 Research Article
Research Article
Acute Chronic Toxicity of Lindane in Channa punctatus
Aradhna Gupta1 and Bechan Sharma2*
1Department of Biochemistry, PDM University, India
2Department of Biochemistry, University of Allahabad, India
Bechan Sharma, Department of Biochemistry, University of Allahabad, India.
Received Date: August 02, 2021; Published Date: August 30, 2021
Abstract
In the present study, the response of the fish, C. punctatus, to different concentrations of lindane (0.1, 0.2, 0.3mg l-1) was found to be largely dependent on the concentration of pesticide and period of exposure (24, 48, 72 and 96h). Toxicity testing within 24h shows 40% mortality at 0.3 mg l-1; within 48h, 60% mortality was observed at 0.3 mgv l-1 and 20% at 0.2 mg l-1; within 72h, 40% at 0.2 and 80% at 0.3mg l-1, respectively. Within 96h, no mortality observed in control, whereas at 0.1, 0.2, and 0.3mg l-1, the mortality observed was 20, 80 and 100%, respectively. The 96h LC50 value of lindane was calculated by two methods (Finney probit analysis; Dede and Karber), the value being 0.15mg l-1. The acute chronic ratio calculated was found to be 11.811 in 30 days, 21.45 in 60 days and 30.82 in 90 days respectively at EC10%. Though the ACR values were within the safety guidelines established by REACH, a rising trend was seen. The results showed that lindane was directly impairing the behavioural and physiological processes in fish.
Keywords: Toxicity; LC50; Mortality; Probit; ACR
Introduction
In recent years interest in the environmental pollution and contamination of natural water has become widespread and acute toxicity has been used extensively to determine the effects of potentially toxic materials (pesticides, heavy metals, and industrial effluents) in the aquatic organism during the short term (96h or less) exposure [1]. The detrimental effects of these pollutants on aquatic ecosystems are either acute or chronic due to lethal/sublethal exposure which slowly results in their bioaccumulation not only in aquatic organisms but also in humans when they consume such fishes [2]. India is at 10th position in the top pesticide using countries, China being the first followed by USA, Argentina, Thailand, Brazil, Italy, France, Canada, Japan, and India [3]. Especially organochlorine pesticides have shown to inhibit physiological processes in target organisms [4]. Because of their persistent, non-biodegradable, and lipophilic nature, ATSDR has put them on top of the toxic substances list. Being a potent neurotoxin and endocrine disruptor, the use of lindane has been banned since 2009 under Stockholm convention for persistent organochlorine pesticides [5, 6]. The 4 modes of toxicity for organic toxicants were as follows (i) non polar narcosis or base line toxicity: in which an inert chemical is metabolized via nonspecific mode of action produces a narcosis effect in the body (ii) polar narcosis: toxicity is due to hydrogen bonding for less inert compunds that are slightly more toxic when compared to base line toxicity (iii) aspecific reactivity: an electrophile reacts with a nucleophile in macromolecules in an irreversible way in covalent bond and (iv) specific reactivity: in which there is a ligand and receptor and bond is non-covalent [7]. Adaptation or acclimatization is the capacity of an organism to survive in unfavourable or new conditions. Any change in the morphological and behavioral characteristics of any fish species upon exposure to any toxicant reflects the manifestations to defend their territory during unfavorable conditions. These responses depend on several factors like environmental conditions, concentration of toxicant in medium, species and size of the organism. The interaction of these toxicants with the olfactory neurons of aquatic organisms leads to impair neuron functionality [8]. The behavioral changes due to predator-prey interactions act as essential link between the effects of toxicants on individuals and results at a higher level of organizations [9]. The assessment of these responses in laboratory conditions provides us the information on how much low the concentration of toxicants is in the water, no matter if not lethal; it will always have its slow and undetectable effects on the aquatic biota.
