 Research Article
Research Article
Determination of Nitrofuran Metabolites in Milk by Liquid Chromatography with Diode-Array Detector
Gotsiridze D2,Topchiyeva ShA3* Baramidze K1, Tsikarishvili K1 and Chikviladze T2
1Globaltest testing laboratory, Tbilisi state medical university, Georgia
2Department of pharmacy and toxicology by Tbilisi state medical university, Georgia
3National Academy of Sciences of Azerbaijan, Institute of Zoology, Baku, Azerbaijan
Topchiyeva ShA, National Academy of Sciences of Azerbaijan, Institute of Zoology, Baku, Azerbaijan.
Received Date:August 30,2022; Published Date: October 18,2022
Abstract
For the analysis of four metabolites of nitrofurans in raw milk - furazolidone, furaltadone, nitrofurazone and nitrofurantoin - a liquid chromatographic method using a diode detector was used. The method complies with the requirements of European Commission Resolution 2002/657 / ЕС. The sample was extracted by ethyl acetate, liquid-liquid extraction method was used, cleaning done by solid phase extraction on a silica gel column, after sample hydrolysis and derivatization with 2-nitrobenzaldehyde. The validation of the method was conducted following the European Union criteria for the analysis of veterinary drug residues in foods. The decision limits (CCα) were 0.14-0.32 μg/kg, and the detection capabilities (CCβ) 0.18-0.39 μg/ kg. The advantage of the method is that with relatively less financial costs it is possible to determine less than the minimum working limit for nitrofuran metabolites set by the EU (MRPL Minimum Required Performance Limit 1 μg /kg). This method is financially acceptable for developing countries.
Keywords: LC/DAD; Nitrofuran metabolites; Furazolidone; Furaltadone; Nitrofurazone; Nitrofurantoin; Milk
Highlights: Food safety requirements. Determination of nitrofurans in milk. Developing cheaper alternative for determination of nitrofuran residues. Developing inexpensive method for developing countries.
Introduction
Nitrofurans belong to the group of broad-spectrum synthetic antibiotics which are effectively used in veterinary medicine for the prevention and treatment of such gastrointestinal infections such as Escherichia coli, Salmonlella spp., Mycoplasma spp., Coccidia spp., Coliforms and protozoa which are found in animal products and water [1-3]. These medicines are rapidly metabolized within a few hours after ingestion, and residual metabolites can remain in the organism for several weeks and possibly months in the form of connected protein [4,5]. It was confirmed that these metabolites of nitrofurans represent a potential risk to human health due to their carcinogenic, teratogenic and mutagenic effects [6,7]. Due to food safety requirements, EU restricts the use of nitrofuran preparats in veterinary medicine due to their toxic, carcinogenic and mutagenic properties. [8,9]. After the EU the use of nitrofurans in veterinary medicine has been restricted in other countries too, in the United States, China, and Japan [4,10] However, due to their low cost and significant effectiveness, the use of nitrofurans is allowed or used illegally as a veterinary drug in some developing countries [11,12]. As of today, in the EU, in poultry and seafood, marginal permissible norm of the mentioned four nitrofurans (MRPL - Minimum Requried Performance Regulation 1442/95) is 1 μg / kg [1,13-15]. Illegal use of nitrofurans in the EU is controlled by official inspection and analysis services in accordance with the requirements of Council of Europe Directive 96/23 / EC. In the interest of exports, third world countries are forced to adopt the MRPL L set by the Council of Europe and thus reach the same threshold as EU laboratories [16]. Given the strict regulations and validation requirements of analytical methods set by the Council of Europe, the development of highly sensitive and specific analysis methods for the determination of nitrofuran residues in foodstuff is becoming an increasingly difficult task. (USP 2022; Guidance for Industry Bioanalytical Method Validation 2001; ICH Q2 A [17]. In accordance with the requirements of the European Union, the use of nitrofurans in stockbreeding is regulated by the legislation of Georgia, in particular, by the Resolution №499 of the Government of Georgia, the technical regulation is approved. Rule for the implementation of methods of