbactam) scaffolds. Still, and critically, none of these available inhibitors demonstrate broadspectrum activity against MBLs or even targeted activity against the New Delhi metallo-β-lactamase 1 (NDM-1), which has emerged as a major threat in recent years [3].
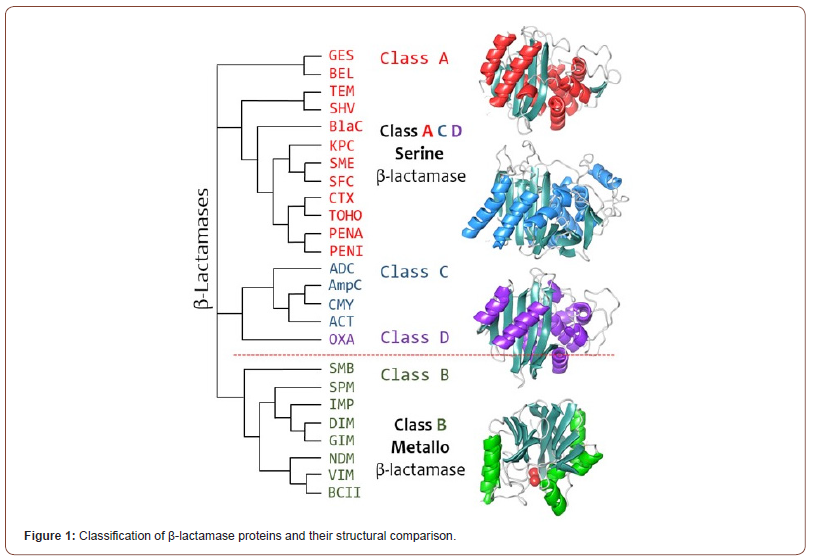
To inhibit MBLs, two strategies that target the active site have been devised: zinc sequestration and direct binding to the zinc in the active site (i.e. competitive inhibition). Two examples that highlight the advantages and subsequent issues of Zn sequestration are N,N,N’N’-ethylenediaminetetraacetic acid (EDTA) and aspergillomarasmine A (AMA). EDTA is a well-known metalchelator with Zn binding constants upwards of 10^16 [4], indicating a far stronger interaction than that of Zn with MBLs [5-7]. When imipenemase (IMP) or Verona imipenemase (VIM) producing P. aeruginosa was treated with EDTA, up to 16-fold reductions in the minimum inhibitory concentration (MIC) of imipenem were observed. Similarily, AMA, a naturally occruing chelator, was also demonstrated to significantly inhibit NDM-1 in vitro and in vivo [8, 9]. However, the same strength of these chelators is also a strong detractor as their ability to chelate Zn is non-specific (i.e. these chelators can target other metallo-enzymes), and several reports have detailed the evolution of new NDM-1-based proteins exhibiting higher Zn affinities and greater antibiotic resistance as being promoted by Zn-limited conditions [3, 9-11] Additionally, AMA does not exhibit broad activity against all MBLs (e.g. IMP).
As a result of these limitations, more attention has been given to the research and development of inhibitors that interact directly with the zinc in the active site without its sequestration. These promise, through the greater selectivity of enzymatic active sites, to be far safer and possibly more effective. Currently, two such inhibitors are in phase 1 and phase 3 clinical trials: QPX7728 and taniborbactam, respectively [12-14]. These are both based on boronic acids and have demonstrated broad spectrum MBL and serine β-lactamase inhibition. Still, these inhibitors are not equally active against all MBL classes, their mechanisms remain unclear and, although highly promising, they have not yet been approved [11].