The present study was carried out to assess the acute toxicity of lindane up to 96h treatment duration and the behavioural changes of C. punctatus under laboratory conditions. The results obtained were then manipulated by ACE software for determining acute chronic toxicity upto 90 days. The need to extrapolate the acute toxicity results into the chronic toxicity is to assess the sensitiveness to aquatic organisms on long term of exposures. To perform chronic toxicity tests for aquatic organisms in laboratories is not easy as it requires maintaining the animals in living condition, laborious and expensive too. ACE (acute to chronic estimation) Software Version 2.0, calculated data are used widely and proved to be accurate in manipulating low level mortality in chronic exposures [10-13]. The maximum admissible toxicant concentration (MATC) is the value which has two limits. The lowest limit is the value (NOEC) which has no observed affect at higher concentrations and highest limit is the value (LOEC) which has lowest observed effect based on statistics analysis of variance with no confidence limits.
Materials and Methods
Healthy Snakehead fish C. punctatus mean length (6cm-8cm) and mean weight (25g-30g) were treated with potassium permanganate (0.1%, w/v) and acclimatized for seven days in water at 18- 220C in separate glass tanks (1x1 ft), each containing 5 fish, under standard laboratory conditions. Lindane (99% pure) technical grade was used in the study. The fish were exposed to 0.3, 0.2, and 0.1mg/l of the compound dissolved in 0.5ml of acetone. In the tank containing a control group, only 0.5ml of acetone (no pesticides) was added. Fish were confirmed dead when there was no more extended response to prodding. The 96h L50 value of lindane was calculated using the arithmetic method of Karber as adopted by Dede and Kaglo [14] and by the Finney probit regression line [15]. The behavioural morphological changes in the model organism fish were also recorded during the course of exposure. Further with the help of ACE software the maximum likelihood values for no effect concentration for 30, 60 and 90 days respectively were used to analyze Acute chronic toxicity ratio (ACR). The NOEC and LOEC values were taken at EC 10% (effective concentration at 10% mortality). The ACR was calculated by formula:
ACR= LCACR= LC50/MATC; MATC=√(NOEC)(LOEC/MATC; MATC=√(NOEC)(LOEC
Results and Discussion
Behavioural and Morphological study
The behavioral response of the fish C. punctatus treated with lindane was found to be largely relying on the concentration of pesticide and period of exposure. The responses were determined when the fish was exposed to different concentrations of lindane (0mg/l-control, 0.1mg/l, 0.2mg/l and 0.3mg/l) for different periods of treatment (24h, 48h, 72h and 96h). The changes in morphological and behavioral responses were recorded and the results are shown in (Table 1).
Table 1: Impact of lindane on the behavioural pattern of C. punctatus exposed for 96h.
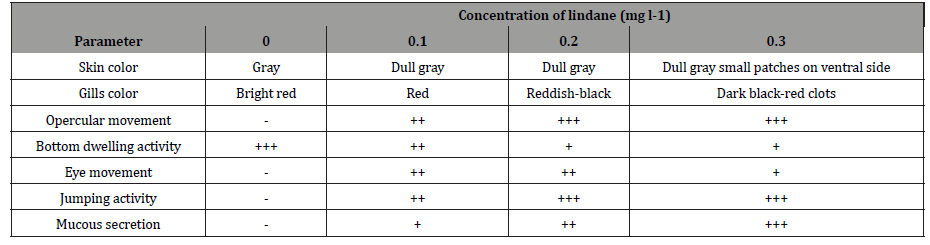
The fish were observed for 96h and behavioral parameters were recorded as suggested by Kumari et al., (1997). (-) sign shows regular activity. Increase or decrease in the (+) sign shows an increase or decrease in the activity. h represents time in an hour. The data indicated a drastic increase in the rate of opercular movement and jumping activity of fish, whereas the bottom-dwelling activity significantly decreased compared to control. Upon increasing the concentration of pesticide in the incubation medium, the responses of the fish also enhanced. However, their eye movement increased up to 0.2mg/l compared to that of 0.3mg l-1. At highest concentration of lindane (0.3mg l-1) the colour of the fish skin turned to dull gray with small dark coloured patches on the ventral side.