analysis and interpretation of results for the examination of certain substances (substances) in animal and food of animal origin and their waste [18] which places quite strict requirements on the analytical methods used for this purpose. As of today, the analytical strategy for the quantification of nitrofurans is based on the determination of 4 stable and steady metabolites which can be released from proteins in the weak acid solution and then derivatized [19,20]. These stable metabolites are 3-amino-2-oxazolidone AOZ, 3-aminomorpholinomethyl- 2-oxazolidinone AMOZ, 1-aminohydantoin AHD and semi carbazide SEM [21]. Many analytical methods for the determination of nitrofuran metabolites have been developed in various matrices, such as seafood [22,23], animal feed [24], meat [25], milk [26], honey [27-29] and others [30,31]. These analytical methods are liquid chromatography-tandem mass spectrometry (LC-MS), [32] high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), [33] ultra-high-performance liquid chromatography- tandem mass spectrometry (UPLC-MS/MS), [34,35] enzyme-linked immunosorbent assay (ELISA), [36], Ultraperformance Liquid Chromatography-Photodiode Array Detection [37] etc. [38,39]. Not all of these methods meet the requirements due to the very low detection threshold required for the study substance MRPL = 1,0 mkg/kg). [40] Therefore, for its high sensitivity and accuracy of HPLC-MS / MS [41]. is mainly used the use of this method of analysis is limited due to the hardware and the high cost of servicing this hardware [31] Which is why it is still not possible to introduce this method in economically developing countries [42]. Therefore, it is relevant to develop a relatively inexpensive and simple analytical method for the determination of nitrofuran metabolites in food of animal origin. Liquid chromatographic method using diode detector is relatively less expensive and convenient compared to other detection methods listed above however, there is almost no data on this which is due to the fact that it is difficult to achieve the required detection limit using diode detector. [39,40,43]. In order to simplify the experimental process according to the requirements of MRPL, after extraction, we used the solid-phase cleaning to reach required detection limit. For solid-phase cleaning, instead of expensive cartridges (columns), we used our own silica gel columns. We have selected milk as a research matrix, as milk control ensures the safety of all dairy products. As a result, we have obtained a sensitive, fast and relatively inexpensive method that meets the requirements of a set MRPL. The method can be successfully used for the simultaneous determination of four metabolites of nitrofurans in milk and guarantees the safe use of milk and consequently products made from this milk. This method is financially affordable and can be used in developing countries.
Materials and Methods
Reagents and Chemicals: Hexane (95%), anhydrous dipotassium hydrogen orthophosphate, 35% hydrochloric acid, sodium hydroxide, 2-nitrobenzaldehyde (2-NBA), acetonitrile (HPLC grade), dimethyl sulfoxide anhydrous, ≥99.9%, ethyl acetate, sodium acetate ACS reagent, ≥99.0% were from Sigma Aldrich Chemical Company (Germany) and Kiselgel 60 from Roth (Germany). Ultrapure water was filtered through a Milli-Q system Millipore (USA). The metabolites AOZ, AMOZ, AHD, and SEM, and the internal standards AOZ-d4, AMOZ-d5, (2-NP-13C3) AHD and 1,2- (13C15 N2) SEM were supplied by Sigma (Aldrich Chemical Company, Germany).
Standard solutions
Individual standard stock solutions of 1 mg/mL were prepared in acetonitrile. Working solutions of 10 ng/mL were diluted by water. All standard stock soutions were stored -20ºC, and the working solutions were stored in refrigerator. The concentration and content of mix standard solution was used to spiked samples with AMOZ, AOZ, AHD and SEM at a 8, 16, 20, 28 and 36 ng/mL respectively. The concentration and content of internal mix standard solutions were used AOZ-d4, AMOZ-d5, (2-NP-13C3) AHD and 1,2- (13C15 N2) SEM at a 40, 40, 100 and 100 ng/mL, respectively.
Preparation of silica gel columns
The silica gel column was prepared by the dry method (18 x 75 mm). The prepared column was washed with 5 ml of hexane, dried under vacuum, then washed with 10 ml of acetonitrile, and again dried under vacuum.