Quercetin: An All-Natural Inhibitor
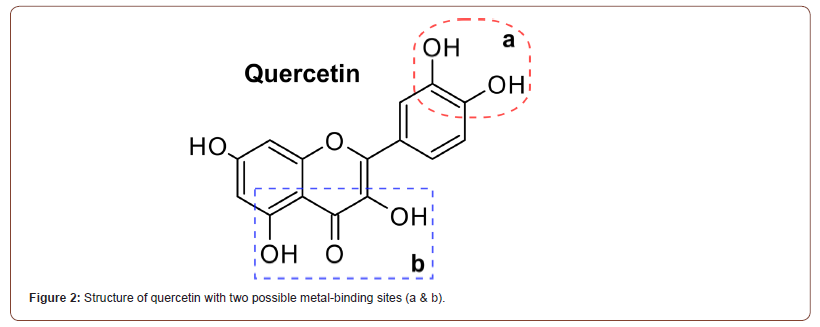
The current lack of approved candidates has prompted researchers to also examine various natural products as possible inhibitors. Typically, potential candidates must be able to (i) act as metal chelators, (ii) exhibit limited-to-no antimicrobial efficacy alone, and (iii) prevent the degradation of β-lactam antibiotics to restore their primary activity. In the search for naturally occurring compounds that can meet these requirements, numerous groups have reported on quercetin, a naturally occurring flavinol, as a potential candidate (Figure 2).
Quercetin, itself, is a yellow, plant-derived flavinol that is insoluble in water but soluble in alcohols and lipids (Figure 2). It has received sporadic attention among several studies suggesting that it may exhibit various beneficial biological properties, including anti-inflammatory, anti-cancer, anti-viral, etc. although its efficacy in these aspects is still debated. Now, however, it has received renewed attention as it has been demonstrated to successfully satisfy the three criteria for MBL inhibition (metal chelation, high MIC, preventing β-lactam cleavage) and has been shown to bind to the active site of NDM-1 [15].
The first of these criteria, metal-chelation, is achieved by the presence of various donor atoms on adjacent carbons (Figure 2). Although two possible binding sites exist (catechol, Figure 2a; ketol, Figure 2b), a Zn-quercetin complex was recently synthesized and demonstrates that quercetin acts as a bidentate ligand via the ketol functionality (Figure 2a) [16]. Importantly, quercetin binds Zn far weaker than other chelators and other metalloenzymes (Kd ~ 10- 4), supporting the notion that it will be effective without disrupting other enzymes [17].
Second, quercetin has been shown to exhibit only a very weak growth inhibitory effect on bacteria when administered alone with minimum inhibitory concentrations (MIC) typically above 128 μg/ mL and, in some cases, above 500 μg/mL [18,19]. This suggests that quercetin by itself cannot expect a significant antibiotic effect, but it can be used appropriately for targeting resistance caused by MBL expression using combination therapy with existing antibiotics.
Reports on the third criterion, inhibition of the enzymatic cleavage of β-lactams, have thus far been scarce although the concept of metallo-enzyme inhibition by quercetin and other flavonoids has been discussed since the 1990’s [20,21]. One of the initial focused studies on the ability of quercetin to act as an MBL inhibitor was published nearly a decade later by West et al. in which quercetin, and a related flavinol—galangin, was demonstrated to inhibit the MBL (L1) expressed by Stenotrophomonas maltophilia [22]. Although promising, the authors were unable to demonstrate the inhibition of meropenem inactivation in bacterial studies.
It was not until just recently, in 2020, that Morellet et al. solved for the first time a flavinol-MBL (NDM-1) structure through the use of solution nuclear magnetic resonance (NMR) spectroscopy (Figure 3, Table 1) [15]. The authors solved the structures for several protein-drug conjugates containing quercetin, myricetin, and morin (three analogous flavinols), not only demonstrating that flavinols could indeed act as competitive NDM-1 inhibitors, but also indirectly validating the earlier, theoretical placement of galangin in the L1 active site (Figure 3).
