 Research Article
Research Article
Bioaccumulation and Biochemical Studies of Toxicants in Fish on AChE
Aradhna Gupta1, Nikhat J Siddiqi2, and Bechan Sharma3*
1Department of Biochemistry, PDM University, India
2Department of Biochemistry, King Saud University, Riyadh, Saudi Arabia
3Department of Biochemistry, University of Allahabad, India
Bechan Sharma, Department of Biochemistry, University of Allahabad, India.
Received Date:January 03, 2022; Published Date: January 24, 2022
Abstract
The rivers Ganges have been worst victim of environmental degradation as many hazardous and toxic substances are carried through sewage and industrial effluents to open water bodies. The present paper deals with the study related to occurrence and bioaccumulation of some organochlorine pesticides and heavy metals in the riverine sediment and the muscles of two cat fish species, Channa punctatus and Aorichthys aor procured from Ganges at Allahabad. The levels of these toxicants were determined to find out the extent of contamination and accumulation in the aforesaid samples from Ganges. Their contents in decreasing order were found to be HCH>DDT>heptachlor>endosulphan>aldrin>endrin for pesticides and Zn>Pb>Cr>Cu>Cd for heavy metals. Though level of these detected were under the permissible level but the results were indicative of rising trend in these samples. The in vivo effect of subacute concentrations of toxicants were also evaluated by treating fish with sublethal levels for 96h and assaying the activity of acetylcholinestearse (AChE) isolated from the muscle of the fish. The data indicated significant alterations in the levels of AChE, a known biomarker of toxicity, which exhibited direct correlation with the doses applied. The results could be used as an indicator for better environmental management with special reference to the water quality and human health.
Keywords: Organochlorine pesticides (OCP); Heavy metals; Accumulation; Toxicity; Acetylcholinesterase (AChE)
Introduction
It is a well-known fact that organochlorine pesticides are dual weapons, but because of their persistent nature they are more detrimental than beneficial. These pesticides are hydrophobic, highly volatile in nature, able for long distance transport and can also bioaccumulate in aquatic organisms and then to higher trophic levels of food chain. Besides different human anthropogenic activities several other natural ways for release of these in different ecological compartments like soil, water, and air and in environment are pesticide drift, soil erosion and rainfall, affecting many other organisms away from the first target [1,2]. Only 0.1% reaches the specific target after their application into the field [3]. The adversities of these pollutants on aquatic ecosystems are either acute or chronic due to gradual accumulation or slow degradation in different ecological compartments or in body tissues [4]. Being slowly metab olized in partially closed surface area they can have much higher adverse effects on the inhabitants of that area [5]. Livings being are always in continuous contact with water through different activities like irrigation, washing, bathing, drinking [6] and can be directly affected by these toxicants. Indirectly humans using these fish resources as food may therefore be highly impacted because it is well known that fish accumulate these contaminants in tissue and organs when exposed to polluted water [7,8]. Organochlorine pesticides like DDT, HCH, endosulphan are put under the persistent organo chlorines pesticides by Stockholm Convention. OCP are also linked to thinning of egg shells of aquatic birds, crocodiles and on reproductive development [9]. Many agencies like Arctic Monitoring and Assessment Program, Integrated Atmospheric Deposition Network, European Monitoring and Evaluation Program [10-12] for monitoring the levels of theses in air, sediment, water, as well as in aquatic organisms provides the current status of these at the local levels [13]. Models like BETR, mechanistics, kinetics, has been useful in assessing the transport and fate of these pesticides in different compartments [14]. Fish has shown to bioaccumulate these toxicants through two major routes ie dermal and ingestion/oral. Currently freshwater fish/ loaches and several other aquatic organisms have been used as model for assessing the bioaccumulation of these at the regional or local levels [15]. Not only pesticides but heavy metals are also the potent polluters of aquatic environment. Several heavy metals are used as cofactors like Zn, Ni, Mn, Cu, Fe but other heavy metals like Cd, Pb, Hg though not useful have found to be tremendously toxic to aquatic organisms [16,17]. Heavy metal toxicity is mainly due to generation of free radicals which can cause locomotry respiratory, osmoregulatory, neurodegenerative or endocrinology related problems in fish [18,19]. Discharge from industries like forging, leather, printing etc contains these metals in the ionic forms which easily pass through the cell membranes of the aquatic organisms and deposited on the mucous membranes sometimes rupturing them [20]. The significant use of different metals like copper in electronics has resulted in aquatic pollution. Chromium is mainly used in tanning industry and small amount required for carbohydrates, lipids and sugar metabolism [21,22]. Entry of toxicants in different ecological compartments is shown in (Figure 1).
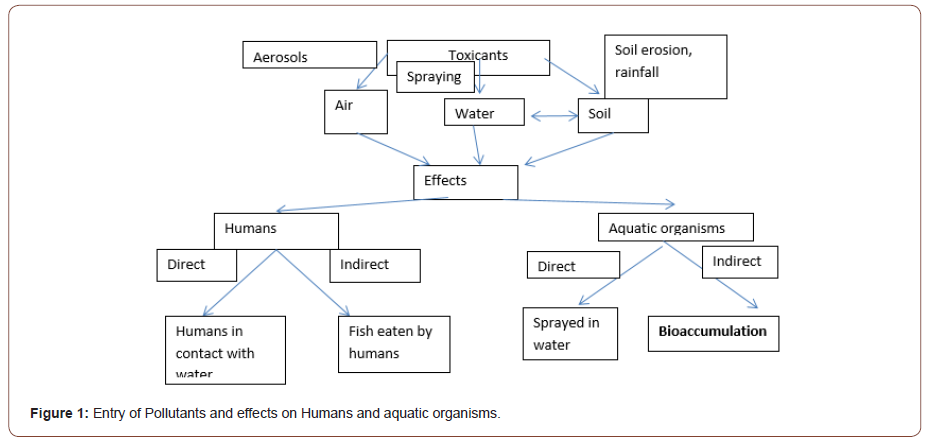
Toxicity caused by these pollutants may be at cellular, molecular or morphological level. The biological changes due to these toxicants can be used as a barometer for assessing the adverse effects of these pollutants [23]. Acetylcholinesterase (AChE) inhibition in fish is used as a biomarker of the species’ exposure to toxicants present in the aquatic biota [24]. It is an enzyme which hydrolyzes the neurotransmitter acetylcholine into acetic acid and choline. The impaired function of this enzyme results in nervous or musculoskeletal system deformities finally to paralysis and death [25,26]. There are numerous studies on bioaccumulation, but very few have been extrapolated in laboratory based on the accumulation data. In this study we have integrated our bioaccumulation results with biomarkers like AChE study in vivo by exposing the fish with two different sublethal concentrations of maximum accumulated pesticides HCH, DDT and heavy metals Zn, Cu, Cr, Cd and Pb detected in the study.