The different behavioural responses shown by fish are in actual the strategies which the organisms used to resist the chemical exposure. These toxicants may be entering the circulatory path by direct diffusion through gill filaments or skin [16]. Gills respond quickly to toxicants due to their large surface area and small diffusion distance between water and blood. The change in color of gills at higher concentration (0.3mg/l) proves that there is some kind of impairment in the respiratory surface resulting in decreased gas exchange and blood flow and increased opercular rate [17]. Minimum secretion of mucous is normal as it protects the fish from the direct interaction with aquatic microbes by colonization of microbes on mucous. It also improves mechanical protection and osmoregulation. The excess mucous secretion in the present study by the fish may have clogged the gill surface resulting in higher gas exchange and respiration [18]. Such fishes have been shown to possess increased rate of pesticide uptake from the aquatic environment which reduces the bottom-dwelling activity of fishes possibly by modifying the behavior of the fish which strives to survive on least expense of energy and exposure of toxicant [19]. Stressed fish uses stored fats and protein to compete with the existing condition of toxicant exposure. Stress can also be correlated to neuronal excitation resulting in decreased neurotransmitters and enzymes. They probably block the GABA-gated chloride metabolism leading to hyperexcitation, convulsions and death. The results of the present study indicated that lindane exerts toxic effects on fish. The change in skin color may be due to less secretion of melanophore stimulating hormone or direct action of pesticide on melanocyte. The jumping or escape behavior, more directly related to swimming patterns, is a prevalent indicator of aquatic pollution endured by aquatic organisms. It has been proved that with increase in exposure period there is increase of reactive oxygen species which later decreases [20]. Based on this finding it can be correlated to absence of responses or no activity shown by fish exposed to contaminants which may be due to their acclimatization potential, and thus non-willingness or lack of energy or impaired decision-making ability. The erratic/ lethargic swimming behavior has been reported in Zebrafish for nano plastics, European Seabass exposed to mercury etc [21, 22].
Acute Toxicity testing
The toxicity testing of lindane in C. punctatus within 24, 48, 72 and 96h is shown in Table 2. Toxicity testing within 24h shows no mortality in control (0 mg l-1) and at 0.1mg l-1 and 0.2mg l-1 concentrations of lindane, whereas 40% mortality was observed at 0.3 mg l-1. After treating the fish for 48h, no mortality was observed in control (0mg l-1) and at (0.1mg l-1), whereas 20% and 60% mortality was observed at 0.2 and 0.3 mg l-1 concentrations, respectively. The toxicity assay after 72h shows no mortality in control (0 mg l-1) and at 0.1mg l-1 concentration of lindane, whereas 40 and 80% mortality was observed at 0.2 and 0.3mg l-1 concentrations of lindane, respectively. When the experiment was extended upto 96h, no mortality was observed in control (0 mg l-1), whereas 20, 80, and 100% mortality was observed at 0.1, 0.2, and 0.3mg l-1, respectively. The 96h LC50 value of lindane was calculated using the arithmetic method of Karber as adopted by Dede and Kaglo (2001) Table 3 and by probit regression line Figure 1. The 96h LC50 value of organochlorine pesticide lindane was calculated to be 0.15mg l-1 in C. punctatus by both methods (Table 2,3 and Figure 1).
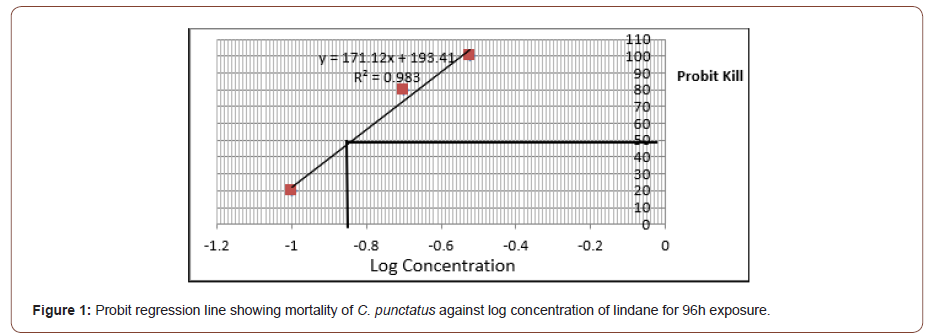
Table 2: Toxicity testing of lindane on C. punctatus up to 96h.