Sample preparation
Nitrofurans and their metabolites were prepared using the same method as previously described, with some modifications [44,45]. The cooled milk was centrifuged at 3500 rpm for 15 minutes at +4°C and the fat layer was carefully removed. 2.0 ± 0.05 g of homogenized raw milk was weighed into a 50 ml polypropylene centrifuge tube. Standard spiking solution mix (50, 100, 150 and 200 μL), internal standard solution mix (100 μL), 5 mL of 0.1 M hydrochloric acid solution and 50μl of a solution of 2-nitrobenzaldehyde in dimethyl sulfoxide (DMSO) (8 mg ± 0.6 mg in 5 ml of DMSO) were added. Thoroughly stirred for 1minute (with vigorous stirring, the extract acquires a jelly-like consistency) and incubated overnight at 37°C to hydrolyze protein-bound NP metabolites and convert the metabolites to their nitrophenyl (NP) derivatives. After the sample solution was cooled to room temperature, 500 μl of di-potassium hydrogen orthophosphate solution, at least 300 μl of sodium hydroxide solution were added to adjust the pH to 7.0 ± 0.5, and 5 ml of ethyl acetate; Thoroughly mixed for 1 minute (with intensive stirring, the extract acquires a jelly consistency); centrifuged at 3500 rpm for 15 minutes at +4°C, ethyl acetate layer transferred to a silica gel column for sample purification at a rate of 5 ml / min. After completion of sampling, the column was washed with 10 ml of ethyl acetate, the column pumped out with a vacuum, the eluates were combined and was evaporated in a moderate flow of nitrogen, the residue was dissolved in 1 ml of hexane, 1 ml of the mobile phase was added, mixed for 1 minute, centrifuged at 3500 rpm for 15 minutes at +4°C, the hexane layer was removed and the resulting aqueous phase was used for chromatography.
Instrumentation
LC/DAD: The LC/DAD system consisted of an Agilent Series 1260 HPLC system (Agilent Technologies, Germany) with DAD detector. The chromatography was performed in a C18 column 3 μm x 2 mm 150 mm (Phenomenex, Torrance, CA, USA), connected to a C18 precolumn 3 μm x 2 mm x 4 mm (Phenomenex, USA). The mobile phase was Acetonitrile: 0.01 M sodium acetate buffer pH 6, 0 - 250:750, λ 376 nm, flow rate of 1 mL/min, Injection volume was 50 μL. The column was thermostated at 300C. All determinations were carried out under standard conditions: Air temperature - (20 ± 5) 0С, atmospheric pressure - 84.0 - 106.7 kPa (630 - 800 mm Hg), air humidity no more than - 80%, mains voltage 198 - 242 V, frequency AC - 50 ± 1 Hz.
Results
Specificity/Selectivity
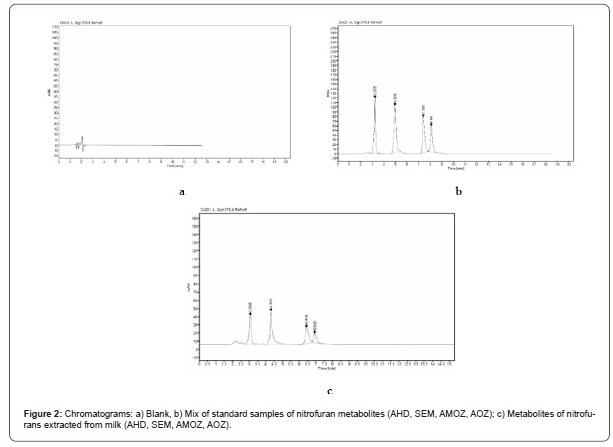
Specificity/selectivity were evaluated via analysis of blank matrix samples fortified with standards of nitrofuran metabolites (concentration of 1 μg/kg each). According to analysis for the studied substances of the nitrofuran group, the specific wavelength is 376 nm (Figure 1), no significant peaks with an S/N (signal to noise) ratios of 3 or more and chromatographic interference were being observed at the retention times of the targeted nitrofuran metabolites (Figure 2); The coefficient of variation of the specificity of the results obtained during the working day is in the range of 0.02-0.17, during the working week - in the range of 0.02-0.26. Which indicates satisfactory, required by Decision 2002/657/EC (Figure 1 and 2).
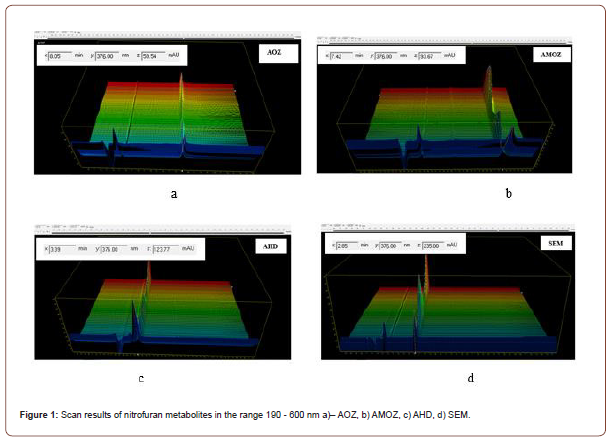
LOD and LOQ
• For furazolidone (AOZ) S/N (signal to noise) at 0,32 μg/kg is 3,2; at 0,91 μg/kg 10,8.