Materials and Methods
Samples of sediment, water and fish Channa punctatus (C.punctatus) and Aoricthys.aor (A.aor) were collected bimonthly throught the year from river Ganges at Allahabad, India. Organochlorine pesticide analysis: The reagents used for pesticide analysis were anhydrous sodium sulphate, petroleum ether (60-800C), acetonitrile, hexane, diethyl ether, florisil (60-100 mesh), and whatmann filter paper number 1 and 42. All reagents used were of Merck.
Pesticide extraction from sediment: The extraction of sediment was performed by procedure given by FAO. Sediment samples were first dried and grinded before extraction. In a beaker 50g grinded soil sample was mixed with 100ml of solvent mixture of hexane:acetone (1:1). After shaking them in beaker, mixture was filtered through whatmann filter paper number 1. First step was repeated for re-extraction of pesticides and then the filtrate was transferred through a seperatory funnel. After vigorous shaking 5ml of 2% NaCl and 300ml of distilled water was added and kept for some time to separate into solvent and aqueous phase. On clear separation lower aqueous portion was discarded and the solvent phase was washed twice with 100ml portion of distilled water. Finally column plugged tightly with 2.5gm anhydrous sodium sulphate and 2gm of 15% activated alumina. The column was eluted with 10ml hexane (HPLC). The concentration of samples was done using flat rotatory evaporator. The dried extract was then dissolved in 2ml of hexane. The cleaned up extract was stored at 40C for analysis on gas chromatograph.
Pesticide extraction from fish muscle: The extraction of pesticides from fish muscle was done in four steps described by USFDA 1994.
Extraction: 50g fish muscle (boneless) was mixed with 250g sodium sulphate and grinded in metallic blender at high speed with three consecutive volumes (200, 150 and 100ml) of petroleum ether. The filtrate from each portion was combined in a 500ml conical flask and was evaporated to near dryness on a rotatory evaporator.
Partitioning: The extract was then partitioned in three steps. In first portion extract was mixed with 30ml saturated solution of acetonitrile in petroleum ether, shaken 100 times and lower portion was transferred to 2nd separating funnel containing 100ml petroleum ether, 40ml of saturated solution of NaCl, 600ml distilled water, shaken 100 times and lower portion of separating funnel 2nd was transferred to 3rd separating funnel containing 100ml petroleum ether, shaken and lower layer discarded. Its upper layer was mixed to separating funnel 2, 100ml distilled water added, shaken, lower layer discarded and upper layer collected and was evaporated to dryness on a rotatory evaporator.
Clean Up: 20g activated florisil was topped with 4g anhydrous Na2SO4. The column was eluted with 200ml of 6% and 15% diethylether. All the glassware’s were cleaned with liquid soap followed by tap water, distilled water and acetone. The glassware’s were then kept in oven at a temperature of 2200C for 24h.
Analysis: Gas liquid Chromatography Analysis: Pesticides residue in all extracts were estimated quantitively by NUCON Gas Chromatograph equipped with Ni63 Electron Capture Detector (ECD).
Operating parameters: Purified nitrogen gas grade1 99.9% pure gas was used as carrier gas, and its flow rate is optimized at usually 60ml/min Temperature of injection port (2100C), column (1900C) and detector (2200C) is adjusted accordingly and stabilized properly. When the instrument is thermally stabilized (3-4h) we injected appropriate volume (2-10μl) of the working standard mixture of pesticides with the help of 10 μl micro syringe (Hamilton) carefully and waited till the component with maximum retention time is detected on chromatogram usually 1-1/2 h. Now as the standard run is over, we injected unknown sample volume concentrated extract (2μl-fish extract, 1ml- sediment extract) on the same column efficiently with many times washed and rinsed microsyringe with hexane. The different peaks of the samples were identified with those of standards. The pesticides for which the GC was standardizes were p-p’- dichlorodiphenyl trichloroethane (p-p’-DDD), p,p’-dichlorodiphenylethane (p-p’-DDE), α, ß, γ isomers of hexachlorocyclohexane, dieldrin, aldrin and endosulphan.
Preparation of standards for GLC: 1mg of pure pesticide standard A.R. grade 100% is dissolved in 10ml hexane. From this different concentration of pesticide is prepared. These standard solutions are kept in vaccum dessicator at -150C for a period of six months in case of organochlorine pesticides.
The fish were exposed to two different sublethal concentrations of effectors for 96h. After the stipulated periods of treatment, the fish were dissected and muscle was excised out. The muscle was thoroughly washed in normal cold saline (0.15M, 4-6oC), blotted dry and quickly weighed. For isolating AChE, a membrane bound enzyme, sodium phosphate buffer (50mM, pH 8.0) containing tritonX- 100 (0.5%, v/v) a non-ionic detergent was used in extraction buffer. The homogenates of each tissue were kept for 30min in cold with intermittent stirring and centrifuged at 10,000 Xg for 30min in a refrigerated high-speed centrifuge. The clear supernatant of each tissue homogenate was thus collected and used as the source of enzymes and other cellular constituents. The estimation of biochemical indices (protein content, AChE activity) were done in these cell free fractions of tissue homogenates.
Acetylcholinesterase: (Acetylcholinesterase, EC 3.1.1.7, AChE) was assayed by the method of Ellman et al., (1961). The reaction mixture contained sodium phosphate buffer (50mM, pH 8.0), acetylthiocholine iodide (ATI) (0.5mM), 5,5’ dithiobis-(2-nitrobenzoic acid) (DTNB, 0.5mM, pH 8.0) and suitable amount of enzyme preparation (100-200μg cytosolic protein). The increase in absorbance was monitored at 412nm for 3min against blank at room temperature (26±20C). The measurements were made in triplicates in each tissue homogenate.
Heavy metal analysis: The reagents used for analysis of were nitric acid, perchloric acid, sulphuric acid and metals/salts of copper (Cu), chromium (Cr), cadmium (Cd), Zinc (Zn) and lead (Pb).
Water collection: Water samples were collected in polyethylene bottles (500ml), acidified with concentrated nitric acid (5ml) and then evaporated on sand bath till volume concentrated to 50ml.
Sediment collection and analysis: Sediment samples were dried, powdered and sieved. 5gm samples were digested with a mixture of concentrated nitric acid (35ml), perchloric acid (5ml) and sulphuric acid (2.5ml) in a ratio of 7:1:0.5 at 75-80oC for 4-5h on heating mantle till a clear solution is obtained.
Fish muscle: Fish samples were rinsed in deionised water to remove surface adherents that could have adsorbed metals. Then 30gm of muscle (boneless) was chopped and kept in 25ml nitric acid overnight. The samples were then digested by adding 10ml sulphuric acid on heating mantle till a clear light yellow solution was obtained.
The volume of all digested samples was then made up to 100ml. heavy metals analyzed in samples by atomic absorption spectrophotometer (GBC AvantaΣ) using different cathode lamps.
Preparation of standards:
Preparation of 1000μg/ml standard Cadmium (Cd): The 1000μg/ml standard Cd was prepared by dissolving 1.0g of cadmium metal in 20ml of 5N hydrochloric acid containing 0.5ml of concentrated nitric acid and diluted to 1 litre.