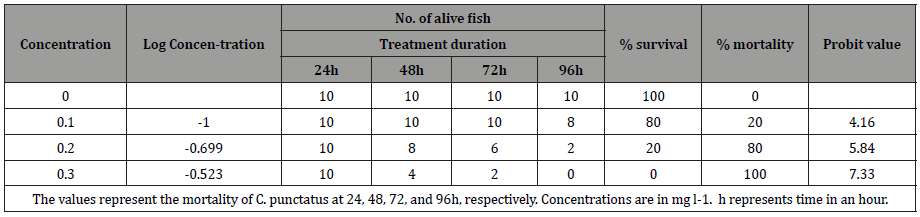
Table 3: 96h LC50 value was determined using Arithmetic method of Karber as shown below.
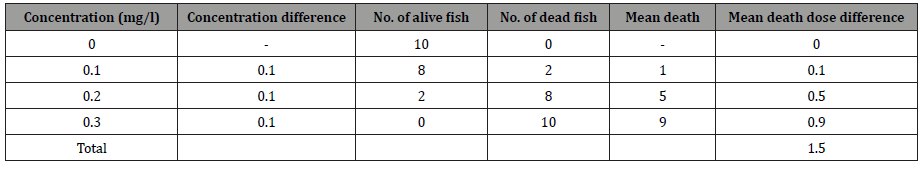
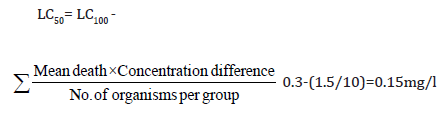
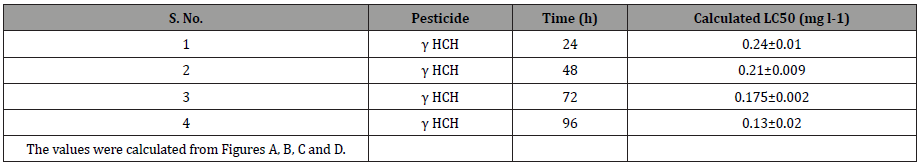
The percent mortality of C. punctatus at different concentrations of lindane for varying treatments period such as 24, 48, 72, and 96h were also recorded. The results presented in Figure 2 (A, B, C, and D) indicated S-shaped curve between percent mortality and the concentration of lindane for C. punctatus exposed to the pesticide for specific treatment duration. The LC50 values for each of the treatment durations were calculated from these graphs. The calculated LC50 values for lindane to C. punctatus at 24, 48, 72, and 96h are demonstrated in Table 4. The results indicated a maximum LC50 value for the smallest treatment duration (24h) and the lowest LC50 value for lindane at maximum treatment duration (96h). The computed LC50 values were found to be 0.24, 0.21, 0.175 and 0.13mg l-1, respectively, for lindane after 24, 48, 72, and 96h (Figure 2 and Table 4).
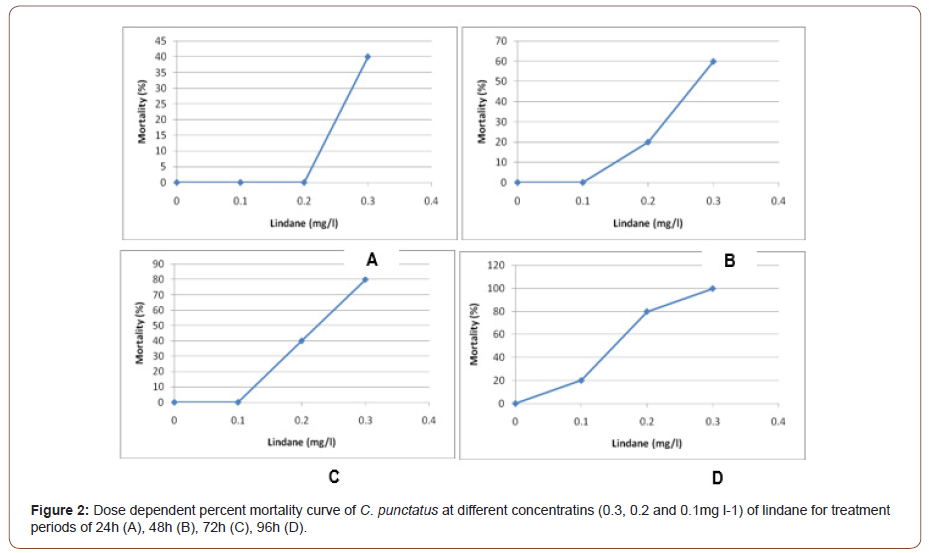
Table 4: LC50 values of lindane at different treatment durations.