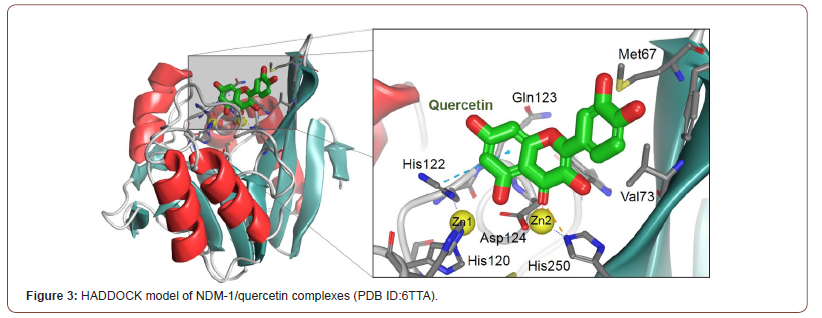
Table 1:Recently reported protein-ligand complex model of quercetin and quercetin analogs with metallo beta-lactamase enzyme.
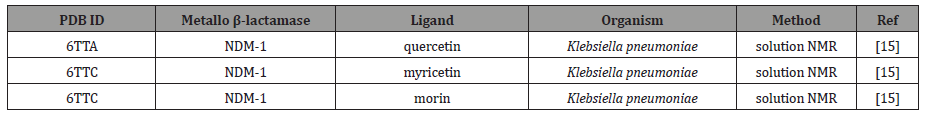
Notably, the use of NMR to solve these structures further demonstrates the potential of quercetin to be an effective and potent inhibitor with NMR. Proteins studied by NMR have the advantage that they can be investigated in a solution state, more closely mimicking the biological environment. This is in contrast to X-ray crystal structures, which are determined from protein crystals that have packed in the solid state and are thus less dynamic than proteins in solution (Table 1).
The authors further demonstrated that quercetin and its analogs, once bound to the NDM-1 active site, could effectively block the hydrolysis of imipenem. Their work joins others in demonstrating the capability of quercetin to act as an effect β-lactamase inhibitor.
Although this recent article, as well as the earlier article by West et al., demonstrated successful enzyme inhibition by quercetin, neither extended these studies to bacterial cell-based analysis. At the same time, various studies have investigated the potential for quercetin to act synergistically with multiple classes of antibiotics in bacteria, but these have not focused on selecting systems that could clearly test the ability of quercetin to block MBL inhibition. Instead, they utilized non-resistant strains or non-β-lactam antibiotics [23-29]. As a result, the list of studies that have more faithfully investigated the ability of quercetin to act synergistically with β-lactam antibiotics is currently short (Table 2).
Table 2:Previously reported results on combination therapies utilizing beta-lactam antibiotics and quercetin.
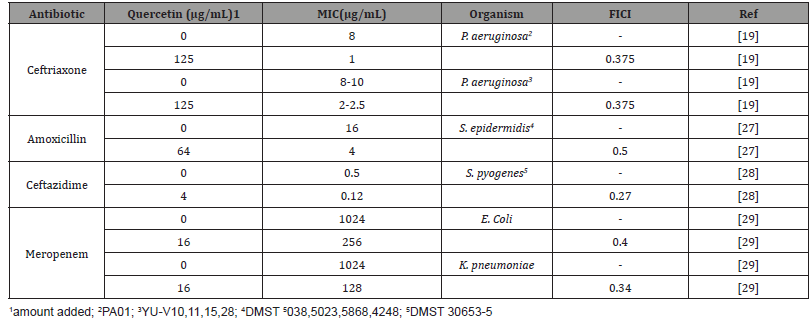
While these studies have successfully demonstrated the ability of quercetin to sensitize MBL producing bacteria towards β-lactam antibiotics, only Eumkeb et al. directly mentioned quercetin as a potential MBL inhibitor, but without reference to any specific MBL. This lack of control over MBL type further represents one of the key gaps in our current understanding for two reasons. First, a quercetin/MBL model only exists, thus far, for NDM-1; the extent and strength with which quercetin will interact with other MBLs (e.g. VIM, IMP, etc.) is not yet clear. Second, there have not yet been any studies describing the ability of quercetin to inhibit NDM-1 in bacterial culture models specifically. It is, as a result, unclear how effective quercetin is at inhibiting MBLs in actual cultures and whether its previously observed synergism may be from another, less obvious target or mechanism. These questions will need to be answered unambiguously in order to gauge the actual usefulness of quercetin as an inhibitor correctly. Despite many advances in studies targeting MBL-based drug resistance over the last decade, it is still a challenging area with few combinational therapies. The in-depth study of quercetin as an MBL targeting inhibitor seems to be a promising lead for expanding the field of novel combinational therapies.