Preparation of 1000μg/ml standard Chromium (Cr): The 1000μg/ml standard chromium was prepared by dissolving 1.0g of chromium metal in 50ml of concentrated hydrochloric acid and diluted to 1 litre.
Preparation of 1000μg/ml standard Copper (Cu): The 1000μg/ml standard copper was prepared by dissolving 1.0g of copper metal in 50ml of 6N nitric acid and diluted to 1 litre.
Preparation of 1000μg/ml standard Lead (Pb): The 1000μg/ ml standard lead was prepared by dissolving 1.0g of lead metal in 20ml of 6N nitric acid and diluted to 1 litre.
Preparation of 1000μg/ml standard Zinc (Zn): The 1000μg/ ml standard zinc was prepared by dissolving 1.0g of zinc metal in 40ml of 5N hydrochloric acid and diluted to 1 litre.
Exposure to subacute concentrations of pesticides (HCH, DDT) and heavy metals (Cu, Cr, Cd, Pb, Zn): The healthy fish were equally distributed in four aquaria of 1x1 ft, The subacute concentrations of toxicants were used for the exposure of C. punctatus and A.aor for 96h. The equal volume of acetone was maintained in control aquaria as pesticide added to the experimental aquaria was dissolved in acetone. All aquaria were constantly aerated during the period of exposure by aerator and the fish were fed properly. The water was changed after 24h each and replenished with fresh toxicants.
Result and Discussion
The aquatic system have been worst victim of environmental degradation as many hazardous and toxic substances such as pesticides and heavy metals and other chemicals are carried through sewage and industrial effluents including urban and agricultural total run off to open water bodies. These substances being highly persistent get deposited either in the sediment or water and ultimately contaminate the whole aquatic cycle. The extensive use of organochlorine pesticides, heavy metals and their ability to accumulate in aquatic food chain pose serious threat to the environmental equilibrium. The present study is related to occurrence and bioaccumulation of these toxicants in the water, sediment and muscles of two cat fish species, Channa punctatus and Aorichthys aor and the sublethal effect of these on the biomarker enzyme acetylcholinesterase AChE in laboratory.
Evaluation of organochlorines in sediments and muscles of fish
The order of annual accumulation of different pesticides in two ecological compartments of aquatic ecosystem ie sediment and fish are HCH>DDT>Endosulphan>Heptachlor>aldrin>endrin. The highest accumulation was of HCH, DDT, endosulphan and lowest of aldrin, endrin in all the compartments. Heptachlor accumulation is on average among all the pesticides. Pesticides accumulation was higher in sediment than fish (Table 1).
Table 1: Annual accumulation of pesticides (μg/Kg wet weight) in the sediment and muscle of the freshwater fish species procured from river Ganges at Allahabad.
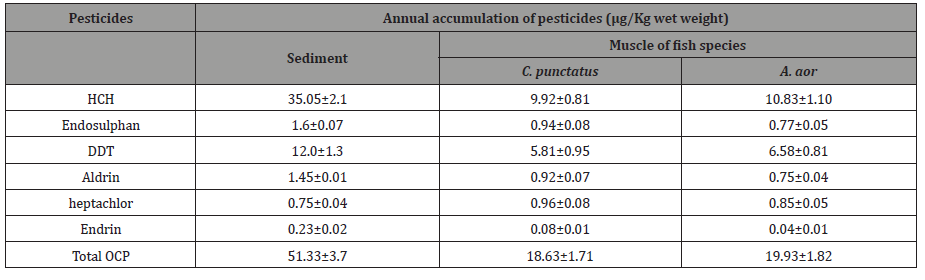
The extraction of organochlorines from sediment and muscles of two fish species and their determination were done as has been described in Materials and methods. The values are the average of three independent experiments. HCH=Hexachlorocyclohexane, DDT=Dichlorodiphenyltrichloroethane
The percentage distribution of pesticides between sediment and fish shows almost 20% increase rate of accumulation for HCH, 10% for DDT in sediment. In fish the accumulation of HCH was higher in C.punctatus and DDT in A.aor. However the other OCP detected in sample was less than 10% in sediment and 20% in fish (Table 2).
Table 2: Percent distribution of organochlorine pesticides in the sediment and the muscles of two freshwater fish species procured from river Ganges at Allahabad.
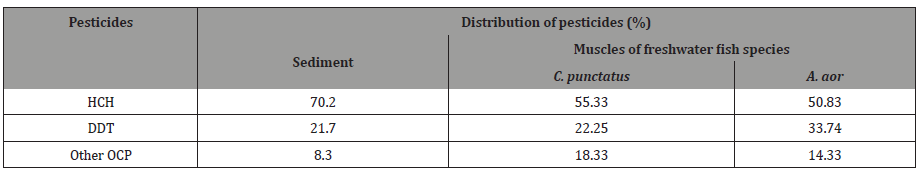
The data indicated that the major contributor of total OCP was DDT and HCH in sediment and fish muscle.
The accumulation of pesticides into the sediment of river and muscles of two fish species were determined as mentioned in the Materials and Methods. Values are the average of three independent experiments.
The results show the highest concentration of pesticide present in sediment as compared to that present in the flesh of two fish species. In fish higher concentration of pesticide accumulation was recorded in the flesh of A. aor than C. punctatus, which is directly related to their feeding behaviour. As organochlorine pesticides are insoluble in water they get settled at the bottom, adsorb to sediments or remain suspended in water and when bottom feeder fish feed on these contaminated zooplankton and plankton, it gets in them.
The bioaccumulation of these pesticides in aquatic organisms depends on species, age, sex, feeding habitat, position in trophic level and the rate of mobilization, uptake and elimination [27,28]. The interaction of these pesticides with sediments depends on the charge, ions, detritus organic matter associated with sediment which sometimes increases the total dissolved solids (TDS) and conductivity of water.
The high levels of HCH in the sediment of river Ganges may have served as a sink to persistent organochlorine pesticides. Drought, leaching of the surface soil during monsoon can also result in increase of OCP in sediment [29-32]. The presence of pesticides in soil is controlled by many factors like adsorption on the solid phase, decomposition and volatilization. The results show that pesticides concentration in sediment is more than that present in fish. There are mainly two ways how these pesticides reach the soil, (1) by fall out on account of crop spraying for insect / pest control or (2) by their direct application for the treatment of soil or for the control of soil dwelling pests, nematodes and pathogens of bacterial and fungal diseases. The organic content in the sediment may be considered as the probable reason for attracting the pesticides from water. The organochlorine pesticides are non-polar and therefore are less soluble in water and tend to remain adsorbed on the suspended solids. Therefore, the pesticide residues in sediment tell the data of not only the pesticides present in sediment but also in water. The movement of the pesticides from water to sediment and to lipid compounds is possible. The presence of pesticides in sediments of Ganges at Allahabad in the present study is in agreement with the studies of Miles and Harris [33], Rajendra and Subramanian [34] and Darko et al. [35]; Sarafioska et al. [36]. A study by Bossi et al. [37] showed high concentrations of pesticides in May-June followed by decreasing concentartions in July-August and then slight increase in September-October. The endosulphan detected in present study may be due to soil leaching. Aldrin can undergo photolysis or metaboloized to dieldrin (more toxic than aldrin) in plants and animals by epoxidation of double bonds carried out by microsomal enzymes making it more polar and less lipid soluble [38,39]. Among pesticides high concentrations of DDT has been reported by [40,41]. Camenzuli et al. [14] reported higher concentrations of pesticides HCH and DDT in sediment may be due to soil organic carbon, pesticide drift, emissions, agriculture use.