Acute toxicity caused by lindane showed a significant positive correlation between dose and mortality because increased concentrations of toxic chemicals in water resulted in more intake or entry of toxic chemicals in the body of the animals. The LC50 values found to be continuously decreased with an increasing period of exposure Table 4. It is usually observed that toxicity of pollutants to fish often increases with temperature, which may be due to reduced oxygen solubility or increased uptake of the toxin [23]. Toxicity to fish decreases as their weight increases because of lipophilic nature of lindane and so less availability to target organs [24, 25].
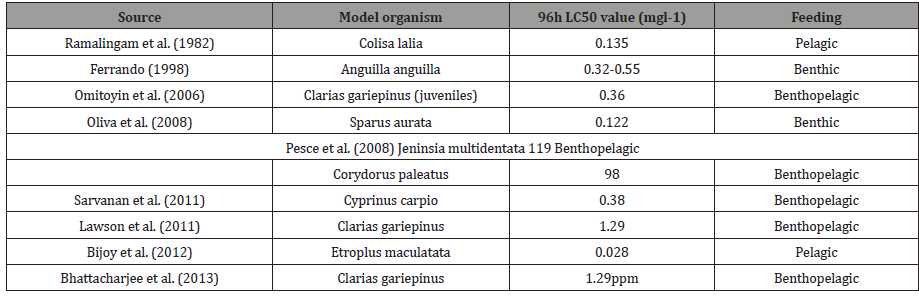
The results of the present study shown in Tables 3 indicated that lindane is quite efficient in causing lethal toxicity to the fish even at very low concentrations. The 96h LC50 value (0.15mg l-1) for C. punctatus suggests that the fish showed a quick response to the toxicant. The reported 96h LC50 values in different model organisms exposed to lindane have been summarised in (Table 5).
Table 5: LC50 of lindane in different model organisms.
Maximum Likelihood estimate for NO Effect concentration
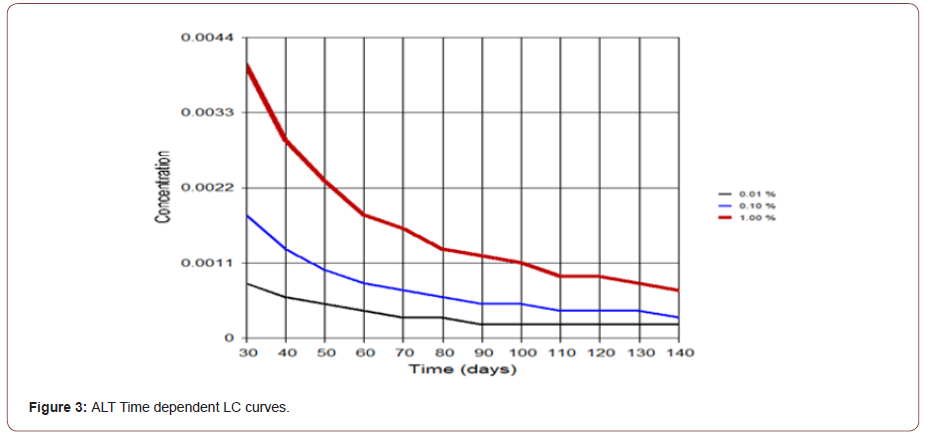
Table 6 shows the statistical output, the maximum likelihood estimates of chronic no-effect concentrations. The analyses were performed for three different chronic times (30, 60 and 90 days). Within each time period are percent level of chronic mortality (0.01–10.0%), predicted toxicant concentration associated with each percentage, standard error of the predicted toxicant concentration, and confidence limits (default is 95% confidence limits). Time dependent LC curves at 0.01%, 0.01% and 1% mortality is shown in (Figure 3 and Table 6). From the Table 6 it can be seen that concentration is decreasing to cause mortality as time is increasing. The MATC values for 30, 60, 90 days were found to be 0.0038, 0.0015, 0.0019 at 10% EC. The calculated ACR (acute chronic toxicity ratio) ratio at EC10% for 30, 60 and 90 days is 11.811, 21.45, 30.82. The results show increase in ACR value on increasing exposure period. The increase in ACR ratio can be due to many factors. Not a single factor can be attributed to the increase of ACR values. It can be due to long time bioaccumulation as some times chemicals are present chronically in the medium or persistence which is evidenced by the erratic behaviour of aquatic organisms. The high ACR values tell us about injury caused to aquatic organisms by pesticides and heavy metals [26]. The ACR values upto 10.5 has been reported for fish which is less than the ACR safety factor of 100 as stated by REACH guidance [27, 28]. The calculated ACR values in the present study are below the ACR safety factor given by REACH guidance.