Conclusion
The newly discovered structure of quercetin bound to NDM- 1 has provided critical information supporting the concept of quercetin as a novel, natural, abundant, and potentially effective MBL inhibitor, which acts by directly binding to the NDM-1 active site. While exciting, there are still numerous open questions remaining, such as how quercetin is effective in bacteria, which β-lactamases can quercetin interact with and inhibit, and can bacteria induce another type of resistance to evade quercetin in vitro? The answers to these questions will ultimately determine whether the concept of quercetin as an MBL inhibitor can realize its current potential.
Acknowledgement
None.
Conflict of interest
None.
References
- Nelson RE, Hatfield KM, Wolford H, Samore MH, Scott RD, et al. (2021) National Estimates of Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clin Infect Dis 72(Suppl 1): S17-S26.
- Lisa MN, Palacios AR, Aitha M, Gonzalez MM, Moreno DM, et al. (2017) A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-beta-lactamases. Nat Commun 8(1): 538.
- Mojica MF, Rossi MA, Vila AJ, Bonomo RA (2021) The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. The Lancet Infectious Diseases 3099(20): 30868-30869.
- Nyborg JK, Peersen OB (2004) That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J 381(Pt 3): e3-4.
- Palzkill T (2013) Metallo-beta-lactamase structure and function. Ann N Y Acad Sci 1277: 91-104.
- Yamaguchi Y, Ding S, Murakami E, Imamura K, Fuchigami S, et al. (2011) A demetallation method for IMP-1 metallo-beta-lactamase with restored enzymatic activity upon addition of metal ion(s). Chembiochem 12(13): 1979-1983.
- Paul-Soto R, Bauer R, Frere JM, Galleni M, Meyer-Klaucke W, et al. (1999) Mono- and binuclear Zn2+-beta-lactamase. Role of the conserved cysteine in the catalytic mechanism. J Biol Chem 274(19): 13242-13249.
- King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, et al. (2014) Aspergillomarasmine A overcomes metallo-beta-lactamase antibiotic resistance. Nature 510(7506): 503-506.
- Rotondo CM, Sychantha D, Koteva K, Wright GD (2020) Suppression of beta-Lactam Resistance by Aspergillomarasmine A Is Influenced by both the Metallo-beta-Lactamase Target and the Antibiotic Partner. Antimicrob Agents Chemother 64(4): e01386-19.
- Bahr G, Vitor-Horen L, Bethel CR, Bonomo RA, Gonzalez LJ, et al. (2018) Clinical Evolution of New Delhi Metallo-beta-Lactamase (NDM) Optimizes Resistance under Zn (II) Deprivation. Antimicrob Agents Chemother 62(1): e01849-17.
- Bahr G, Gonzalez LJ, Vila AJ (2021) Metallo-beta-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design. Chem Rev 121(13): 7957-8094.
- Tsivkovski R, Totrov M, Lomovskaya O (2020) Biochemical Characterization of QPX7728, a New Ultrabroad-Spectrum Beta-Lactamase Inhibitor of Serine and Metallo-Beta-Lactamases. Antimicrob Agents Chemother 64(6): e00130-20.
- Liu B, Trout REL, Chu GH, McGarry D, Jackson RW, et al. (2020) Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-beta-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J Med Chem 63(6): 2789-2801.
- Hecker SJ, Reddy KR, Lomovskaya O, Griffith DC, Rubio-Aparicio D, et al. (2020) Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-beta-lactamases. J Med Chem 63(14): 7491-7507.
