 Case Report
Case Report
Caplacizumab Treatment in a Case of Resistant Thrombotic Thrombocytopenic Purpura
Zarrin Hossein-Zadeh1*, Behnam Rafiee1, Bebu Ram1, Jonathan Weltz1 and Mark Friedman1
1Department of Pathology, NYU Langone Long Island, USA
Zarrin Hossein-zadeh, Department of Pathology, NYU Langone Long Island, USA.
Received Date: December 06, 2021; Published Date: December 17, 2021
Abstract
A 27-year-old G2P1 woman, with no significant past medical history admitted at 37 weeks of gestation after complaints of nausea, vomiting, diarrhea, and vaginal bleeding. An emergent vaginal delivery was performed which was complicated by significant blood loss requiring massive transfusion. On admission, laboratory values showed hemoglobin 6.4 g/dL and platelets 7 K/uL. Following delivery and transfusion, the patient’s hemoglobin and platelets increased to 9.8 g/dL and 22 K/uL, respectively. Peripheral blood smear showed schistocytosis. Thrombotic thrombocytopenic purpura was thought to be the most probable cause. Stat daily TPE was initiated. The ADAMTS-13 enzyme activity level results confirmed severe deficiency (<5%, reference range ≥61%) with an inhibitor level of 2.8 [reference range ≤0.4]). Despite daily therapeutic plasma exchange (TPE) and prednisone, the patient was not maintaining a sustained response. Thus, additional measures were taken including use of daily caplacizumab. Notably, after administration of caplacizumab, the platelet count consistently increased and remained stable. On discharge, the platelet count was 248 K/uL.
Keywords: Neurology; Acute medicine; Critical care medicine; Emergency medicine
Introduction
Thrombotic thrombocytopenic purpura (TTP) classically characterized by microangiopathic hemolytic anemia, thrombocytopenia, renal failure, fever and neurologic symptoms is a life-threatening condition, which even with proper treatment, has a 20% mortality rate and a relapse rate of up to 36%. TTP in pregnancy is an uncommon but serious disorder associated with high maternal and perinatal complications. TTP during pregnancy can increase the risk of stillbirth especially in second trimester, thus timely diagnosis and management of TTP in pregnancy would decrease stillbirth rate significantly. Here we present a case on the utilization of caplacizumab in resistant pregnancy induced TTP, rather than a first line treatment. Author and co-authors of this study declare that they do not have an affiliation, financial or otherwise, with a pharmaceutical company. They have no conflict of interest to disclose. This study is in accordance with all ethical standards of the responsible committee on human experimentation (institutional and national). This article does not contain any studies with animal subjects performed by the any of the authors. A verbal informed consent was obtained from patient included in the study in accordance of the institutional review board committee. This study contains no patient’s health information (PHI), does not required IRB approval.
Case Presentation
Our patient is a 27-year-old G2P1 woman, with no significant past medical history, who was admitted at 37 weeks of gestation after complaints of nausea, vomiting, diarrhea, and vaginal bleeding. An emergent vaginal delivery was performed which was complicated by significant blood loss requiring activation of the massive transfusion protocol. Admission laboratory values, including complete blood count (CBC), comprehensive metabolic panel, lactate dehydrogenase (LDH), and haptoglobin, were significant for Coombs-negative hemolytic anemia (hemoglobin hgb] 6.4 g/dL) and severe thrombocytopenia (platelets 7 K/uL). Following the delivery and transfusion, the patient’s hemoglobin and platelets increased to 9.8 g/dL and 22 K/uL, respectively. Peripheral blood smear confirmed severe thrombocytopenia and showed 2+ schistocytosis. Of note, the patient’s prior platelet count at 26 weeks gestation was 275 K/uL. The working differentials included HELLP syndrome, thrombotic thrombocytopenic purpura (TTP), immune thrombocytopenic purpura, hemolytic uremic syndrome, disseminated intravascular coagulation, fatty liver of pregnancy, and pre-eclampsia. However, given normal fibrinogen, prothrombin time (PT), activated partial thromboplastin time (aPTT), liver function tests, creatinine, and the degree of thrombocytopenia, TTP was thought to be the most probable cause. The calculated PLASMIC score of 7, predicted a high risk for severe ADAMTS-13 deficiency. Stat daily therapeutic plasma exchange (TPE) was initiated (1 plasma volume and 2 g of calcium gluconate). The ADAMTS-13 enzyme activity level results confirmed severe deficiency (<5%, reference range ≥61%) with an inhibitor level of 2.8 [reference range ≤0.4]). Prednisone oral immunosuppression (75mg daily) was added. The Figure below tracks the patient’s daily pre- and post-TPE platelet counts, hgb levels, and LDH. As depicted, despite daily TPE, the patient was not maintaining a sustained response. Thus, additional measures were taken, which included increasing the replacement volume from 1 to 1.5 plasma volumes, use of cryodepleted plasma, rituximab, and addition of daily caplacizumab. The patient was discharged 1-day post-TPE #20 on prednisone, rituximab, and caplacizumab. Notably, after administration of caplacizumab, the platelet count consistently increased and remained stable despite refractory thrombocytopenia to other lines of treatment. On discharge, the platelet count was 248 K/uL. At 1 month follow up, caplacizumab was discontinued and the ADAMTS-13 enzyme activity was 41% with a platelet count of 278 K/uL. Prednisone was discontinued at 2 months with an ADAMTS-13 level of 82%. At 3 months, ADAMTS-13 was 87% and all other laboratory parameters remained normal.