• For furaltadone (AMOZ) S/N (signal to noise) at 0,35 μg/kg is 2,8; at 0,95 μg/kg- 9,9.
• For nitrofurantoin (AHD) S/N (signal to noise) at 0,29 μg/kg is 3,0; at 0,91 μg/kg - 10,5.
• For nitrofurazone (SEM) S/N (signal to noise) at 0,38 μg/kg is 2,6; at 0,99 μg/kg - 10,8.
The value of LOD and LOQ are presented in (Table 1).
Table 1:The mean value recovery, LOD, and LOQ of four compounds (n = 6).
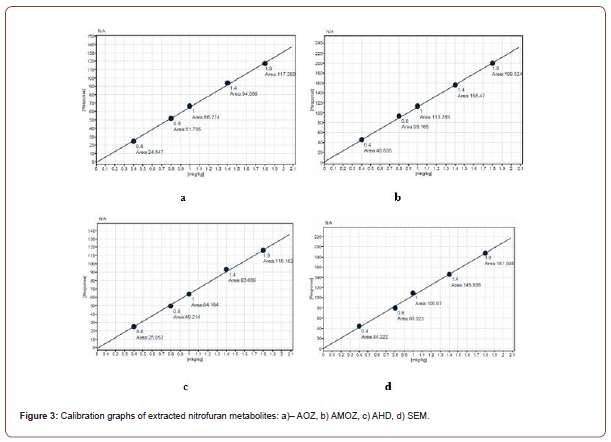
Linearity
To construct calibration graphs, we used 5 concentrations: 0.4, 0.8, 1, 1.4 and 1.8 μg/kg for all four metabolites. The graphs are linear in the indicated range and are acceptable as long as the correlation coefficient r2 is above 0.999 (Figure 3).
Recovery
The method recoveries and RSDs were determined from 6 replicates at four concentration levels spiking blank samples over three days. The recovery results were observed in acceptable range of 70-110%. All the data relating to method recovery and precision were summarisedin (Table 4); mean recoveries ranging and CV% values were satisfactory, required by Decision 2002/657/EC [14]. The mean value of recovery, LOD, and LOQ were presented in (Table 2).
Table 2:The mean value CCα and CCβ.

Decision limit (CCα) and detection capability (CCβ)
The CCα and CCβ for banned substances were calculated with the application of the following formula:
CCα = C1 + 2.33 x SDwIR
where in C1 is lowest concentration level of the validation study (MRPL) and SDwIR is the standard deviation from within-laboratory reproducibility.
CCβ = CCα + 1.64 x SDwIR,CCα
where in SDwIR,CCα is standard deviation at CCα concentration.
For each compound of CCα and CCβ were calculated from the linearity study.
The mean value CCα and CCβ were presented in (Table 2).
Discussion
Sample purification is important for increasing the sensitivity of the method, however, there are works in which analysis without purification is carried out. Fatih Alkan et al. nitrofuran metabolites in honey, milk, poultry and fish samples were subjected to acid hydrolysis followed by derivatization with nitrobenzaldehyde and liquid-liquid extraction with ethyl acetate, and then without purification, LC-MS/MS determination [46]. In rare cases, liquid cleaning is used. An LC-ESI-MS-MS method for the analysis of metabolites of four nitrofurans (furazolidone, furaltadone, nitrofurazone and nitrofurantoin) in raw milk has been developed. The samples were achieved by hydrolysis of the protein-bound drug metabolites, derivatization with 2-nitrobenzaldehyd (2-NBA) and clean-up extraction liquid-liquid with ethyl acetate [47]; Bock et al. liquid-liquid separation is used to purify the obtained extracts [48,49]. Most often, solid-phaze cleaning is used through a variety of expensive cartridges. Mottier et al. [50] quantitative determinated of four nitrofuran metabolites in meat by isotope dilution liquid chromatography– electrospray ionisation–tandem mass spectrometry. This study used liquid–liquid extraction method and clean-up on a polymeric solid phase extraction cartridge (SPE) are then performed before LC–MS/MS analysis by positive electrospray ionisation (ESI) Barbosa et al. Determination of nitrofurans in animal feeds by liquid chromatography-UV photodiode array detection and liquid chromatography-ionspray tandem mass spectrometry. Following ethyl acetate extraction at mild alkaline conditions and purification on NH2 column (SPE), the nitrofurans are determined using liquid chromatography with photodiode-array detection (LC-DAD) [51] Tomasz Śniegocki et al. After incubation the sample was purified by solid phase extraction technique [52] De La Calle et al. The sample was extracted with hydrochloric acid and derivatized with 2-nitrobenzaldehyde, with 1,2[(15)N2(13)C] SEM as the internal standard. The extract was neutralized and purified on a solid-phase extraction cartridge. In this study, a polystyrene–divinylbenzene copolymer (SDB–L) was used as sorbent material, solid phase extraction (SPE) with polymeric sorbent SEM was determined by reversed-phase LC with detection by MS/MS [53] The use of SPE technique