- Riviere G, Oueslati S, Gayral M, Crechet JB, Nhiri N, et al. (2020) NMR Characterization of the Influence of Zinc (II) Ions on the Structural and Dynamic Behavior of the New Delhi Metallo-beta-Lactamase-1 and on the Binding with Flavonols as Inhibitors. ACS Omega 5(18):10466-10480.
- Halevas E, Mavroidi B, Pelecanou M, Hatzidimitriou AG (2021) Structurally characterized zinc complexes of flavonoids chrysin and quercetin with antioxidant potential. Inorg Chim Acta 523: 120407.
- Bhuiya S, Haque L, Pradhan AB, Das S (2017) Inhibitory effects of the dietary flavonoid quercetin on the enzyme activity of zinc(II)-dependent yeast alcohol dehydrogenase: Spectroscopic and molecular docking studies. Int J Biol Macromol 95: 177-184.
- Hossion AM, Zamami Y, Kandahary RK, Tsuchiya T, Ogawa W, et al. (2011) Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase IV as novel antibacterial agents. J Med Chem 54(11): 3686-3703.
- Vipin C, Saptami K, Fida F, Mujeeburahiman M, Rao SS, et al. (2020) Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS One 15(11): e0241304.
- Parellada J, Suarez G, Guinea M (1998) Inhibition of zinc metallopeptidases by flavonoids and related phenolic compounds: structure-activity relationships. J Enzyme Inhib 13(5): 347-59.
- Parellada J, Guinea M (1995) Flavonoid inhibitors of trypsin and leucine aminopeptidase: a proposed mathematical model for IC50 estimation. J Nat Prod 58(6): 823-829.
- Denny BJ, Lambert PA, West PW (2002) The flavonoid galangin inhibits the L1 metallo-beta-lactamase from Stenotrophomonas maltophilia. FEMS Microbiol Lett 208(1): 21-24.
- Lin RD, Chin YP, Hou WC, Lee MH (2008) The effects of antibiotics combined with natural polyphenols against clinical methicillin-resistant Staphylococcus aureus (MRSA). Planta Med 74(8): 840-846.
- Sahyon HA, Ramadan ENM, Mashaly MMA (2019) Synergistic Effect of Quercetin in Combination with Sulfamethoxazole as New Antibacterial Agent: In Vitro and In Vivo Pharmaceutical Chemistry Journal 53(9): 803-813.
- Hirai I, Okuno M, Katsuma R, Arita N, Tachibana M, et al. (2010) Characterisation of anti-Staphylococcus aureus activity of quercetin. International Journal of Food Science & Technology 45(6): 1250-1254.
- Abreu AC, Serra SC, Borges A, Saavedra MJ, McBain AJ, et al. (2015) Combinatorial Activity of Flavonoids with Antibiotics Against Drug-Resistant Staphylococcus aureus. Microb Drug Resist 21(6): 600-609.
- Siriwong S, Teethaisong Y, Thumanu K, Dunkhunthod B, Eumkeb G (2016) The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol Toxicol 17(1): 39.
- Siriwong S, Thumanu K, Hengpratom T, Eumkeb G (2015) Synergy and Mode of Action of Ceftazidime plus Quercetin or Luteolin on Streptococcus pyogenes. Evid Based Complement Alternat Med 2015: 759459.
- Pal A, Tripathi A (2020) Demonstration of bactericidal and synergistic activity of quercetin with meropenem among pathogenic carbapenem resistant Escherichia coli and Klebsiella pneumoniae. Microb Pathog 143: 104120.
-
Bogdan M. Benin, Trae Hillyer, Woo Shik Shin. Quercetin, A Potential Metallo-β-Lactamase Inhibitor for Use in Combination Therapy Against β-Lactam Antibiotic-Resistant Bacteria. Open J Pathol Toxicol Res. 1(1): 2021. OJPTR.MS.ID.000503.
-
Quercetin, Flavinols, Anti-Microbials, Combination Therapy, Metallo-β-Lactamase Inhibitor, Sulbactam, Tazobactam, Clavulanate
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