These pesticides in water may be present in dissolved, precipitated (when present in excess) and suspended forms (when adsorbed on suspended particles). The solubility of pesticides in water besides their chemical structure (polarity) depends on pH, temperature, salt concentration and organic matter content on medium and on partition between water and sediment phases and biotic activity. Although in aquatic ecosystems especially in rivers the pesticides joining the course move and distribute into various inter compartments viz. sediments, aquatic flora and fauna etc.
The predominant concentration of HCH in all the fish samples in the present study suggests that different HCH containing formulations of pesticides are used or as vector control. These pesticides reach the water by direct or indirect application. The indirect sources include runoff from agricultural fields, spray drift, rain water, sewage and effluents from various industries manufacturing pesticides or using them in their process, whereas direct application include the control of unwanted weeds, insect’s pest infecting water plants, undesirable fish in fish culture ponds to restock with more desirable fishes. Germen et al. [2] reported the trend of accumulation of organochlorine pesticides to be PCB>DDT>HCH and Afful et al. [7] to be of γ-HCH, δ-HCH, heptachlor, aldrin, γ-chlordane, α-endosulfan, dieldrin and p,p’-DDT respectively, in different fishes. In contrast findings by Buah-Kowfie et al. [23] showed higher accumulation in fish than sediment which was directly related to the feeding habitat of fish Clarias gariepinus [42].
Evaluation of heavy metals in water, sediments and muscles of fish
The range of heavy metal accumulation in all the three ecological compartments were water 0.001-0.043, sediment 0.08-14.62 and muscles of fish C. Punctaus 0.001-9.50, A.aor 0.001-14.13. The decreasing trend in all the cases were Zn>Pb>Cr>Cu>Cd. Highest accumulation of Zn was seen in sediment and fish A.aor. In water the concentration detected was almost negligible (Table 3).
Table 3: Annual accumulation of heavy metals (μg/Kg wet weight) in the water, sediment and fish muscle.
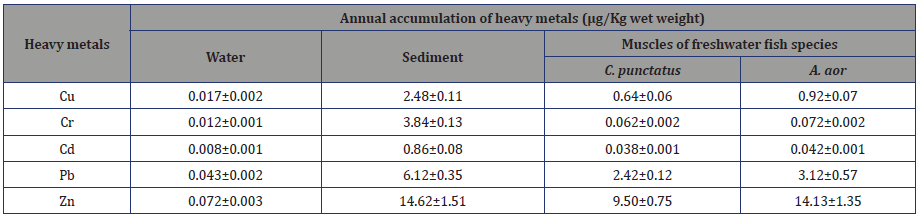
The extraction of heavy metals from water, sediment and muscles of two fish species and their determination were done as described in Materials and methods. The values are the average of three independent experiments. Cu=Copper, Cr=Chromium, Cd=- Cadmium, Pd= Lead, Zn=Zinc
The trend of percent distribution was same as that of accumulation of heavy metals. The difference in percent accumulation of Zn with Cr and Cd was around more than 70% in fish. Almost similar level of percentage difference seen for Zn with Cu but variable for Pb. Around 30% difference (decrease) was seen in sediment than fish for Zn and less than 5% between sediment and water (Table 4 & Table 5).
Table 4: Percent distribution of heavy metals in water, sediment and the muscles of two freshwater fish species procured from river Ganges at Allahabad.
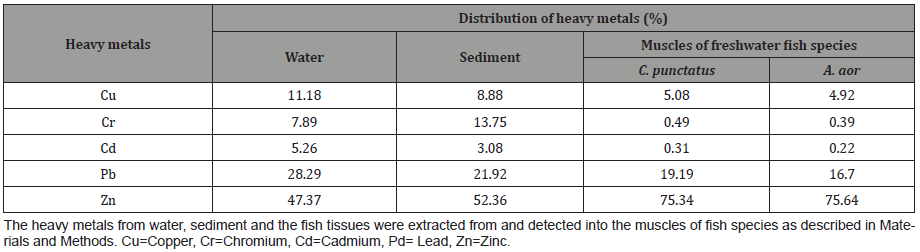
Table 5: Reference levels of heavy metals and Organochlorine Pesticides.
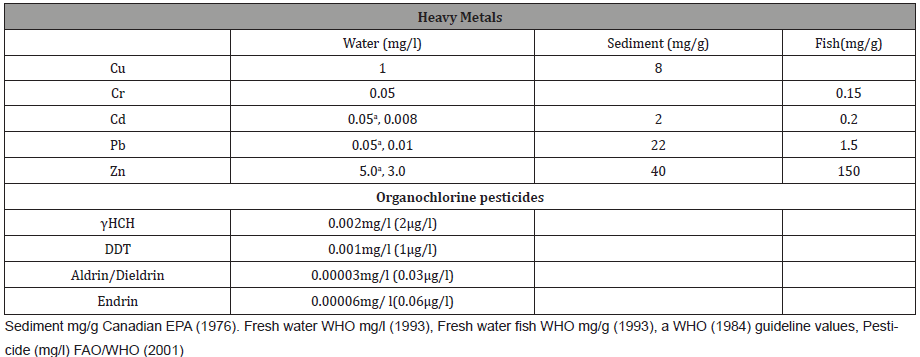
The levels of metals detected in the study can be due to many reasons: (i) precipitation in the alkaline pH, (ii) increase in temperature proportionately decreases the oxygen concentration of water and hence toxicity increases resulting in physiological and morphological imbalances (iii) decreased particle size and increased organic matter results in adsorption and deposition (iv) metal contaminated feeds due to discharge of contaminants into the riverine system without any pretreatment.
Bioaccumulation studies of heavy metals in aquatic organisms were done by several researchers like Bonsignore et al. [43], Bawuro et al. [44], Rajkumar et al. [45] etc. The trend of heavy metals accumulation in the presented study was Zn>Pb>Cr>Cu>Cd which is different from the findings given by Rajeshkumar etal. [45] were Pb>Cu>Cr>Cd and by Bawuro et al. [44] Zn>Cu>Pb>Cd in carnivores and herbivore fish. These metals have toxic effect on animal reproduction, development and immunological function and are capable of producing acute and sub chronic toxic effects in mammals [46-49]. The copper concentration of 100mg/l has been detected in mining areas [50]. Bottom dwelling fishes are found to exhibit higher concentration of heavy metals than pelagic fishes. These fish are found in large quantity and so more susceptible to biomagnification [51]. The prolonged thermal and chemical protective actions of these substances against the pests create a risk of contaminating the environment and agricultural products. All the toxicants detected in the study were below the permissible limits (Table 6).