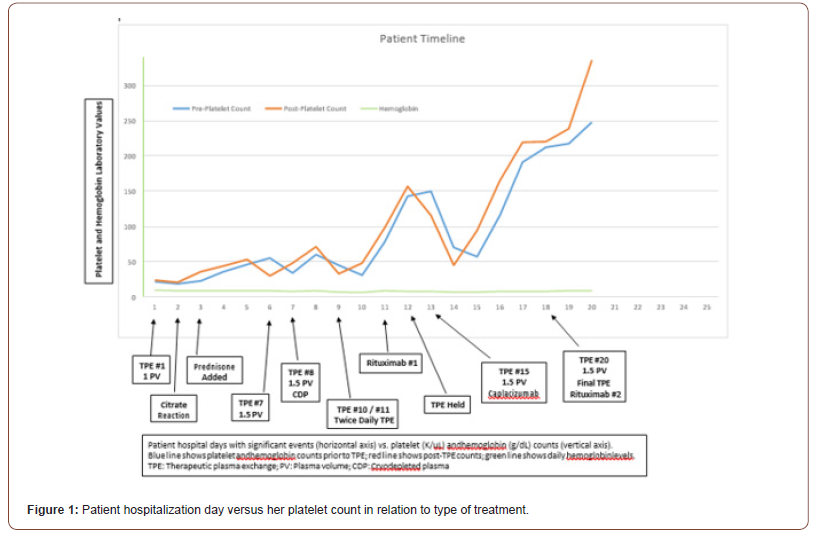
Discussion
during pregnancy can increase the risk of stillbirth especially in second trimester; thus, timely diagnosis and management of TTP in pregnancy would decrease the stillbirth rate significantly [1,2]. A calculated score based on platelet count, evidence of hemolysis, active cancer, history of solid organ transplant, MCV, INR, and creatine levels (PLASMIC Score) is used to risk stratify individuals for further workup and treatment of TTP [3]. The first line treatment for TTP is daily TPE and glucocorticoids. In case of refractory disease, additional measures, including increased replacement volume, use of cryodepleted plasma, twice-daily TPE, and rituximab, may be recommended. Nonetheless, if none of the above-mentioned treatments are effective, caplacizumab, a novel nanobody agent which attaches to A1 domain of vWF and prevents binding of vWF to glycoprotein Ib on the platelets, can be instilled [4]. Multiple studies have shown the benefits of caplacizumab use in management of patients with TTP including the TITAN study (2010-2014), which evaluated the effect of caplacizumab in addition to the standard-of-care treatment. The study results showed a faster improvement and less episodes of relapse in TTP patients who received caplacizumab plus standard treatment [5].
Several prospective randomized control trials have concluded that caplacizumab induced a faster resolution of the acute TTP episode than the placebo. Additionally, the platelet-protective effect of caplacizumab was maintained during the treatment period [5]. A study by Coppo and colleagues that treated ninety TTP patients with a triplet regimen (TPE, corticosteroids, rituximab, and caplacizumab versus conventional treatment) concluded that caplacizumab was associated with fewer adverse effects, faster platelet count recovery, and shorter hospitalization [6-11].
Our patient had reduced ADAMTS-13 activity, schistocytosis, normal liver function tests, and thrombocytopenia which increased the likelihood of TTP. After administration of caplacizumab the platelet count consistently increased and have remained stable in follow up.
Acknowledgement
None.
Conflict of interest
None.
References
- Martin JN Jr, Bailey AP, Rehberg JF, Owens MT, Keiser SD, et al. (2008) Thrombotic thrombocytopenic purpura in 166 pregnancies: 1955–2006. Am J Obstet Gynecol 199(2): 98-104.
- Burrows RF, Kelton JG (1993) Fetal thrombocytopenia and its relation to maternal thrombocytopenia. N Engl J Med 329(20): 1463-1466.
- Oliveira DS, Lima TG, Benevides FLN, Barbosa SAT, Oliveira MA, et al. (2019) Plasmic score applicability for the diagnosis of thrombotic microangiopathy associated with ADAMTS13-acquired deficiency in a developing country. Hematol Transfus Cell Ther 41(2): 119-124.
- Scully M, Cataland SR, Peyvandi F, Coppo P, Knöbl P, et al (2019) Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med 380(4): 335-346.
- Peyvandi F, Scully M, Kremer Hovinga JA, Knöbl P, Cataland S, et al. (2017) Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost 15(7): 1448-1452.
- Coppo P, Bubenheim M, Azoulay E, Galicier L, Malot S, et al. (2021) A regimen with caplacizumab, immunosuppression and plasma exchange prevent unfavorable outcomes in immune-mediated TTP. Blood 137(6): 733-742.
- Budhathoki N, Timilsina S, Ram B, Marks D (2021) Bone marrow necrosis and fat embolism syndrome: a near fatal complication in previously undiagnosed sickle beta + thalassaemia. BMJ Case Rep 14(1): e238317.
- Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, et al. (2016) TITAN Investigators Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. 374(6): 511-522.
- Chang JC (2018) TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J 16: 20.
- Wada H, Matsumoto T, Suzuki K, Imai H, Katayama N, et al (2018) Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb J 16: 14.
- Masias C, Cataland SR (2017) Novel therapies in thrombotic thrombocytopenic purpura. Res Pract Thromb Haemost. 2(1): 19-26.
-
Zarrin Hossein-Zadeh, Behnam Rafiee, Bebu Ram, Jonathan Weltz, Mark Friedman etc all. Caplacizumab Treatment in a Case of Resistant Thrombotic Thrombocytopenic Purpura. Open J Pathol Toxicol Res. 1(1): 2021. OJPTR.MS.ID.000504.
-
Neurology, Acute medicine, Critical care medicine, Emergency medicine, Patient’s health information, Human experimentation, Lactate dehydrogenase, Prothrombin time
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