Table 6: Maximum Likelihood estimate for “NO Effect” concentration by Accelerated Life Testing (ALT) Theory.

Conclusion
The results of present study can be helpful in developing a risk analysis model to different organochlorine pesticides and their mixtures to understand the latent effects of the pesticide under sub-lethal concentrations. It also indicated that adequate risk assessment to aquatic life must be taken into account before the broad-scale application of lindane as consistent exposure to lindane may be lethal to fish. Its use must be strictly controlled and regulated by appropriate legislation to prevent its bioaccumulation in the environment and to avoid immediate disastrous consequences in the aquatic ecosystem. The assessment of the responses of fish as recorded upon lindane exposure in laboratory conditions renders the information on how much low the concentration of toxicants could be in the water, no matter if not lethal; it would always have its slow and undetectable effect on the morphological and physiological indices of the aquatic biota.
Acknowledgement
Authors are grateful to the Department of Biochemistry, University of Allahabad for providing research facilities for the work.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Connon RE, Geist J, Werner I (2012) Effect-Based Tools for Monitoring and Predicting the Ecotoxicological Effects of Chemicals in the Aquatic Environment. Sensors 12(9):12741-12771.
- Vijgen J, de Borst, B Weber, R Stobiecki T, Forter M (2019) HCH and lindane contaminated sites: European and global need for a permanent solution for a long-time neglected issue. Environ Pollut.
- (2018) World Atlas.
- Martyniuk CJ, Mehinto AC, Denslow ND (2020) Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol Cell Endocrinol 507: 110764.
- Legradi J, Di Paolo C, Kraak M, Van Der Geest H, Schymanski E, et al. (2018) An ecotoxicological view on neurotoxicity assessment. Environ SciEur 30(1): 46.
- UNEP (2007) Stockholm Convention on Persistent Organic Pollutants (POPs). UN Environ Program.
- Verhaar HJM, Van Leeuwen CJ, Hermens JLM (1992) Classifying environmental pollutants. Chemosphere 25(4): 471-491.
- Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, et al. (2010) Olfactory toxicity in fishes. Aquat Toxicol 96(1): 2-26.
- Faria M, Wu X, Luja-Mondragón M, Prats E, Gómez-Oliván LM, et al. (2020) Screening anti-predator behaviour in fish larvae exposed to environmental pollutants. Sci Total Environ 714: 136759.
- Mayer FL, Ellersieck MR, Krause GF, Sun K, Lee G, et al. (2002) Time-concentration-effect models in predicting chronic toxicity from acute toxicity data. In Crane M, Newman MC, Chapman PF, Fenlon J, (Eds). Risk Assessment with Time to Event Models. Pp. 39-67.
- Mayer FL, Ellersieck MR, Asfaw A (2009) Risk Assessment Tools: Software and User’s Guide. SETAC Pensacola, FL, USA.
- Mayer FL, Ellersieck MR, Slaughter AR (2011) Accuracy assessment of time-concentration-effect models in predicting chronic lethality from acute toxicity data. Environmental Toxicology and Chemistry 30(3): 757-762.