Table 6: Effect of pesticides and heavy metals on the activity of acetylcholinesterase (AChE) in muscles of freshwater fish.
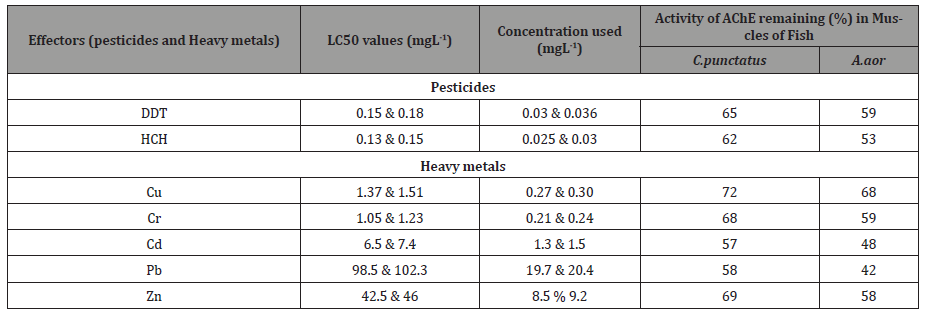
Effect of Effectors on the AChE activity in fish muscle
The conclusions drawn from Table 7 on the basis of acute toxicity test were that HCH was more toxic than DDT. The inhibition was found to be between 38-47% in fish exposed to pesticides. For heavy metals the range of inhibition was between 28-52%. Maximum inhibition was in A.aor (48%) for Pb and lowest for Cu (28%) in C. punctatus. The AChE activity at two different subacute levels of effectors (pesticides and heavy metals) was found to show a stochastic decrease in activity at higher concentrations. The trend of % decrease in AChE activity for heavy metals in C.punctatus was Pb>Cd>Cr>Zn>Cu and A.aor was Pb>Cd>Zn>Cr>Cu. The % decrease for pesticides in both the fish was HCH>DDT.
The extraction of AChE from the pesticides and heavy metals treated fish tissues and the determination of enzyme activity in the tissue homogenates were done as described in Materials and Methods. The values are the average of three independent experiments. Cu=Copper, Cr=Chromium, Cd=Cadmium, Pd= Lead, Zn=Zinc AChE is an enzyme which helps in cholinergic transmission of neurotransmitter acetyl cholein at the post synaptic junction, and hydrolysed to acetyl Co-A and choline, where choline can again be used for synthesis of acetyl choline [52-54]. Potential sources of variation which can affect AChE activity are as follows: (i) differences in age, sex, reproductive status, and stressors such as water temperature, dissolved oxygen concentration, exposure to multiple contaminants [55] (ii) due to catecholamines or acetylcholine levels which through the adenylcyclase system, can increase cAMP affecting enzymes of glycogen breakdown and glycogen synthesis [56] (iii) due to higher accumulation of acetylcholine resulting in hyperpolarization of post synaptic membranes which causes disruption in the transmission of nerve impulses. The other reasons for AChE inhibitions are movement and seasons. Baslow, [25] reported increased inhibitions in active fish than sluggish fish. Seasonal variations were studied by Sumith et al. [57] in fish exposed to organophosphates and showed, increased inhibition during drier months ie july-september (Yala), when compared with Maha seasons. Similar findings reported by Menedez et al., [58] in fresh water fish Cenesterodon decemmaculatus where activity decreased upto 80% in summers and upto 60% in winters. Decrease in AChE activity in fish exposed to pesticides were reported by Marigoudar et al. [59] in Labeo rohita for cypermethrin, Milegla et al. [60] in Clarius gariepinus for organo phosphates and carbamates, Pereira et al. [61] in zebra fish muscle exposed to endosulphan whereas Ezemonye and Ikpesu [62] reported no change in activity in serum of Clarias gariepinuss exposed to endosulphan. In contrast increased activity were reported by Moraes et al. [63] in brain of fish upon exposure to imazapic and imazethapyr herbicides, Toni et al. [64] for commercial herbicide bispyribac sodium after seven days of exposure. However, these authors have shown reduction in AChE activity in fish brain and muscle after prolonged (72 days) of exposure.
Heavy metals have shown to cause changes in circadian behavior and inverse relationship between exposure time and activity in addition to decreased AChE activity. Decreased activity by heavy metals exposure were reported by Cunha et al. [65] in marine gastropods for cadmium and copper, [66] for mercury and lead in brain of zebra fish, Haverroth et al. [67] in Zebra fish for Copper, Leitemperge et al. [68] in silver catfish Rhamdia quelen for copper, Kim and Kang et al. [22] for cadmium in brain and muscles of juvenile rock fish Sebastes schgeleii, Pan et al. [69], Zhang et al. [70] in fish Danio rerio for cadmium chloride and deltamethrin, Lee and Freeman [71] documented lead toxicity in Zebra fish, Green et al. [72].
The toxicity to aquatic biota by chromium is due to ionic states of these heavy metals which can easily pass through the cell membranes. Cr (Vl) can easily pass plasma membrane with the help of anion transporter phosphate whereas Cr (lll) cannot. Hexavalent state of chromium has been much toxic than trivalent stage.
The lethality of Cu in fish is due to several reasons; it mimics the sodium ion, blocks ions by ATP dependent enzymes, decreases the levels of sodium and chloride ions as a result increased diffusion loss takes place leading to osmoregulatory disturbances and death [73]. Sublethal toxic effects of copper in fish include degeneration of gill cells, decreased RBC and increased hematocrit at different sublethal levels of exposure [74,75]. Copper (ll) and (l) results in generation of reactive oxygen specie through lipid peroxidation and genotoxic [76]. Zinc toxicity in aquatic organisms is linked to embryo damage, fecundity, low hatching rate and high mortality. Zebra fish has the genes for AChE responsible for acetylcholine degradation, in brain [77]. Zinc esposure of more than 1ppm caused different circadian rhythms, decreased activity during day which increases in dark [78]. Lead and other stressors at sublethal levels showed muscular and neurodegenerative change, reproductive inhibitions in aquatic organisms, hypo demethylation of DNA [79]. Studies by Kumar et al. [80] showed lead toxicity in cat fish Pangasius hypophthalmus, can be mitigated to some extent when fish were fed on zinc diet. Cadmium toxicity decreases as water hardness increases [81]. Sublethal levels exposure may cause alterations to appetite and metabolism. It is an endocrine disruptor and cause DNA damage and stress in common carp Cyprinus carpio [82-90]. However it cannot be predicted that whether the OCPs and heavy metals acts as reversible or irreversible inhibitors, but yes the results prove that subacute concentrations of these effectors are also highly toxic to the fish. The bioaccumulation of pesticides and heavy metals were in the permissible limits and is directly related to the feeding habitat.
The data obtained from the present study gives an indication of the extent of aquatic contamination that may help to understand the behavior and fate of these persistent chemicals in the aquatic environment. The results may be important form public health and ecological standpoint and is useful for better water quality management and environmental health risk assessment.