was previously described by Leitner et al. [54] and they indicated that sample preparation protocol including cleanup with polymeric sorbent is simple and robust; Kaufmann et al. used an ultra-high performance liquid chromatography based method, coupled with high resolution mass spectrometry (UHPLC-HRMS), was developed to permit the detection and quantification of various nitrofuran and chloramphenicol residues in a number of animal based food products. This method is based on the hydrolysis of covalently bound metabolites and derivatization with 2-nitrobenzaldehyde. Clean-up is achieved by a liquid/liquid and a reversed phase/solid phase extraction [28] The method used by Pak-Sin et al. involves overnight acid hydrolysis and simultaneous derivatization of the released side chains with 2-nitrobenzaldehyde. During hydrolysis, the bound metabolites are hydrolyzed to the side chains. After pH adjustment and solid-phase extraction cleanup, the derivatives are detected and quantitated using a liquid chromatography−tandem mass spectrometry [55]. In accordance with the MRPL requirement We attempted to achieve the required detection limit by solid phase purification of the sample after extraction. For solid-phase cleaning, instead of expensive cartridges, we used columns with silica gel of our own preparation. As a result, we have obtained a sensitive, fast and relatively inexpensive method that meets the requirements of MRPL. The method can be successfully used for the simultaneous determination of four metabolites of nitrofurans in milk and guarantees the safe use of milk and consequently products made from this milk in developing countries and regions.
Evaluation
This method has been developed and tested internally in accordance with the requirements of European Commission Decision 2002/657/EC [14]. The effectiveness of the method developed by us, is confirmed by the results of professional testing, implemented in the testing laboratory “GlobalTest” and accredited according to ISO 17025 by the Accreditation Agency of Georgia.
Conclusion
Developed methods indicate accordance with Decision 2002/657/EC [14,56-58]. The CCα and CCβ are below the MRPL of 1 μg kg-1. Due to its Cost effectiveness, it is available in developing countries and can be successfully used for the simultaneous determination of four metabolites of nitrofurans in milk, and guarantees the safe use of milk and, consequently, products made from this milk in developing countries and regions.
Acknowledgement
The authors are grateful to the study participants for their invaluable contribution to this research project.
Conflict of interests
The authors have no conflict of interests to declare.
References
- An H, Parrales L, Wang K, Cain T, Hollins R, et al. (2015) Quantitative Analysis of Nitrofuran Metabolites and Chloramphenicol in Shrimp Using Acetonitrile Extraction and Liquid Chromatograph-Tandem Mass Spectrometric Detection: A Single Laboratory Validation. J AOAC Int 98(3): 602-608.
- El-Demerdash A, Song F, Reel RK, Hillegas J, Smith RE (2015) Simultaneous Determination of Nitrofuran Metabolites and Chloramphenicol in Shrimp with a Single Extraction and LC-MS/MS Analysis. J AOAC Int 98(3): 595-601.
- Gotsiridze D, Baramidze K, Chikviladze T, Otarashvili T (2022) Nitrofurans and their metabolites in food. Journal of Experimental and Clinical Medicine Georgia: 4.
- De la Torre CAL, Blanco JE, Silva JT, Paschoalin VM, Conte Júnior CA (2015) Chromatographic detection of nitrofurans in foods of animal origin. Arq Inst Biol 82: 1-9.
- Johnston L, Croft M, Murby J (2015) Fortified versus incurred residues: extraction of furazolidinone metabolite from prawn. Anal Bioanal Chem 407(16): 4535-4540.
- Kulikovskii AV, Gorlov IF, Slozhenkina MI, Vostrikova NL, Ivankin AN, et al. (2019) Determination of Nitrofuran Metabolites in Muscular Tissue by High-Performance Liquid Chromatography with Mass Spectrometric Detection. J Anal Chem 74: 906-912.
- Zuma NH, Aucamp J, N’Da DD (2019) An update on derivatisation and repurposing of clinical nitrofuran drugs. Eur J Pharm Sci 140: 1051092.