- Ellersieck MR, Asfaw A, Mayer FL, Krause GF, Sun K, et al. (2003) Acute-to-chronic estimation (ACE v 2.0) with time-concentration-effect models: User manual and software. EPA/600/R-03/107. U.S. Environmental Protection Agency, Washington, DC.
- Dede EB, Kaglo HD (2001) Aqua-toxicological effects of water-soluble fractions (WSF) of diesel fuel on O. Niloticus fingerlings. J Appl Sci Environ Manage 5: 93-96.
- Finney DJ (1952) Probit Analysis. (2nd Edn). By Cambridge University Press, New York J Am Pharm Assoc (Scientific Ed.) 41(11): 627.
- Gupta A, Rai DK, Pandey RS, Sharma B (2008) Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ Monit Assess 157(1-4):449-458.
- Fernandes MN, Paulino MG, Sakuragui MM, Ramos CA, Pereira CDS, et al. (2013) Organochlorines and metals induce changes in the mitochondria-rich cells of fish gills:An integrative field study involving chemical, biochemical and morphological analyses. Aquat Toxicol 126: 180-190.
- Paulino MG, Sakuragui MM, Fernandes MN (2012) Effects of atrazine on the gill cells and ionic balance in a neotropical fish, Prochilodus lineatus. Chemosphere 86(1): 1-7.
- Kuppulakshmi C, Prakash M, Gunasekaran G, Manimegalai G, Sarojini S (2008) Antibacterial properties of fish mucus from Channa punctatus and Cirrhinus mrigala. Eur Rev Med Pharmacol 12(3): 149-153.
- Piskac-Collier AL, Smith MA (2009) Lindane-Induced Generation of Reactive Oxygen Species and Depletion of Glutathione do not Result in Necrosis in Renal Distal Tubule Cells. J oxicol Environ Health A 72(19): 1160–1167.
- Chen Q, Gundlach M, Yang S, Jiang J, Velki M, et al. (2017) Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci Total Environ 584-585: 1022-1031.
- Barboza LG A, Vieira LR, Guilhermino L (2018) Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchuslabrax): Changes in behavioural responses and reduction of swimming velocity and resistance time. Environ Pollut 236: 1014-1019.
- Claësson D, Wang T, Malte H (2016) Maximal oxygen consumption increases with temperature in the European eel (Anguilla anguilla) through increased heart rate and a rteriovenous extraction. Conserv Physiol 4(1): cow027.
- Geyer HJ, Scheuneti I, Briiggemann R, Langer D, Korte F, et al. (1997) Half-lives and bioconcentration of lindane (γ-HCH) in different fish species and their relationship with lipid content. Chemosphere 35(2): 343-351.
- Lee JW, Choi H, Hwang UK, Kang JC, Kang YJ, et al. (2019) Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ Toxicol Pharmacol 68: 101-108.
- Kenaga EE (1982) Predictability of chronic toxicity from acute toxicity of chemicals in fish and aquatic invertebrates. Environmental Toxicology and Chemistry 1(4): 347-358.
- Ahlers J, Riedhammer C, Vogliano M, Ebert RU, Kuhne R, et al. (2006) Acute to Chronic Ratios in Aquatic Toxicity-Variation Across Trophic Levels and Relationship with Chemical Structure. Environmental Toxicology and Chemistry 25 (11): 2937–2945.
- (2008) ECHA (European Chemicals Agency) Characterisation of Dose [Concentration]–Response for Environment. Chapter R.10 in Guidance on Information Requirements and Chemical Safety Assessment. Helsinki.
-
Aradhna Gupta, Bechan Sharma. Acute Chronic Toxicity of Lindane in Channa punctatus. Open J Pathol Toxicol Res. 1(1): 2021. OJPTR.MS.ID.000501.
-
Toxicity, LC50, Mortality, Probit, ACR, Environmental Pollution, Potentially Toxic Materials, Heavy Metals, Industrial Effluents, Aquatic Ecosystems, Aquatic Biota, Neurotransmitters Enzymes, Lethargic Swimming
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