Acknowledgement
AG acknowledges the University Grants Commission-New Delhi for providing financial support in the form of a Research Fellowship. The author (NJS) would like to thank the Research Center, Female Center for Scientific and Medical Colleges, King Saud University for the financial support. BS gratefully appreciates the support provided by the Department of Biochemistry of University of Allahabad and CIFRI-Allahabad for carrying out present research.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Gupta A, Rai DK, Pandey RS, Sharma B (2009) Analysis of heavy metals in the riverine sediments and fish from river Ganges at Alahabad. Environ Monit Assess 157(1): 449-458.
- German AV, Zakonnov VV, Mammantov AA (2010) Organochlorine pesticides in the bottom sediments, benthos and fish in the Volka pool of the Rybinsk Reservoir. Water Resources 37(1): 84-88.
- Aguiar LH, Moraes G, Avilez IM, Altran AE, Corrêa CF (2004) Metabolic effects of Folidol 600 on the neotropical freshwater fish matrinxa, Brycon cephalus. Environmental Research. 95(2): 224-230.
- Lukyanova ON, Tsygankov VY, Boyarova MD, Khristoforova NK (2016) Bioaccumulation of HCHs and DDTs in organs of Pacific salmon (genus Oncorhynchus) from the Sea of Okhotsk and the Bering Sea. Chemosphere 157: 174-180.
- Barakat AO, Khairy M, Aukaily I (2016) Bioaccumulation of organochlorine contaminants in fish species from Lake Qarun, a protected area of Egypt. Toxicological & Environmental Chemistry 99(1): 117-133.
- Yang Y, Yun X, Liu M, Jiang Y, Li QX, et al. (2014) Concentrations, distributions, sources, and risk assessment of organochlorine pesticides in surface water of the East Lake, China. Environ Sci Pollut Res Int 21(4): 3041-3050.
- Afful S, Anim AK, Serfor-Armah Y (2010) Spectrum of organochlorine pesticide residues in fish samples from the Densu Basin. Research Journal of Environmental and Earth Sciences 2(3): 133-138.
- Khairy MA, Noonan GO, Lohmann R (2019) Uptake Of Hydrophobic Organic Compounds, Including Ocps And Pbdes, And Perfluoroalkyl Acids (Pfaas) In Fish And Blue Crabs Of The Lower Passaic River (NJ, USA). Environmental Toxicology and Chemistry 38(4): 872-882.
- Sherwin BD, Mudge JF, Cañas-Carrell JE, Lanza HA, Rainwater TR, et al. (2016) Organochlorine pesticide residues in caudal scutes of Belize Morelet's crocodiles (Crocodylus moreletii). J Herpetol 50(4): 552-558.
- Hung H, Kallenborn R, Breivik K, Su Y, Brorstrom-Lunden E, et al. (2010) Atmospheric monitoring of organic pollutants in theArctic under the Arctic Monitoring and Assessment Programme (AMAP): 1993-2006. Sci Total Environ 408(15): 2854-2873.
- Venier M, Hites RA (2010) Time trend analysis of atmospheric POPs concentrations in the Great Lakes region since 1990. Environ Sci Technol 44: 8050-8055.
- Tørseth K, Aas W, Breivik K, Fjæraa A, Fiebig M, et al. (2012) Introduction to the Europe an Monitoring and Evaluation Programme (EMEP) and observed atmospheric composition change during 1972e2009. Atmos Chem Phys 12.
- Nøstbakken OJ, Hove HT, Duinker A, Lundebye AK, Berntssen MHG, et al. (2015) Contaminant levels in Norwegian farmed Atlantic salmon (Salmo salar) in the 13-year period from 1999 to 2011. Environ Int 74: 274-280.
- Camenzuli L, Scheringer M, Hungerbühler K (2016) Local organochlorine pesticide concentrations in soil put into a global perspective. Environmental Pollution 217: 11-18.
- Zhang H, Lu X, Zhang Y, Ma X, Wang S, et al. (2016) Bioaccumulation of organochlorine pesticides and polychlorinated biphenyls by loaches living in rice paddy fields of Northeast China. Environmental Pollution 216: 893-901.
- Miao L, Yan W, Zhong L, Xu W (2014) Effect of heavy metals (Cu, Pb, and As) on the ultrastructure of Sargassum pallidum in Daya Bay, China. Environmental Monitoring & Assessment 186(1): 87-95.
- Chen P, Miah MR, Aschner M (2016) Metals and neurodegeneration. F1000Research 5: 366.
- Kuykendall JR, Miller KL, Mellinger KN, Cain AV (2006) Waterborne and dietary hexavalent chromium exposure causes DNA-protein cross link (DPX) formation inerythrocytesof largemouth bass (Micropterus salmoides). Aquat Toxicol 78: 27-31.
- Kim JH, Kang JC (2015) The arsenic accumulation and its effect on oxidative stress responses in juvenile rockfish, Sebastes schlegelii, exposed to waterborne arsenic (As3+). Environmental Toxicology and Pharmacology 39(2): 668-676.
- Nazimabashir, Manoharan V, Miltonprabu S (2015) Cadmium induced cardiac oxidative stress in rats and its attenuation by GSP through the activation of Nrf2 signaling pathway. Chemico-Biological Interactions 242: 179-193.
- He H, Zhu T, Liu Z, Chen M, Wang L (2015) Treatment of cadmium-containing wastewater in zinc smelting using sodium dimethyl dithio carbamate. Environmental Protection of Chemical Industry 35(3): 293-296.
- Kim JH, Kang JC (2016) Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium (Cr6+) exposure. Ecotoxicology and Environmental Safety 125: 78-84.
- Buah-Kwofie A, Humphries MS, Pillay L (2018) Bioaccumulation and risk assessment of organochlorine pesticides in fish from a global biodiversity hotspot: iSimangaliso Wetland Park, South Africa. Science of The Total Environment 621: 273-281.
- Richardson N, Gorodon AK, Muller WJ, Petschchke Bl, Whitfield AK (2010) The use of liver histpathology, lipid peroxidation and acetylcholinesterase assays as biomarker of contaminant induced stress in Cape stumpnose, Rhabdosargus holubi (Teleostei: Sparidae), from selected South African estuaries. Water SA. 36(4).
- Baslow MH, Nigrelli RF (1961) Muscle acetylcholinesterase level as an index of general activity in fishes. Copeia 1: 8-11.
- Van Dyk JS, Pletschke B (2011) Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 82(3): 291-307.
- Crosly RW, Donald DB, Block HO (1998) Trends and seasonality in alpha and gamma hexachlorocyclo hexane in Western Canadian surface waters. Environ Pollut 103: 277-285.
- Eqani S, Malik RN, Cincinelli A, Zhang G, Mohammad A, et al. (2013) Uptake of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) by river water fish: The case of River Sci Chenab. Sci Total Environ 450-451: 83-91.
- Akan JC, Sodipo OA, Mohammed Z, Abdulrahman F (2014) Determination of organochlorine, organophosphorus and pyrethroid pesticide residues in water and sediment samples by high performance liquid chromatography (HPLC) with UV/visible detector. J Anal Bioanal Tech 5: 226.