- Chang GR, Chen HS, Lin FY (2016) Analysis of banned veterinary drugs and herbicide residues in shellfish by liquid chromatography-tandem mass spectrometry (LC/MS/MS) and gas chromatography-tandem mass spectrometry (GC/MS/MS). Mar Pollut Bull 113(1): 579-584.
- García V, Montero I, Bances M, Rodicio R, Rodicio MR (2017) Incidence and Genetic Bases of Nitrofurantoin Resistance in Clinical Isolates of Two Successful Multidrug-Resistant Clones of Salmonella enterica Serovar Typhimurium: Pandemic “DT 104” and pUO-StVR2. Microb Drug Resist 23(4): 405-412.
- Sheng LQ, Chen MM, Chen SS, Du NN, Liu ZD, et al. (2013) High-performance liquid chromatography with fluorescence detection for the determination of nitrofuran metabolites in pork muscle. Food Addit Contam Part A 30(12): 2114-2122.
- Shi Z, Li Q, Xu D, Huai, Q, Zhang H (2016) Graphene-based pipette tip solid-phase extraction with ultra-high performance liquid chromatography and tandem mass spectrometry for the analysis of carbamate pesticide residues in fruit juice. J Sep Sci 39(22): 4391-4397.
- Zhao H, Guo W, Quan W, Jiang J, Qu B (2016) Occurrence and levels of nitrofuran metabolites in sea cucumber from Dalian, China. Food Addit Contam Part A 33(11): 1672-1677.
- Commission Decision (2002) Commission Decision 2002/657/EC of 12 August 2002 implementing Council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal of the European Communities L221: 8-36.
- Commission Decision (2003) Commission Decision 2003/181/EC of 13 March 2003 amending Decision 2002/657/EC as regards the setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin. Official Journal of the European Communities L71: 17-18.
- Yu Y, Li N, Jin Q, Ji Z, Sun Z, et al. (2019) Novel fluorescence labeling reagent 4-(carbazole-9-yl)-benzyl chloroformate and its application in the determination of nitrofuran metabolites compounds in foodstuffs by high performance liquid chromatography with fluorescence detection. Microchem J 145: 9-17.
- Commission Directive (2004) Commission Directive 2004/1/EC of 6 January 2004 amending Directive 2002/72/EC as regards the suspension of the use of azodicarbonamide as blowing agent. Official Journal of the European Communities L7: 45-46.
- (1997) ICH Q2 A (CPMP/ICH/381/95), Validation of analytical procedure: Methodology, London UK.
- (2016) Resolution No. 499 of the Government of Georgia "Technical Regulation - Rules for the Analysis of the Results of the Study of Certain Substances (Substances) in Live Animals and Food of Animal Origin and the Use of Methods for the Interpretation of the Results of the Study.
- Alkan F, Kotan A, Ozdemir N (2016) Development and Validation of Confirmatory Method for Analysis of Nitrofuran Metabolites in Milk, Honey, Poultry Meat and Fish by Liquid Chromatography-Mass Spectrometry. Maced Vet Rev 39: 15-22.
- Diblikova I, Cooper KM, Kennedy DG, Franek M (2005) Monoclonal antibody-based ELISA for the quantification of nitrofuran metabolite 3-amino-2-oxazolidinone in tissues using a simplified sample preparation. Anal Chim Acta 540(1): 285-292.
- Du NN, Chen MM, Sheng LQ, Chen SS, Xu HJ, et al. (2014) Determination of nitrofuran metabolites in shrimp by high performance liquid chromatography with fluorescence detection and liquid chromatography–tandem mass spectrometry using a new derivatization reagent. J Chromatogr A 1327: 90-96.
- Øye BE, Couillard FD, Valdersnes S (2019) Complete validation according to current international criteria of a confirmatory quantitative method for the determination of nitrofuran metabolites in seafood by liquid chromatography isotope dilution tandem mass spectrometry. Food Chem 300: 125175.
- Gotsiridze D, Baramidze K, Chikviladze T, Otarashvili T, Ioramashvili H (2020) Development and validation of HPLC method for the determination of nitrofuran metabolites in fish. Collection of scientific works. Tbilisi state medical university 54: 42-45.
- Li J, Liu JX, Wang JP (2009) Multi determination of Four Nitrofurans in Animal Feeds by a Sensitive and Simple Enzyme-Linked Immunosorbent Assay. J Agric Food Chem 57(6): 2181-2185.
- Violante FGM, de Oliveira Rosas C, de Freitas Guimaraes E, de Carvalho Vital H, Zuniga NOC, et al. (2018) Feasibility study for the development of a certified reference material of nitrofuran metabolites in chicken breast muscle from incurred samples. Measurement 129: 368-374.