- Pérez Parada A, Goyenola G, Teixeira de Mello F, Heinzen H (2018) Recent advances and open questions around pesticide dynamics and effects on freshwater fishes. Current Opinion in Environmental Science & Health.
- Arisekar U, Shakila RJ, Jeyasekaran G, Shalin R, Kumar P, et al. (2018) Accumulation of organochlorine pesticide residues in fish, water, and sediments in the Thamirabarani river system of southern peninsular India. Environmental Nanotechnology, Monitoring & Management.
- Baqar M, Sadef Y, Ahmad SR, Mahmood A, Zhang G, et al. (2018) Organochlorine pesticides across the tributaries of River Ravi, Pakistan: Human health risk assessment through dermal exposure, ecological risks, source fingerprints and spatio-temporal distribution. Sci Total Environ 618: 291-305.
- Miles IRW, Harris CR (1970) Insecticde residues in stream and control drainage system in agricultural areas of South –Western Ontario. Pestic Monit J 5: 289-294.
- Rajendran RB, Subramanian AN (1999) Chlorinated pesticides residues in surface sediments from the river Kaveri, South India. J Environ Sci Hlth B 34: 269-288.
- Darko G, Osei A, Caleb O (2008) Persistent organochlorine pesticide residues in fish, sediments and water from Lake Bosomtwi, Ghana. Chemosphere 72(1): 21-24.
- Sarafioska E, Jordanoski M, Semo M, Patceva S (2010) The presence of organochlorine pesticides in the sediment, water system in Lake Dojran. BALWOIS. Ohrid, Republic of Macedonia - 25.
- Bossi R, Vorkamp K, Skov H (2016) Concentrations of organochlorine pesticides, polybrominated diphenyl ethers and perfluorinated compounds in the atmosphere of North Greenland. Environmental Pollution 217: 4-10.
- National Research Council (NRC) (US) Committee on Toxicology. An Assessment of the Health Risks of Seven Pesticides Used for Termite Control. Washington (DC): National Academies Press (US); 1982.
- World Health Organisation, United Nations Environmental Program (WHO) (1989) In: Aldrin, Dieldrin (Eds.) Environmental Health Criteria.
- Ping Gong, Xiao-ping Wang, Sheng-hai Li, Wu-sheng Yu, Jiu-le Li, et al. (2014) Atmospheric transport and accumulation of organochlorine compounds on the southern slopes of the Himalayas, Nepal Environ Pollut 192: 44-51.
- Nawab J, Wang X, Khan S, Tang YT, Rahman Z, et al. (2020). New insights into the bioaccumulation of persistent organic pollutants in remote alpine lakes located in Himalayas, Pakistan. Environmental Pollution. Environ Pollut: 114952.
- Barnhoorn IEJ, van Dyk JC, Genthe B, Harding WR, Wagenaar Barnhoorn GM (2015) Organochlorine pesticide levels in Clarias gariepinus from polluted freshwater impoundments in South Africa and associated human health risks. Chemospher 120: 391-397.
- Bonsignore M, Salvagio Manta D, Mirto S, Quinci EM, Ape F, et al. (2018) Bioaccumulation of heavy metals in fish, crustaceans, molluscs and echinoderms from the Tuscany coast. Ecotoxicol Environ Saf 162: 554-562.
- Bawuro AA, Voegborlo RB, Adimado AA (2018) Bioaccumulation of Heavy Metals in Some Tissues of Fish in Lake Geriyo, Adamawa State, Nigeria. J Environ Public Health: 1854892.
- Rajeshkumar S, Xiaoyu Li (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5: 288-295.
- Ecobichon DJ, Dovis JE, Dovill J, Enrich MR, Millian P, et al. (1990) Neurotoxic effects of pesticides. In the effects of pesticides on human health (Baker, S.R., Wilkinson, C.F, eds) Princeton. NJ, Princenton Scientific Publishing: 131-199.
- Tanner RW, Lanston JW (1990) Do Environmental toxins cause parkinsons disease: A critical review. Neurology 40: 17-30.
- Rosentock L, Keifer M, Daniell WE, McConnell R, Claypoole K (1991) Chronic central nervous system effects of acute organophosphate pesticide intoxication. The pesticides health status study groups. Lancet 338: 223-227.
- Adelekan BA, Abegunde KD (2011) Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. International Journal of Physical Sciences 6(5): 1045-1058.
- Grosell M (2012) Copper In: Wood CM, Farrell AP, Brauner CJ (Eds.) Homeostasis and Toxicology of Essential Metals—Fish Physiology. Elsevier, SanDiego, CA. pp: 54-135.
- Akoto O, Azuure AA, Adotey KD (2016) Pesticide residues in water, sediment and fish from Tono Reservoir and their health risk implications. SpringerPlus 5(1): 1849.
- Singh RK, Sharma B (2004) In vivo alteration in protein metabolism by subacute carbofuran intoxication in the freshwater teleost, Clarias batrachus. Bull Environ Contam Toxicol 73(5): 919-926.
- Singh RK, Sharma B (2005) Sub-acute toxicity of carbofuran on acetylcholinesterase activity in the freshwater catfish, Clarias batrachus. J Environ Occup Medicine 22 (5): 403-407.
- Scholz NL, Truelove NK, Labenia JS, Baldwin DH, Collier TK (2006) Dose-additive inhibition of chinook salmon acetylcholinesterase activity by mixtures of organophosphate and carbamate insecticides. Environ Toxicol Chem 25(5): 1200-1207.
- Santana MS, Sandrini-Neto L, Di Domenico M, Prodocimo MM (2021) Pesticide effects on fish cholinesterase variability and mean activity: A meta-analytic review. Science of The Total Environment 757: 143829.
- Das BK, Mukherjee SC (2000) A histopathological study of carp Labeo rohita exposed to hexachlorocyclohexane. Veterinaraski Arhiv 70(4):169-180.
- Sumith JA, Hansani PLC, Weeraratne TC, Munkittrick KR (2012) Seasonal exposure of fish to neurotoxic pesticides in an intensive agricultural catchment, Uma-oya, Sri Lanka: Linking contamination and acetylcholinesterase inhibition. Environmental Toxicology and Chemistry 31(7): 1501-1510.
- Menéndez Helman RJ, Ferreyroa GV, dos Santos Afonso M, Salibián A (2015) Circannual rhythms of acetylcholinesterase (AChE) activity in the freshwater fish Cnesterodon decemmaculatus. Ecotoxicol Environ Saf 111: 236-241.
- Marigoudar SR, Nazeer Ahmad R, David M (2009) Cypermethrin induced: In vivo inhibition of the acetylchoinesterase activity in functionally different tissues of the freshwater teleost, Labeo rohita (Hamilton). Toxicological and Environmental chemistry 91(6): 1175-1182.
- Milegla H, Mosha R, Sandvik M, Slcaary U (2010) Assessment of acetylchoinesterase activity in Clarias gariepinus as a biomarker of organophosphate and carbamate exposure. Ecotoxicology.
- Pereira VM, Bortolotto JW, Kist LW, Azevedo MB de, Fritsch RS, et al. (2012) Enndosulfan exposure inhibits brain AChE activity and impairs swimming performance in adult zebrafish (Danio rerio). NeuroToxicology 33(3): 469-475.
- Ezemonye LIN, Ikpesu TO (2011) Evaluation of sub-lethal effects of endosulfan on cortisol secretion, glutathione S-transferase and acetylcholinesterase activities in Clarias gariepinus. Food Chem Toxicol 49(9): 1898-1903.
- Moraes BS, Clasen B, Loro VL, Pretto A, Toni AC, et al. (2011) Toxicoogical response of Cyprinus carpio exposure to a commercial herbicide containing imazethapryl and imazapic. Ectoxicol Environ Saf 74(3): 328-335.
- Toni C, Menezes CC, Loro V, Clasen B, Cattaneo R, et al. (2010) Oxidative stress biomarkers in Cyprinus carpio exposed to commercial herbicide bispyriac-sodium. J Appl Toxicol 30(6): 590-595.
- Cunha I, Mangas Ramirez E, Guilhermino L (2007) Effects of copper and cadmium on cholinesterase and glutathione-S-transferase activities of two marine gastropods (Monodonta lineata and Nucella lapillus). Biochem Physiol C Toxicol Pharmacol 145(4): 648-657.
- Richetti SK, Rosemberg DB, Ventura-Lima J, Monserrat JM, Bogo MR, et al. (2011) Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 32(1): 116-122.
- Haverroth GMB, Welang C, Mocelin RN, Postay D, Bertoncello KT, et al. (2015) Copper acutely impairs behavioral function and muscle acetylcholinesterase activity in zebrafish (Danio rerio). Ecotoxicol Environ Saf 122: 440-447.
- Leitemperger J, Menezes C, Santi A, Murussi C, Lópes T, et al. (2016) Early biochemical biomarkers for zinc in silver catfish (Rhamdia quelen) after acute exposure. Fish Physiol Biochem 42(3): 1005-1114.
- Pan H, Zhang X, Ren B, Yang H, Ren Z, et al (2017) Toxic Assessment of Cadmium Based on Online Swimming Behavior and the Continuous AChE Activity in the Gill of Zebrafish (Danio rerio). Water Air & Soil Pollution 228(9).
- Zhang T, Yang M, Pan H, Li S, Ren B, et al. (2017). Does time difference of the acetylcholinesterase (AChE) inhibition in different tissues exist? A case study of zebra fish (Danio rerio) exposed to cadmium chloride and deltamethrin. Chemosphere 168: 908-916.
- Lee J, Peterson SM, Freeman JL (2017) Sex-specific characterization and evaluation of the Alzheimer's disease genetic risk factor sorl1 in zebrafish during aging and in the adult, brain following a 100 ppb embryonic lead exposure: characterization of zebrafish sorl1. J Appl Toxicol 37(4): 400-407.
- Green AJ, Planchart A (2018) The neurological toxicity of heavy metals: A fish perspective. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 208: 12-19.
- Simonato JD, Mela M, Doria HB, Guiloski IC, Randi MAF (2016) Biomarkers of waterborne copper exposure in the Neotropical fish Prochilodus lineatus. Aquat Toxicol 170: 31-41.
- Mazon AF, Cerqueira CC, Fernandes MN (2002) Gill cellular changes inducedby copper exposure in the south american tropical freshwater fish Prochilodusscrofa. Environ Res 88(1): 52-63.
- Martins AE, Bianchini A (2011) Toxicity tests aiming to protect Brazilian aquaticsystems: current status and implications for management. J Environ. Monit 13(7): 1866-1875.
- Mustafa SA, Davies SJ, Jha AN (2012) Determination of hypoxia and dietarycopper mediated sub-lethal toxicity in carp, Cyprinus carpio, at different levelsof biological organization. Chemosphere 87(4): 413-422.
- Colovic MB, Krstic DZ, Lazarevic Pasti TD, Bondzic AM, Vasic VM (2013) Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr Neuropharmacol 11(3): 315-335.
- Sarasamma S, Audira G, Juniardi S, Sampurna B, Liang ST (2018) Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int J Mol Sci 19(10): 3195.
- Kumar N, Gupta S, Chandan, NK, Aklakur M, Pal AK, et al. (2014) Lipotropes Protect against Pathogen-Aggravated Stress and Mortality in Low Dose Pesticide Exposed Fish. PLoS one 9(4): e93499.
- Kumar N, Krishnani KK, Kumar P, Jha AK, Gupta SK, et al. (2017) Dietary zinc promotes immuno-biochemical plasticity and protects fish against multiple stresses. Fish Shellfish Immunol 62: 184-194.
- Som Niyogi, Rebecca Kent, Chris M Wood (2008) Effects of water chemistry variables on gill binding and acute toxicity of cadmium in rainbow trout (Oncorhynchus mykiss): A biotic ligand model. Comparative Biochemistry and Physiology Part C 148(4): 305-314.
- Jia X, Zhang H, Liu X (2011) Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp Cyprinus carpio var color. Fish Physiol Biochem 37(1): 97-103.
- Mdegela RH, Mosha RD, Sandvik M, Skaare JU (2010) Assessment of acetylcholinesterase activity in Clarias gariepinus as a biomarker of organophosphate and carbamate exposure. Ecotoxicology 19(5): 855-863.
- FAO (1975) Manual of methods in Aquatic Environment Research Part1 Method for detection, measurement and monitoring of water pollution. FAO Fish tech 137: 69-76.
- Ellman GL, Courtney Kd, Andres v, Featherstone RM (1961) A new and rapid colourimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88-95.
- S. Food and Drug Administration (1994) Pesticide analytical manual, Vol.1 (PAM I). (3rd edn.) U.S. Food and Drug Administration, Washington, D.C.
- FAO/WHO (2001) Pesticide residues in food — 2000. Evaluations — 2000. Part II — Toxicology. Geneva, World Health Organization, Joint FAO/WHO Meeting on Pesticide Residues (WHO/PCS/01.3).
- EPA (1976) Heavy metals in the sediments of Port Phillip Bay and Input Streams. Environmental Protection Authority, Victoria. Report No. 16/76.
- World Health Organization (WHO) (1993) Revision of WHO guidelines for water quality. WHO Geneva.
- World Health Organization (1984) Guidelines for drinking-water quality (Vol. 1). Geneva, Switzerland: Recommendations, World Health Organization.
-
Aradhna Gupta, Nikhat J Siddiqi, and Bechan Sharma. Bioaccumulation and Biochemical Studies of Toxicants in Fish on AChE. Open J Pathol Toxicol Res. 1(1): 2021. OJPTR.MS.ID.000505.
-
Organochlorine pesticides (OCP), Heavy metals, Accumulation, Toxicity, Acetylcholinesterase (AChE), Hexachlorocyclohexane, Dichlorodiphenyltrichloroethane, Total dissolved solids, Decomposition, Volatilization, Predominant concentration, Glycogen, Herbicide bispyribac sodium, Zinc esposure
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