- Li J, Ren X, Diao Y, Chen Y, Wang Q, et al. (2018) Multiclass analysis of 25 veterinary drugs in milk by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem 257: 259-264.
- Shendy AH, Al-Ghobashy MA, Gad Alla SA, Lotfy HM (2016) Development and validation of a modified QuEChERS protocol coupled to LC-MS/MS for simultaneous determination of multi-class antibiotic residues in honey. Food Chem 190: 982-989.
- Kaufmann A, Butcher P, Maden K, Walker S, Widmer M (2015) Determination of nitrofuran and chloramphenicol residues by high resolution mass spectrometry versus tandem quadrupole mass spectrometry. Anal Chim Acta 862: 41-52.
- Gotsiridze D, Baramidze K, Chikviladze T, Otarashvili T, Ioramashvili H (2021) Development and validation of HPLC method for the determination of nitrofuran metabolites in honey. Collection of scientific works. Tbilisi state medical university 55: 60-63.
- Zhang Y, Qiao H, Chen C, Wang Z, Xia X (2016) Determination of nitrofurans metabolites residues in aquatic products by ultra-performance liquid chromatography–tandem mass spectrometry. Food Chem 192: 612-617.
- Wang Y, Chan W (2016) Automated In-Injector Derivatization Combined with High-Performance Liquid Chromatography-Fluorescence Detection for the Determination of Semicarbazide in Fish and Bread Samples. J Agric Food Chem 64(13): 2802-2808.
- Tribalat, L, Paisse O, Dessalces G, Loustalot MF (2006) Advantages of LC-MS-MS compared to LC-MS for the determination of nitrofuran residues in honey. Anal Bioanal Chem 386(7): 2161-2168.
- Park MS, Kim KT, Kang JS (2017) Development of an analytical method for detecting nitrofurans in bee pollen by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B 1046: 172-176.
- Aldeek F, Hsieh KC, Ugochukwu ON, Gerard G, Hammack W (2018) Accurate Quantitation and Analysis of Nitrofuran Metabolites, Chloramphenicol, and Florfenicol in Seafood by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry: Method Validation and Regulatory Samples. J Agric Food Chem 66(20): 5018-5030.
- Gong J, Li J, Yuan H, Chu B, Lin W, et al. (2020) Determination of four nitrofuran metabolites in gelatin Chinese medicine using dispersive solid phase extraction and pass-through solid phase extraction coupled to ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B 1146: 122018.
- Cháfer-Pericás C, Maquieira Á, Puchades R (2010) Fast screening methods to detect antibiotic residues in food samples. TrAC Trends Anal. Chem 29(9): 1038-1049.
- Kangkang Wang, Yuli Kou, Meng Wang, Xin Ma, Jide Wang (2020) Determination of Nitrofuran Metabolites in Fish by Ultraperformance Liquid Chromatography-Photodiode Array Detection with Thermostatic Ultrasound-Assisted Derivatization. ACS Omega 5(30): 18887-18893.
- Horne E, Cadogan A, O’Keeffe M, Hoogenboom LAP (1996) Analysis of Protein-bound Metabolites of Furazolidone and Furaltadone in Pig Liver by High-performance Liquid Chromatography and Liquid Chromatography-Mass Spectrometry. Analyst 121: 1463-1468.
- Chumanee S, Sutthivaiyakit S, Sutthivaiyakit P (2009) New Reagent for Trace Determination of Protein-Bound Metabolites of Nitrofurans in Shrimp Using Liquid Chromatography with Diode Array Detector. J Agric Food Chem 57(5): 1752-1759.
- Luo X, Sun Z, Wang X, Yu Y, Ji Z, et al. (2019) Determination of nitrofuran metabolites in marine products by high performance liquid chromatography–fluorescence detection with microwave-assisted derivatization. New J Chem 43: 2649-2657.
- Shi Z, Li Q, Xu D, Huai, Q, Zhang H (2016) Graphene-based pipette tip solid-phase extraction with ultra-high performance liquid chromatography and tandem mass spectrometry for the analysis of carbamate pesticide residues in fruit juice. J Sep Sci 39(22): 4391-4397.
- Ong X, Sun Z, Liu S, Luo X, Li G (2019) Determination of Semicarbazide in Foodstuffs by HPLC with Fluorescence Detection Using 2-Formylphenylboronic Acid as Derivatization Reagent. Chromatographia 82: 1051-1058.
- Fernando R, Munasinghe DMS, Gunasena ARC, Abeynayake P (2017) Determination of nitrofuran metabolites in shrimp muscle by liquid chromatography-photo diode array detection. Food Control 72: 300-305.
- Śniegocki T, Posyniak A, Żmudzki J (2008) Determination of nitrofuran metabolite residues in eggs by liquid chromatography-mass spectrometry. Bull Vet Inst Pulawy 52: 421.
- Irma EA Bongers, Milou GM van de Schans, Coen VM Nibbeling, Ingrid JW Elbers, Bjorn JA Berendsen, et al. (2021) A single method to analyse residues from five different classes of prohibited pharmacologically active substances in milk. J Food Additives & Contaminmants: Part A Chem Anal Control Expo Risk Assess 38(10): 1717-1734.
- Fatih Alkan, Arzu Kotan, Nurullah Ozdemir (2016) Development and Validation of Confirmatory Method for Analysis of Nitrofuran Metabolites in Milk, Honey, Poultry Meat and Fish by Liquid Chromatography-Mass Spectrometry Macedonian Veterinary Review. 39(1).
- Lech Rodziewic (2008) Determination of nitrofuran metabolites in milk by liquid chromatography-electrospray ionization tandem mass spectrometry Journal of chromatography. B Analytical technologies in the biomedical and life sciences 864(1): 156-60.
- Bock C, Stachel C, Gowik P (2007) Validation of a confirmatory method for the determination of residues of four nitrofurans in egg by liquid chromatography–tandem mass spectrometry with the software InterVal. Anal Chim Acta 586(1): 348-358.
- McCraken RJ, Spence DE, Floyd SD, Kennedy DG (2001) Evaluation of the residues of furazolidone and its metabolite, 3-amino-2-oxazolidone (AOZ), in eggs. Food Addit Contam 18(11): 954-961.
- Mottier P, Khong SP, Gremaud E, Richoz J, Delatour T, et al. (2005) Quantitative determination of four nitrofuran metabolites in meat by isotope dilution liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr A 1067(1): 85-91.
- Barbosa J, Moura S, Barbosa R, Ramos F, Silveria MIN (2007) Determination of nitrofurans in animal feeds by liquid chromatograpgy-UV photodiode array detection and liquid chromatography-ionspray tandem mass-spectrometry. Anal Chim Acta 586(1): 359-365.
- Tomasz Śniegocki, Marta Giergiel, Bartosz Sell, Andrzej Posyniak (2018) New Method of Analysis of Nitrofurans and Nitrofuran Metabolites in Different Biological Matrices Using UHPLC-MS/MS. J Vet Res 62(2): 161-166.
- De La Calle MB, Szilagyi Z (2006) Determination of semicarbazide in fresh egg and whole egg powder by liquid chromatography/tandem mass spectrometry: interlaboratory validation study. J AOAC Int 89(6): 1664-1671.
- Leitner A, Zoller P, Linder W (2001) Determination of the metabolites of nitrofuran antibiotics in animal tissue by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 939(1): 49-58.
- Pak-Sin Chu, Mayda I Lopez (2007) Determination of Nitrofuran Residues in Milk of Dairy Cows Using Liquid Chromatography−Tandem Mass Spectrometry. Journal of Agricultural and Food Chemistry 55 (6): 2129-2135.
- Chang GR, Chen HS, Lin FY (2016) Analysis of banned veterinary drugs and herbicide residues in shellfish by liquid chromatography-tandem mass spectrometry (LC/MS/MS) and gas chromatography-tandem mass spectrometry (GC/MS/MS). Mar Pollut Bull 113(1): 579-584.
- (2001) Guidance for Industry Bioanalytical Method Validation U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM).
- Zuma NH, Aucamp J, N’Da DD (2019) An update on derivatisation and repurposing of clinical nitrofuran drugs. Eur J Pharm Sci 140: 1051092.
-
Gotsiridze D,Topchiyeva ShA* Baramidze K, Tsikarishvili K and Chikviladze T. Determination of Nitrofuran Metabolites in Milk by Liquid Chromatography with Diode-Array Detector. Open J Pathol Toxicol Res. 1(3): 2022. OJPTR.MS.ID.000511.
-
Liquid extraction, Broad-spectrum synthetic antibiotics, Carcinogenic, Teratogenic, Mutagenic, Chromatography-photodiode, Diode detector, Solid-phase cleaning, Milli-Q system Millipore, Standard spiking, Dimethyl sulfoxide, Hydrolyze protein-bound
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






